Abstract
Pattern recognition receptors (PRRs) and their signaling pathways have essential roles in recognizing various components of pathogens as well as damaged cells and triggering inflammatory responses that eliminate invading microorganisms and damaged cells. The zebrafish relies heavily on these primary defense mechanisms against pathogens. Here, we review the major PRR signaling pathways in the zebrafish innate immune system and compare these signaling pathways in zebrafish and humans to reveal their evolutionary relationship and better understand their innate immune defense mechanisms.
Similar content being viewed by others
Introduction
Zebrafish (Danio rerio) is extensively used as a model organism in many fields, including developmental biology, cancer and immunology.1, 2, 3 There is a clear temporal separation between the innate and adaptive immune responses of this organism. Zebrafish relies more on the innate immune system than mammals because it does not have a morphologically and functionally mature adaptive immune system until 4 weeks after fertilization. As a result, the zebrafish model can provide new insights into the function and evolution of innate immune responses.
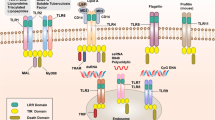
As the first line of host defense, the innate immune system relies on a large family of pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs) derived from various microbial pathogens, including viruses, bacteria, fungi, parasites and protozoa,4 and danger-associated molecular patterns (DAMPs) that are present in aberrant locations or abnormal molecular complexes as the consequence of infection, inflammation or cellular stress.5, 6 Currently, the four best characterized groups of PRRs include the Toll-like receptors (TLRs), the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and the absent in melanoma-2 (AIM-2)-likereceptors (ALRs). PRRs can be localized at the cell surface (TLRs, CLRs), within the cytoplasm (NLRs, RLRs and ALRs) or in endosomes (TLRs). The cell surface PRRs are responsible for surveying the extracellular environment, whereas the cytoplasmic PRRs sense intracellular pathogens or danger signals. Endosomal PRRs are used to detect microbes that have entered the phagolysosome. On PAMP or DAMP recognition, PRRs activate signaling cascades leading to the NF-κB and interferon (IFN) response factor (IRF) transcription factors, thus leading to the induction of proinflammatory cytokines, chemotactic cytokines and antimicrobial responses.
Zebrafish rely on cytokine and IFN production, complement activation, innate immune cell activation and cellular cytotoxic stimulation as host defense mechanisms against pathogens.1 Several classical receptor families that are involved in primary immune responses have been identified in the zebrafish genome. Counterparts of the majority of vertebrate PRRs and downstream signaling components have been identified in zebrafish, and some of these have been functionally characterized (Table 1). In this review, we focus on the pattern recognition receptors and their signaling pathways in zebrafish and compare them with their counterparts in mammals, including humans, to reveal their evolutionary relationship and provide new insight into innate immune defense mechanisms.
Pattern recognition receptors
Toll-like receptors
The mammalian TLR family consists of 12 members that are integral glycoproteins possessing an extracellular (or intra-endosomal) ligand-binding domain with a leucine-rich repeat (LRR) motif and a cytoplasmic signaling Toll/Interleukin-1 (IL-1) receptor homology (TIR) domain.7, 8 Some TLRs (TLR1, -2, -4, -5, -6 and -10) are expressed at the cell surface, whereas others (TLR3, -7, -8, -9, -11 and -13) are located almost exclusively in intracellular compartments, including endosomes and lysosomes. TLRs are principally responsible for the recognition and response to pathogen ligands such as lipopolysaccharide (LPS) from Gram-negative bacteria (TLR4 ligand),9 lipoteichoic acid from Gram-positive bacteria (TLR2 ligand)10 and the flagellin protein (TLR5 ligand).11 Nucleic acids such as dsRNA, ssRNA and single-stranded unmethylated CpG motif-containing DNA are recognized by TLR3, TLR7 and TLR9, respectively, in antiviral or antibacterial pathways.12, 13, 14, 15, 16 In a typical mammalian TLR signaling cascade, ligand binding to the extracellular leucine-rich repeats region causes TLRs to dimerize and undergo conformational changes and/or oligomerization of their intracellular TIR domains, which in turn recruit and activate cytosolic TIR domain-containing adaptor molecules such as MyD88, MyD88 adaptor-like protein (MAL/TIRAP), TRIF/TICAM1 and TRIF-related adaptor molecule (TRAM/TICAM2) to transduce signals from the membrane surface to the cytosol and then to the nucleus via the activation of downstream transcription factors such as ATF, NF-κB, AP-1, IRF and the STAT family. The MyD88-dependent signaling pathway recruits downstream IRAKs and activates TRAF6 and the IκB kinase (IKK), leading to the translocation of NF-κB to the nucleus and the secretion of anti-infection molecules and inflammatory cytokines and type-I IFN production in dendritic cells (DCs). In the MyD88-independent signaling pathways, the non-typical IKKs IKKi/IKKɛ and TBK1 mediate the activation of IRF3 downstream of TRIF,17 which is responsible for type-I IFN responses in non-DCs.
