Abstract
Chromosomal missegregation is a common feature of many human tumors. Recent studies have indicated a link between nucleoporin RanBP2/Nup358 and chromosomal segregation during mitosis; however, the molecular details have yet to be fully established. Observed through live cell imaging and flow cytometry, here we show that RNA interference-mediated knockdown of RanBP2 induced G2/M phase arrest, metaphase catastrophe and mitotic cell death. Furthermore, RanBP2 down-modulation disrupted importin/karyopherin β1 as well as the expression and localization of the Ran GTPase activating protein 1. We found that N-terminal of RanBP2 interacted with the N-terminal of importin β1. Moreover, at least a portion of RanBP2 partially localizes at the centrosome during mitosis. Notably, we also found that GTPase Ran is also involved in the regulation of RanBP2–importin β1 interaction. Overall, our results suggest that mitotic arrest and the following cell death were caused by depletion of RanBP2. Our findings point to a crucial role for RanBP2 in proper mitotic progression and faithful chromosomal segregation.
Similar content being viewed by others
Main
The nuclear pore complex (NPC) is the major gatekeeper of macromolecular traffic between the nucleus and cytoplasm. It is composed of multiple subunits comprising ≈30 proteins called nucleoporins. During cell division, transmembrane nucleoporins and nuclear envelope membrane fragments move to the endoplasmic reticulum, while other nucleoporins are disassembled into subcomplexes that are distributed throughout the mitotic cytoplasm to kinetochore, spindle and centrosome regions.1, 2, 3 As mitosis nears completion, the NPC is sequentially reassembled from the dispersed cytosolic components.4 Our previous findings demonstrated that several NPC proteins, such as Rae1,5, 6, 7, 8 Nup98,9 Tpr10 and Nup88,11 do not simply disperse into the mitotic cytoplasm but instead become preferentially associated with kinetochores and/or spindle pole regions, where they are crucial in maintaining spindle bipolarity and thus prevent aneuploidy and carcinogenesis.4, 12
RanBP2, a Ran binding protein with four Ran binding domains, is a major cytosolic component of filaments that are derived from the cytoplasmic ring of the NPC13, 14 during interphase. RanBP2 is composed of 3224 residues in humans. It can be divided into several domains: an N-terminal TPR domain, an α-helical region, four Ran-binding domains, eight tandem zinc fingers, a SUMO E3 ligase domain, and a C-terminal domain that displays sequence homology to cyclophilins.15, 16 RanBP2 was initially described as a regulator of nucleocytoplasmic transport because of its link with the small G protein, Ran, and because it possesses docking motifs for nuclear transport receptors.17, 18, 19 However, the multi-domain configuration of RanBP2 indicates a more pleiotropic function for this huge nucleoporin; indeed, RanBP2 is now recognized as a regulator of numerous cellular activities.20, 21, 22, 23, 24, 25, 26, 27 Besides, RanBP2-RanGAP1*SUMO1/Ubc9 was identified as a multi-subunit SUMO E3 ligase.28 Interestingly, RanBP2 has also been implicated in the delivery and integration of the genomic material of HIV-1.29, 30, 31, 32 RanBP2 was also shown to potentiate the translation of a subset of mRNA encoding secretory proteins.33
RanBP2 aids proper nuclear envelope assembly34 and is involved in mitosis.35, 36, 37 Notably, together with Ran GTPase activating protein 1 (RanGAP1), RanBP2 relocates to kinetochores and mitotic spindle microtubules during mitosis.38, 39 Whether normal mitosis also proceeds in the presence of altered RanBP2 expression is unclear.
To illustrate the participation of RanBP2 in cell-cycle regulation and to understand the underlying molecular mechanism thereof, we examined the effects of modulating RanBP2 expression on cell-cycle progression. Here, we show that knockdown of RanBP2 causes an aberrant metaphase and mitotic arrest in G2/M phase, leading to abnormal chromosome segregation, aneuploidy and metaphase catastrophe. We also found that at least a portion of RanBP2 localizes at the spindle pole/centrosome regions. Using RNAi-depletion assays, flow cytometry and live cell imaging studies, we were able to document a novel regulatory mechanism involving RanBP2 and importin β1/karyopherin-β1. Our biochemical characterization further confirms their direct interaction, while ectopic expression of the interaction domain revealed the role of the RanBP2-RanGAP1-importin β1 subcomplex in maintaining proper bipolarity segregation, thus preventing mitotic catastrophe. In addition to its activity as a GTPase, Ran might also regulate RanBP2–importin β1 interactions. These findings suggest a crucial role for RanBP2 in proper mitotic progression and chromosomal segregation, both of which prevent apoptosis.