The TLR protein family is conserved from insects to mammals, but TLR signaling pathways in fish exhibit different features than those in mammals.18 TLRs are highly expressed in the skin of zebrafish, which suggests a prominent role in the defense against pathogens. Zebrafish has an almost complete set of 20 putative TLR variants.19, 20 Among them, 10 are orthologs of human TLR family members, and TLR22 belongs to a fish-specific subfamily that is closely related to the Drosophila melanogaster toll-9 gene. TLR21 is common to birds, amphibians and fish. Zebrafish TLR9 and TLR21 were found to have similar expression profiles and antimicrobial activities toward CpG-ODNs.21 Because the genome of teleost fish was duplicated during evolution, zebrafish have two counterparts of some mammalian TLRs, including tlr4ba/tlr4bb for the LPS-specific TLR4 and tlr5a/tlr5b for TLR5, and tlr8a/tlr8b. Homologs of mammalian TLR6 and TLR10 are absent from fish, but TLR14 and TLR18 are non-mammalian. It has been reported that Japanese flounder TLR14 shares some features with TLR1, TLR6 and TLR10, suggesting that TLR14 might be a functional substitute for mammalian TLR6 and TLR10.22 TLR18 in zebrafish and channel catfish is the homolog of human TLR123, 24 and may correspond to TLR14 in other fish.18 In addition, Peitretti et al.25 identified TLR20 in zebrafish and common carp, finding that this protein is similar to TLR11 and TLR12 in mice. It is not clear when such gene duplications or deletions occurred during evolution, suggesting that the innate immune system of fish may be more complex than that of mammals. The ancient jawless vertebrate Japanese lamprey has no more than 16 TLR genes,26 whereas there are up to 20 putative TLR variants in zebrafish. Ohno proposed that two rounds of whole-genome duplication have occurred during early vertebrate evolution, with a third fish-specific genome duplication occurring later in a basal teleost.27 A fourth whole-genome duplication even occurred in some cyprinids 8–21 million years ago,28, 29 resulting in the appearance of paralogs of ancestral genes and the development of neofunctionalization.30 With respect to the TLR repertoire, it is of interest to note that, except for a single tlr21 gene, two tlr23 genes and tlr22-related genes, most TLR genes seem absent from the Atlantic cod,31 which may be the result of positive selection to generate neofunctionalization.32 However, whether the increase in the number of TLRs in fish is associated with diversification in ligand recognition is still unknown.
The ligands of some zebrafish TLR receptors have been identified (Table 2). For example, TLR2 can form heterodimers that are responsible for the recognition of bacterial lipoproteins/lipopeptides or Pam3CSk4.20, 33 TLR3, -5 and -9 recognize dsRNA, flagellin and unmethylated CpG DNA, respectively.11, 13, 16, 34, 35 However, zebrafish TLR4 is not responsive to LPS stimulation, despite the fact that MD1 and Rp105, which mediate ligand delivery and/or recognition, have been identified. Biochemical and functional studies indicate that MD1 binds both Rp105 and TLR4 in zebrafish,36 but accessory molecules such as LBP, CD14 and MD2 have not been isolated from fish,30, 37, 38 suggesting that TLR4 ligand specificity is not conserved in zebrafish.39 The knockdown of tlr4a, tlr4b and myd88 did not disrupt the zebrafish immune response to LPS exposure,40 suggesting other unidentified roles of TLR4 signaling in PAMP responses. In addition, zebrafish lacks a clear ortholog of caspase-11, which serves as an intracellular LPS receptor in mice. On one hand, Zebrafish Caspy 2 shows the highest homology to human caspase-5 and a preference for caspase-5-like substrates, and Caspy 2 also induced apoptosis in mammalian cells that was inhibited by general caspase inhibitors;41 on the other hand, the Caspy 2 does not contain a CARD domain as found at the N-terminal of caspases. Actually the N-terminal domain of Caspy 2 is most homologous to the N-terminal domain of human NLRP3 (46% similarity), a pyrin-domain-containing NOD-family protein,41 thus it remains to be seen whether fish Caspy 2, which is similar to human caspase-4/5 (caspase-11), can interact with LPS directly and activate the inflammasome. The above discussion suggests that the mechanism of LPS recognition in fish could be different from that in mammals, and it is possible that ancestral or other genes are involved in sensing LPS. In addition, the fish-specific TLR22 recognizes dsRNA viruses or PolyI:C; this is followed by the recruitment of TRIF to induce IFN expression. Thus, TLR22 could be a functional homolog of mammalian TLR3.35 In fact, zebrafish also has TLR3, and TLR3 and TLR22 recruit a common adaptor TRIF to augment the local IFN response to viral infection.35
The innate immune signaling molecules downstream of TLRs are conserved in zebrafish as well, and include orthologous MyD88, SARM1, Tollip, IKAP (IKK complex associated protein), NEMO (NF-κB essential modulator), TIRAP, TRIF and the central intermediator IRFs, the signal transducers and activators of transcription (StatS), AP-1,42 and all of the TRAF family members (traf1 to traf7).43
MyD88, the most-studied TLR adaptor in zebrafish, has important roles in host defense against microbial infections.44 In fact, all adaptor molecules except for TICAM2 (TRAM) have been identified in zebrafish. Although mammals have two copies of TICAM, TICAM2/TRAM was lost specifically from teleost fish, and the only homologous gene is distantly related to TICAM1 or TICAM2, indicating that an ancestral gene subsequently diverged to two copies. Zebrafish TICAM1 localizes to the Golgi apparatus and lacks the N-terminal and C-terminal proline-rich domains found in the mammalian protein,45 despite being able to activate NF-κB promoters and IRF3- and IRF7-mediated pathways.46, 47 The partial function attenuation of TIRAP in zebrafish, which may weaken its ability to recruit MyD88,18, 48 leads to low sensitivity to LPS. Overexpression of the adaptor TRIF induces IFN production in zebrafish, suggesting that zebrafish trif is a true homolog of the mammalian gene.45 In conclusion, TLR adaptors are highly conserved between mammals and teleosts, suggesting that the signaling downstream of TLRs is highly conserved between the innate immune responses of mammals and fish.
The zebrafish has unique features in the components of its TLR downstream pathways, including some gene duplications or losses. With regard to IFN response factors, all of the nine IRF orthologs of mammals have been identified in fish. There are two additional IRFs in zebrafish, namely IRF10 and IRF11, which are absent in mammals.49 Furthermore, zebrafish traf1 differs from mammalian traf1 in that it contains a single zinc finger motif. In addition, traf4 and traf2 are duplicated in zebrafish.50
The zebrafish is now being utilized as a model for infectious disease because of the ease of using particular stages to examine immune responses to infection as well as host–microbe interactions. TLRs are the best understood innate immune receptors that respond to infection in fish. In 2002, Neely et al.51 established a zebrafish model of Streptococcus infection. Their studies suggested that the principles underlying host–pathogen relationships in fish are very similar to those in humans. TLR3 mRNA was upregulated in zebrafish upon infection with Gram-negative bacteria.52 Mycobacterial infection of zebrafish results in elevated TLR1, TLR2, TLR5a/5b and TLR18 expression and also induces the expression of fish-specific TLR20a and TLR22. These studies suggest that the microbial PAMP recognition mechanism is already established in the common vertebrate ancestor and is conserved in mammals and teleosts.
The zebrafish MAL orthologs that are downstream of TLRs also show increased expression, whereas the expression levels of other TIR domain-containing adaptor genes such as MyD88, TRIF and SARM are not responsive to Mycobacterial infection,24 suggesting that the signaling pathways downstream of fish TLRs are different from those in mammals. Of course, infecting the entire animal with a live pathogen induces alterable changes in gene expression due to the presence of multiple PAMPs. As a result, it is difficult to distinguish the correlation between the upregulation of TLR signaling genes and the recognition of specific pathogen-derived ligands.
NOD-like receptors and inflammasome pathways
Intracellular monitoring is performed by several families of receptors to detect those pathogens that evade extracellular and endosomal surveillance. These include the NLRs for different PAMPs and DAMPs,53 RIG-like helicase receptors (RLRs) for viral RNA,54 and the ALRs for cytosolic DNA.55, 56, 57, 58 The innate immune signaling pathways associated with the NLRs and RLRs are largely conserved in mammals and teleost fish, but the signaling pathways for the ALRs are not. The expression of ALRs is restricted to and conserved among mammalian species, and the loss of ALRs in fish suggests they have evolved alternate mechanisms to cope with DNA viruses and intracellular bacteria.59
Owing to the importance of inflammasomes in the innate immune defense system, numerous studies were extended to vertebrates to uncover their functions. At present, the function of NLRs in lower vertebrates and invertebrates is less well understood than that in mammals. With nearly 421 NLR family members in zebrafish,60 it is predicted that at least one prototype gene exists in lower organisms. With events such as gene loss, duplication and acquisition in various species, this unique gene family is likely to have formed gradually in vertebrates during evolution. By analyzing the molecular phylogeny and expression of NLR subfamilies in zebrafish, three NLR subfamilies have been identified, with the first subfamily (NLR-A) containing eight genes that resemble mammalian NODs, the second subfamily (NLR-B) containing nine genes that resemble mammalian NACHT-, LRR- and PYD-containing proteins (NLRP), and the third subfamily (NLR-C) containing 405 NLR genes that are unique to teleost fish.60, 61 Recent reports have indicated that several members of the mammalian NLR family, namely NOD1, NOD2 and NLRC3, are conserved in zebrafish.62 NOD2 cooperates with the dual oxidase (DUOX) enzyme to produce bactericidal reactive oxygen species in epithelial cells in mammals.63 Similarly, the morpholino-mediated depletion of zebrafish NOD1 or NOD2 significantly decreases zebrafish DUOX expression, which reduces the ability of embryos to control systemic infection in a Salmonella infection model64 and suggests that NOD-like receptors are also important for innate antibacterial immunity in teleost fish.
In contrast, NLRP3, the most extensively studied mammalian NLR in the inflammasome, is not conserved in zebrafish,61, 65, 66 and there are no direct NLRP3 orthologs in fish. Although Boyle identified a gene consisting of an NTPase domain, leucine-rich repeats and a C-terminal PRY-SPRY domain on zebrafish chromosome 17 using human NLRP3 in a BLASTP search, the lack of an N-terminal effector domain distinguished this gene from mammalian NLRP3.66 In addition, there are not any other putative NLRP3 orthologs in the genomes of other fish. Stein et al.65 has suggested that these NLRPs in lower vertebrates are not similar to the NLRs in mammals and are not the origin of inflammasome components, as these genes provide diverse responses to infection and injury in different species. Such differences between fish and mammals are interesting and may encourage scientists to reconsider the mechanism and evolution of the inflammasome. In brief summary, NLRPs are not functionally conserved in fish, and whether other zebrafish-specific NLRs may function through inflammasome-like molecular complexes awaits future investigation.
Despite these differences, some components of innate inflammatory pathways are conserved in zebrafish. As a key adaptor molecule in the inflammasome pathways, apoptosis-associated speck-like protein containing a CARD (ASC) links upstream receptors (such as NLRs/PYHINs) and downstream signaling caspases through homotypic or heterotypic protein–protein interactions to form the inflammasome complex, a granular structure formed in the perinuclear region of the cytosol upon inflammasome activation that ultimately leads to the processing of pro-IL-1β and pro-IL-18.67, 68 ASC is highly conserved in vertebrates but is not present in invertebrates. Zebrafish has a single ortholog of ASC (Figure 1, accession number NM_131495) that is also composed of an N-terminal PYD domain and a C-terminal CARD domain. zASC has been observed to form SPECKs in vitro and in the embryo (Li and Jin, unpublished data), suggesting a conserved function of ASC in inflammasome assembly.
Evolutionary relationships of ASC. ASC sequences were obtained from the NCBI database (http://www.ncbi.nlm.nih.gov). Sequences were aligned with Clustal-Omega, and the molecular phylogeny tree was inferred using the Neighbor-Joining method in MEG6.0. The scale bar reflects 0.1-nt substitutions per site. ASC, apoptosis-associated speck-like protein containing a CARD.
In addition to the canonical inflammasomes that activate caspase-1, a non-canonical caspase-11-dependent inflammasome pathway was recently discovered.69, 70, 71 The N-terminal CARD domain of caspase-11 serves as a direct receptor for intracellular LPS. LPS binding induces conformational changes and the oligomerization of caspase-11, activating caspase-11. Activated caspase-11 cleaves gasdermin D to induce pyroptosis.72, 73, 74 In zebrafish, there are two caspase genes, namely Caspy and Caspy 2. Interestingly, their N-terminal regions share higher sequence similarity with the PYD than the CARD domain of mammalian caspases, both of which belong to the death-fold superfamily. These zebrafish caspases function as inflammatory rather than apoptotic mediators.41 The sequence of the zebrafish Caspy PYD domain is similar to the N-terminal domains of caspases from non-mammals such as tetrapod, suggesting their functional similarity. Comparative alignment of the PYD or CARD domains of zebrafish shows that the N-terminal domain of Caspy has sequence similarity to the zebrafish ASC PYD domain, and the sequence of the zebrafish ASC CARD domain is similar to the CARD domain from zebrafish NLP3 (NACHT, LRR and PYD domains-containing protein 3-like isoform X2; Figure 2). Caspy performs the function of human caspase-1, and Caspy 2 shows the highest sequence similarity to human caspase-4/5, both of which have substrate specificity.41 Only Caspy can be activated through interaction with zASC. Although the function of these fish caspases is poorly defined, they appear to be essential for the morphogenesis of the jaw and gill-bearing arches. It is not yet clear whether fish Caspy 2, which is similar to human caspase-4/5 (mouse caspase-11), can directly interact with LPS and activate the inflammasome or cleave the relevant substrates and induce, pyroptosis as its orthologs do in mammals. Interestingly, the zebrafish dfna5 gene (AY603655) also contains a gasdermin family domain, a recently identified caspase-4/5 substrate and the cleavage of which induce pyroptosis in mammals. But the role of zebrafish dfna5 in pyroptosis awaits further studies.
Zebrafish also express IL-1, which is similar to IL-1β in mammals.75 Zebrafish IL-1 represents a single ancestral gene for the IL-1 superfamily in mammals and functions similarly to IL-1β in mammals. Zebrafish IL-1 also requires proteolytic cleavage for maturation into an activated form.76 However, the processing and cleavage mechanism of pro-IL-1β is less clear in fish because fish IL-1β lacks the conserved caspase-1 cleavage site.75 With respect to function, IL-1β behaves the same in teleost and mammalian immunity. A study demonstrated the caspase-1-mediated processing of IL-1β at an aspartic residue distinct from the cleavage site of mammalian IL-1β in the European sea bass,77 suggesting that fish have a more sophisticated inflammasome activation mechanism than mammals. Thus, more research is needed to illuminate the innate immune mechanisms in fish.
RIG-like helicase receptors
The mammalian RIG-I-like receptor (RLR) family consists of retinoic acid-inducible gene-I (RIG-I), melanoma differentiation-associated factor 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2), and has a major role in initiating antiviral immunity against RNA virus infection.78 The three RLR members are broadly expressed in most tissues, but are typically maintained at low levels in resting cells. Their expression levels are greatly increased upon IFN treatment or viral infection.79, 80 RIG-I and MDA5 share similar domain structures, including the N-terminal tandem CARD domains for protein–protein interactions, a central DExD/H box RNA helicase domain with the ability to hydrolyze ATP and bind RNA ligands, and a C-terminal domain involved in ligand binding and auto-inhibition.80, 81 However, LGP2, another member of the RLR family, lacks the N-terminal CARD domain and is thought to function as a negative regulator of RIG-I and MDA5 signaling.
The RLR family is well conserved among vertebrates. Most of the signaling components of the RLR pathways, including RIG-I, MDA5, LGP2, MAVS and TBK1, have been identified in the zebrafish genome. Although zebrafish have two variants of RIG-I and MDA5, they appear to participate in the immune pathways in a manner similar to that of their mammalian homologs.46 The analysis of the evolutionary pattern of RIG-I and MDA5 key domains has indicated that RLRs are conserved from lower organisms to higher mammals. Recent reports have shown the presence of STING/MITA orthologs in zebrafish, demonstrating that RLR-mediated IFN activation is conserved from fish to mammals.82 Nie et al.83 were the first to identify a RIG-I homolog from zebrafish that contained the complete RIG-I sequence with all of the functional domains (N-terminal tandem CARD, central DExD/H box, an RNA helicase domain, and CTD with RD) and demonstrated the conserved functional involvement of zebrafish RIG-I in the IFN-I and NF-κB signaling pathways and the conserved regulatory role of TRIM 25 in the RIG-I signaling pathway. Mammalian RIG-I contains three important lysine residues (K99, K169 and K172) involved in K63-Ub binding or CARD–CARD interaction in ubiquitin-mediated antiviral signaling.84 Two of these three ubiquitination sites (namely K169 and K172) are conserved in zebrafish RIG-I (Figure 3a), indicating that RIG-I-mediated antiviral innate immunity is conserved and may originate as early as teleosts.
(a) Multiple sequence alignment of RIG-I CARDs around the ubiquitin sites. Identical (*) residues are shown below the alignment. K99, K169 and K172 are the crucial ubiquitination sites for human RIG-I, whereas zebrafish lacks the K99 site. Conserved lys residues are shown in bold red and in a black box. (b) Schematic representation of the zebrafish RLRs and their adaptor MAVS. RIG-I, retinoic acid-inducible gene-I; RLR, RIG-I-like receptor.
An MDA5 ortholog was also identified in zebrafish, and the length of zebrafish mda5 is comparable to that of mammals, ranging from 987 residues to 1285 residues, suggesting that MDA5 might possess a conserved function. Zebrafish MDA5 has a conserved DExD/H domain and a helix C domain but lacks an N-terminal CARD domain, which may not be directly involved in downstream signaling. Recently, Gabor et al.85 reported the role of zebrafish MDA5 in protection against snakehead rhabdovirus infection, suggesting the conservation of this antiviral signaling pathway in lower vertebrates and the possession of recognition strategies for a wide array of viruses by fish early in evolution.
Two isoforms of LGP2 have been identified in zebrafish and rainbow trout.86, 87 Because this molecule has both inhibitory and stimulatory effects, the role of LGP2 in the innate antiviral response is still unclear in fish as well as in mammals. Zebrafish LGP2 has a domain structure similar to zMDA5. In the zebrafish genome, MAVS, the adaptor downstream of RLR signaling, is composed of a conserved N-terminal CARD domain and a functional mitochondrial transmembrane domain but lacks the proline-rich domain and the TRAF6/TRAF3 binding motif found in mammalian MAVS (Figures 3 and 4).
Comparison of RIG-I-like receptor (RLR) pathways in humans and zebrafish. RIG-I and MDA5 are responsible for the detection of different types of viral RNAs. On binding of the RNA ligand, homotypic CARD–CARD interactions between RLRs and MAVS recruit TRAFs/TRADD/TANK and kinase complexes (TBK1 and IKKɛ) to facilitate IRF3 phosphorylation and IκB kinase activation, resulting in the production of type-I IFN. In zebrafish, the production of IFNs in turn induces a second wave of IFN through the STAT pathway and IRF7 phosphorylation. IRF10 and a MAVS variant (MAVS_tv2) negatively regulate IFN production by targeting IRF7. IRF, interferon response factor.
The virus-induced IFNs in zebrafish are classified into group I and group II IFNs.88, 89 Although fish IFNs are not the true homologs of mammalian type-I IFNs and their receptors differ from mammalian type-I IFN receptors, their expression is activated through similar mechanisms, such as the STAT pathway.90 In zebrafish, viral RNA induces the expression of IFN that is most similar to the mammalian IFN-alpha (type I).91 It is intriguing to observe that the initial production of IFNs in zebrafish induces the expression of IRF3 through the STAT pathway and activates IRF7 through phosphorylation,82 both of which in turn induce a second wave of IFN. This establishes a unique positive feedback loop. In addition, zebrafish IRF10 inhibits IRF3-mediated IFN induction, and IRF7 is targeted by MAVS_tv2 for the negative regulation of type-I IFN expression;92 this demonstrates special immune mechanisms to balance IFN responses in fish (Figure 4).
Similar to their mammalian counterparts, zebrafish RLRs have important roles in antiviral immunity. The overexpression of MAVS or RIG-I CARDs in zebrafish leads to the constitutive induction of both IFNs and IFN-stimulated genes. This results in the inhibition of viral replication and protection against viral infection,91 suggesting that zebrafish possess a robust antiviral system and share a common evolutionary origin with mammals.
In addition to the above examples, other new PRRs such as cytosolic DNA sensor DNA-dependent activator of IFN-regulatory factors93 and a cytosolic DNA receptor named AIM-256, 58 have been described in mammals. Their orthologs in zebrafish have not been identified.
Conclusions
Counterparts of the major vertebrate PRRs and their downstream signaling components have been identified in zebrafish; these counterparts are functionally similar and more diverse compared with their mammalian counterparts. Due to their importance in innate immunity, we compared the conservation and diversity of the zebrafish pattern recognition receptors and their signaling components with those in mammals in this review. Nearly all of the TLRs, NLRs and RLRs identified in mammals have been described in fish; however, there are still differences between them.
Compared with other teleost fish, the zebrafish genome encodes unique TLRs. Zebrafish-specific TLR4 appears to be non-responsive to LPS, and changes in ligand recognition specificities reflect differences in aquatic and terrestrial pathogens or divergence that occurred during vertebrate evolution. Zebrafish TLR5 harbors two isoforms (TLR5a and TLR5b); it is not clear how these tandem copies of TLR5 function, such as whether both are involved in immune responses and have similar roles or one functions as a co-receptor or an antagonistic receptor. In contrast, functional RLR-triggered IFN antiviral signaling pathways are highly conserved from lower organisms to mammals. Interestingly, although genes closely related to MDA5 and MAVS have been identified in Branchiostoma, the products of these genes could not induce IFN production. Additional knowledge of vertebrate evolution will help uncover the evolutionary history of the RLR family. Furthermore, although the NLRP receptors in the classic NLRP inflammasome signaling pathway are specific to mammals, other NLR family members may have evolved for inflammasome activation in zebrafish.
In conclusion, although mammals diverged from fish 450 million years ago, the PRRs in zebrafish provide functional and evolutionary insights into innate immune signaling pathways. Additional studies in this field will not only enrich our understanding of the immune response in zebrafish itself but will also provide unique perspectives on the evolution of the immune system.
References
Trede NS, Langenau DM, Traver D, Look AT, Zon LI . The use of zebrafish to understand immunity. Immunity 2004; 20: 367–379.
de Jong JL, Zon LI . Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet 2005; 39: 481–501.
Sullivan C, Kim CH . Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol 2008; 25: 341–350.
Akira S, Uematsu S, Takeuchi O . Pathogen recognition and innate immunity. Cell 2006; 124: 783–801.
Beg AA . Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol 2002; 23: 509–512.
Matzinger P . Matzingerl: a renewed sense of self. Science 2002; 296: 301–305.
Migpin SM, Palsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K et al. Effects of combined TLR stimulation on interleukin-27 mRNA expression in human monocyte-derived dendritic cells. Cytokine 2007; 39: 27–28.
Blasius AL, Beutler B . Intracellular toll-like receptors. Immunity 2010; 32: 305–315.
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282: 2085–2088.
Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ . Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 1999; 274: 17406–17409.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410: 1099–1103.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408: 740–745.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA . Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature 2001; 413: 732–738.
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C . Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004; 303: 1529–1531.
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004; 303: 1526–1529.
Ohto U, Shibata T, Tanji H, Ishida H, Krayukhina E, Uchiyama S et al. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015; 520: 702–705.
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003; 301: 640–643.
Zhang J, Kong XH, Zhou CJ, Li L, Nie GX, Li XJ . Toll-like receptor recognition of bacteria in signal pathways fish: ligand specificity and signal pathways. Fish Shellfish Immunol 2014; 41: 380–388.
Jault C, Pichon L, Chluba J . Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol Immunol 2004; 40: 759–771.
Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, Ewa Snaar-Jagalska B et al. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol 2004; 40: 773–783.
Yeh DW, Liu YL, Lo YC, Yuh CH, Yu GY, Lo JF et al. Toll-like receptor 9 and 21 have different ligand recognition profiles and cooperatively mediate activity of CpG-oligodeoxynucleotides in zebrafish. Proc Natl Acad Sci USA 2013; 110: 20711–20716.
Hwang SD, Kondo H, Hirono I, Aoki T . Molecular cloning and characterization of Toll-like receptor 14 in Japanese flounder, Paralichthys olivaceus. Fish Shellfish Immunol 2011; 30: 425–429.
Zhao F, Li YW, Pan HJ, Shi CB, Luo XC, Li AX et al. Expression profiles of toll-like receptors in channel catfish (Ictalurus punctatus) after infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol 2013; 35: 993–997.
Meijer AH, Krens SFG, Rodriguez IAM, He SN, Bitter W, Snaar-Jagalska BE et al. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol 2004; 40: 773–783.
Pietretti D, Scheer M, Fink IR, Taverne N, Savelkoul HF, Spaink HP et al. Identification and functional characterization of nonmammalian Toll-like receptor 20. Immunogenetics 2014; 66: 123–141.
Kasamatsu J, Oshiumi H, Matsumoto M, Kasahara M, Seya T . Phylogenetic and expression analysis of lamprey toll-like receptors. Dev Comp Immunol 2010; 34: 855–865.
Ohno S . Gene duplication and the uniqueness of vertebrate genomes circa 1970-1999. Semin Cell Dev Biol 1999; 10: 517–522.
Li JT, Hou GY, Kong XF, Li CY, Zeng JM, Li HD et al. The fate of recent duplicated genes following a fourth-round whole genome duplication in a tetraploid fish, common carp (Cyprinus carpio). Sci Rep 2015; 5: 8199.
David L, Blum S, Feldman MW, Lavi U, Hillel J . Recent duplication of the common carp (Cyprinus carpio L.) genome as revealed by analyses of microsatellite loci. Mol Biol Evol 2003; 20: 1425–1434.
Pietretti D, Wiegertjes GF . Ligand specificities of Toll-like receptors in fish: indications from infection studies. Dev Comp Immunol 2014; 43: 205–222.
Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrom M, Gregers TF et al. The genome sequence of Atlantic cod reveals a unique immune system. Nature 2011; 477: 207–210.
Sundaram AY, Kiron V, Dopazo J, Fernandes JM . Diversification of the expanded teleost-specific toll-like receptor family in Atlantic cod, Gadus morhua. BMC Evol Biol 2012; 12: 256.
Yang S, Marin-Juez R, Meijer AH, Spaink HP . Common and specific downstream signaling targets controlled by Tlr2 and Tlr5 innate immune signaling in zebrafish. BMC Genomics 2015; 16: 547.
Barton GM, Medzhitov R . Toll-like receptors and their ligands. Curr Top Microbiol Immunol 2002; 270: 81–92.
Matsuo A, Oshiumi H, Tsujita T, Mitani H, Kasai H, Yoshimizu M et al. Teleost TLR22 recognizes RNA duplex to induce IFN and protect cells from birnaviruses. J Immunol 2008; 181: 3474–3485.
Candel S, Sepulcre MP, Espin-Palazon R, Tyrkalska SD, de Oliveira S, Meseguer J et al. Md1 and Rp105 regulate innate immunity and viral resistance in zebrafish. Dev Comp Immunol 2015; 50: 155–165.
Kaiser J, Normile D . Bioethics. Embryo engineering study splits scientific community. Science 2015; 348: 486–487.
Sullivan C, Postlethwait JH, Lage CR, Millard PJ, Kim CH . Evidence for evolving Toll-IL-1 receptor-containing adaptor molecule function in vertebrates. J Immunol 2007; 178: 4517–4527.
Sullivan C, Charette J, Catchen J, Lage CR, Giasson G, Postlethwait JH et al. The gene history of zebrafish tlr4a and tlr4b is predictive of their divergent functions. J Immunol 2009; 183: 5896–5908.
Sepulcre MP, Alcaraz-Perez F, Lopez-Munoz A, Roca FJ, Meseguer J, Cayuela ML et al. Evolution of lipopolysaccharide (LPS) recognition and signaling: fish TLR4 does not recognize LPS and negatively regulates NF-kappaB activation. J Immunol 2009; 182: 1836–1845.
Masumoto J, Zhou W, Chen FF, Su F, Kuwada JY, Hidaka E et al. Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J Biol Chem 2003; 278: 4268–4276.
Ordas A, Hegedus Z, Henkel CV, Stockhammer OW, Butler D, Jansen HJ et al. Deep sequencing of the innate immune transcriptomic response of zebrafish embryos to Salmonella infection. Fish Shellfish Immunol 2011; 31: 716–724.
Stein C, Caccamo M, Laird G, Leptin M . Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol 2007; 8: R251.
van der Sar AM, Stockhammer OW, van der Laan C, Spaink HP, Bitter W, Meijer AH . MyD88 innate immune function in a zebrafish embryo infection model. Infect Immun 2006; 74: 2436–2441.
Fan S, Chen S, Liu Y, Lin Y, Liu H, Guo L et al. Zebrafish TRIF, a Golgi-localized protein, participates in IFN induction and NF-kappaB activation. J Immunol 2008; 180: 5373–5383.
Poynter S, Lisser G, Monjo A, DeWitte-Orr S . Sensors of infection: viral nucleic acid PRRs in fish. Biology (Basel) 2015; 4: 460–493.
Zhang YB, Gui JF . Molecular regulation of interferon antiviral response in fish. Dev Comp Immunol 2012; 38: 193–202.
Liu YH, Li MZ, Fan S, Lin YQ, Lin B, Luo F et al. A unique feature of Toll/IL-1 receptor domain-containing adaptor protein is partially responsible for lipopolysaccharide insensitivity in zebrafish with a highly conserved function of Myd88. J Immunol 2010; 185: 3391–3400.
Huang B, Qi ZT, Xu Z, Nie P . Global characterization of interferon regulatory factor (IRF) genes in vertebrates: glimpse of the diversification in evolution. BMC Immunol 2010; 11: 22.
Kedinger V, Alpy F, Tomasetto C, Thisse C, Thisse B, Rio MC . Spatial and temporal distribution of the traf4 genes during zebrafish development. Gene Expr Patterns 2005; 5: 545–552.
Neely MN, Pfeifer JD, Caparon M . Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun 2002; 70: 3904–3914.
Phelan PE, Mellon MT, Kim CH . Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio). Mol Immunol 2005; 42: 1057–1071.
Schroder K, Zhou R, Tschopp J . The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010; 327: 296–300.
Wilkins C, Gale M . Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 2010; 22: 41–47.
Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES . AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009; 458: 509–513.
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009; 458: 514–518.
Schroder K, Muruve DA, Tschopp J . Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol 2009; 19: R262–R265.
Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 2012; 36: 561–571.
Cridland JA, Curley EZ, Wykes MN, Schroder K, Sweet MJ, Roberts TL et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol Biol 2012; 12: 140.
Howe K, Schiffer PH, Zielinski J, Wiehe T, Laird GK, Marioni JC et al. Structure and evolutionary history of a large family of NLR proteins in the zebrafish. Open Biol 2016; 6: 160009.
Laing KJ, Purcell MK, Winton JR, Hansen JD . A genomic view of the NOD-like receptor family in teleost fish: identification of a novel NLR subfamily in zebrafish. BMC Evol Biol 2008; 8: 42.
Sha Z, Abernathy JW, Wang S, Li P, Kucuktas H, Liu H et al. NOD-like subfamily of the nucleotide-binding domain and leucine-rich repeat containing family receptors and their expression in channel catfish. Dev Comp Immunol 2009; 33: 991–999.
Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 2009; 122 (Pt 19): 3522–3530.
Oehlers SH, Flores MV, Hall CJ, Swift S, Crosier KE, Crosier PS . The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis Model Mech 2011; 4: 832–841.
Stein C, Caccamo M, Laird G, Leptin M . Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol 2007; 8: R251.
Boyle JP, Bryant CE, Monie TP . Cell swelling and the NLRP3 inflammasome. Immunity 2013; 38: 399.
Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007; 14: 1590–1604.
Broz P, von Moltke J, Jones JW, Vance RE, Monack DM . Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 2010; 8: 471–483.
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011; 479: 117–121.
Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013; 341: 1246–1249.
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014; 514: 187–192.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 2015; 25: 1285–1298.
Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015; 526: 666–671.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665.
Secombes CJ, Wang T, Bird S . The interleukins of fish. Dev Comp Immunol 2011; 35: 1336–1345.
Vojtech LN, Scharping N, Woodson JC, Hansen JD . Roles of inflammatory caspases during processing of zebrafish interleukin-1beta in Francisella noatunensis infection. Infect Immun 2012; 80: 2878–2885.
Reis MI, do Vale A, Pereira PJ, Azevedo JE, Dos Santos NM . Caspase-1 and IL-1beta processing in a teleost fish. PLoS One 2012; 7: e50450.
Loo YM, Gale M . Immune Signaling by RIG-I-like Receptors. Immunity 2011; 34: 680–692.
Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 2004; 23: 1789–1800.
Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 2005; 175: 2851–2858.
Fujita T, Onoguchi K, Onomoto K, Hirai R, Yoneyama M . Triggering antiviral response by RIG-I-related RNA helicases. Biochimie 2007; 89: 754–760.
Sun F, Zhang YB, Liu TK, Shi J, Wang B, Gui JF . Fish MITA serves as a mediator for distinct fish IFN gene activation dependent on IRF3 or IRF7. J Immunol 2011; 187: 2531–2539.
Nie L, Zhang YS, Dong WR, Xiang LX, Shao JZ . Involvement of zebrafish RIG-I in NF-kappa B and IFN signaling pathways: Insights into functional conservation of RIG-I in antiviral innate immunity. Dev Comp Immunol 2015; 48: 95–101.
Peisley A, Wu B, Xu H, Chen ZJ, Hur S . Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature 2014; 509: 110–114.
Gabor KA, Charette JR, Pietraszewski MJ, Wingfield DJ, Shim JS, Millard PJ et al. A DN-mda5 transgenic zebrafish model demonstrates that Mda5 plays an important role in snakehead rhabdovirus resistance. Dev Comp Immunol 2015; 51: 298–304.
Wang WW, Asim M, Yi LZ, Hegazy AM, Hu XQ, Zhou Y et al. Abortive infection of snakehead fish vesiculovirus in ZF4 cells was associated with the RLRs pathway activation by viral replicative intermediates. Int J Mol Sci 2015; 16: 6235–6250.
Chang MX, Collet B, Nie P, Lester K, Campbell S, Secombes CJ et al. Expression and functional characterization of the RIG-I-like receptors MDA5 and LGP2 in rainbow trout (Oncorhynchus mykiss). J Virol 2011; 85: 8403–8412.
Zou J, Secombes CJ . Teleost fish interferons and their role in immunity. Dev Comp Immunol 2011; 35: 1376–1387.
Aggad D, Mazel M, Boudinot P, Mogensen KE, Hamming OJ, Hartmann R et al. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J Immunol 2009; 183: 3924–3931.
Aggad D, Stein C, Sieger D, Mazel M, Boudinot P, Herbomel P et al. In vivo analysis of Ifn-gamma 1 and Ifn-gamma 2 signaling in zebrafish. J Immunol 2010; 185: 6774–6782.
Biacchesi S, LeBerre M, Lamoureux A, Louise Y, Lauret E, Boudinot P et al. Mitochondrial antiviral signaling protein plays a major role in induction of the fish innate immune response against RNA and DNA viruses. J Virol 2009; 83: 7815–7827.
Lu LF, Li S, Lu XB, Zhang YA . Functions of the two zebrafish MAVS variants are opposite in the induction of IFN1 by targeting IRF7. Fish Shellfish Immunol 2015; 45: 574–582.
Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007; 448: 501–505.
Gautam JK, Ashish, Comeau LD, Krueger JK, Smith MF Jr . Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J Biol Chem 2006; 281: 30132–30142.
Sahoo BR, Basu M, Swain B, Maharana J, Dikhit MR, Jayasankar P et al. Structural insights of rohu TLR3, its binding site analysis with fish reovirus dsRNA, poly I:C and zebrafish TRIF. Int J Biol Macromol 2012; 51: 531–543.
Sahoo BR, Dikhit MR, Bhoi GK, Maharana J, Lenka SK, Dubey PK et al. Understanding the distinguishable structural and functional features in zebrafish TLR3 and TLR22, and their binding modes with fish dsRNA viruses: an exploratory structural model analysis. Amino Acids 2015; 47: 381–400.
Yeh DW, Liu YL, Lo YC, Yuh CH, Yu GY, Lo JF et al. Toll-like receptor 9 and 21 have different ligand recognition profiles and cooperatively mediate activity of CpG-oligodeoxynucleotides in zebrafish. Proc Natl Acad Sci USA 2013; 110: 20711–20716.
Acknowledgements
TJ is supported by the Fundamental Research Funds for the Central Universities and the 100 Talents Program of the Chinese Academy of Sciences. YJL is supported by the China Postdoctoral Science Foundation. We express our appreciation to Tsan Sam Xiao at Case Western Reserve University and Bin Lin at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA for proofreading and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Li, Y., Li, Y., Cao, X. et al. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cell Mol Immunol 14, 80–89 (2017). https://doi.org/10.1038/cmi.2016.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.50
Keywords
This article is cited by
-
Evaluation of the efficacy of cystinosin supplementation through CTNS mRNA delivery in experimental models for cystinosis
Scientific Reports (2023)
-
Modeling oncolytic virus dynamics in the tumor microenvironment using zebrafish
Cancer Gene Therapy (2021)
-
Applications of atomic force microscopy in immunology
Frontiers of Medicine (2021)
-
Research progress on gut health of farmers teleost fish: a viewpoint concerning the intestinal mucosal barrier and the impact of its damage
Reviews in Fish Biology and Fisheries (2020)
-
Non-murine models to investigate tumor-immune interactions in head and neck cancer
Oncogene (2019)