Results
RanBP2 partially localizes at the centrosome and interacts with importin β1 to form a transient complex during mitosis
To understand the physiological role of RanBP2 in cell-cycle regulation, we first examined the kinetics of endogenous RanBP2 expression during cell-cycle progression by using α-tubulin, a mitotic spindle marker. We found that RanBP2 transiently co-localized with α-tubulin during the metaphase–anaphase transition (Figure 1a). In contrast to the results of previous RanBP2 mitotic studies,38, 39 in metaphase mitotic HeLa cells RanBP2 was visible along the spindle microtubules, and signals were also detected, to a lesser extent, at the kinetochores (Supplementary Figure S1) and even at the centrosome regions (Figure 1a). Confocal microscopy confirmed the RanBP2 centrosome staining pattern, which showed the partial association of γ-tubulin (spindle pole/centrosome marker) with RanBP2 from prophase to anaphase. In γ-tubulin immunoprecipitates from mitotic HeLa cells, low levels of RanBP2 and importin β1 were detected (Supplementary Figure S1). Our RanBP2 centrosome localization data are also supported by recent proteomic studies involving liquid chromatography–MS of the mitotic Drosophila centrosome, which identified RanBP2 in the Drosophila centrosome.40
RanBP2 is associated with importin β1 during mitosis. (a) Confocal microscopic images of HeLa cells at different mitotic stages, stained with anti-RanBP2 (green) and anti-α-tubulin (red) antibodies. Chromatin was stained with 4',6-diamidino-2-phenylindole (DAPI; blue). Bars=5 μm. In lanes marked ‘Input,’ 20 μl of the 500 μl of extract used per IP were analyzed directly. (b) Asynchronized (upper panel) and synchronized mitotic (lower panel) HeLa cells (G2/M) were prepared via double thymidine block and confirmed by flow cytometry. (c) IP of mitotic HeLa cell extracts incubated with anti-RanBP2 or nonspecific rabbit antibodies (IgG) were analyzed by IB with anti-Imp-β1, anti-RanGAP1, anti-TopoIIα and anti-Lamin A/C antibodies. (d) IP of mitotic HeLa cell extracts incubated with anti-Imp-β1 or nonspecific rabbit antibodies (IgG) were analyzed by IB with the indicated antibodies. (e) Confocal microscopic images of HeLa cells at different mitotic stages, stained with anti-RanBP2 (green) and anti-Imp-β1 (red) antibodies. Chromatin was stained with DAPI (blue). Bars=5 μm
Given that the regulatory functions of Ran are mediated by the dissociation of importin β1 complexes41 and that importin β1 also localizes at the mitotic spindle39 and spindle pole region,42 we hypothesized that the contribution of RanBP2 (a Ran binding protein) is to spatiotemporally regulate the recruitment of importin β1 during mitosis. We therefore examined whether RanBP2 was associated with the importin β1 complex in mitotic HeLa cells. HeLa cells were synchronized and released from the double thymidine block treatment, the mitotic HeLa cells were harvested (∼88%) and then confirmed by flow cytometry (Figure 1b). Immunoblotting (IB) of anti-RanBP2 immunoprecipitates showed the coprecipitation of importin β1, TopoIIα and RanGAP1 but not Lamin A/C (Figure 1c and Supplementary Figure S2). Using anti-importin β1 antibodies, we immunoprecipitated RanBP2 and RanGAP1 but not TopoIIα or Lamin A/C (Figure 1d). This interaction was also found in a lysate prepared from asynchronous HeLa cells, and it was not regulated by phosphorylation (Supplementary Figures S2a–c). Furthermore, RanBP2 and importin β1 colocalized in mitotic HeLa cells (Figure 1e). Taken together, these observations suggest dynamic changes in the cellular localization of RanBP2 during mitosis and the potential role of this protein in the regulation of mitotic progression.
Knockdown of importin β1 in vertebrate cells reduces kinetochore localization of RanBP2
To further analyze whether importin β1 regulates the function of RanBP2 or vice versa, we used an importin β1 siRNA knockdown approach (Figure 2a). IB analysis of HeLa cells subjected to importin β1 siRNA treatment for 3 days revealed an 85% reduction in the level of importin β1 compared with controls (Figure 2b and Supplementary Figure S3). The same IB membrane was re-probed with α-tubulin and β-actin to ensure equivalent loading (Figure 2b). Moreover, there were no significant changes in the levels of TopoIIα (another reported binding partner of RanBP2) or the membrane nucleoporin Pom121 or LaminA/C (Figure 2c). Notably, RanBP2 levels were reduced significantly and RanGAP1 proteins were non-sumoylated, in contrast to mock-treated (control siRNA) cells (Figure 2c and Supplementary Figure S3). Moreover, we found that impaired chromosome alignments were increased fourfold compared with control siRNA mitotic cells (n=300 mitotic cells) (Figures 2d–e). We next assessed chromosomal defects and RanBP2 localization in importin-β1-depleted HeLa cells. The depletion of importin β1 reduced RanBP2 localization at the kinetochores, mitotic spindles and spindle poles (Figure 2e). RanGAP1 localization was also examined in importin-β1-depleted cells. Consistent with our prediction, the knockdown of importin β1 also reduced the RanGAP1 kinetochore and spindle localization pattern (Figures 2f–h). These effects were also observed in cells treated with another siRNA against importin β1 (Supplementary Figures S3c–e). Taken together, these observations demonstrated that the knockdown of importin β1 reduces the mitotic localization of RanBP2 and RanGAP1 in vertebrate cells.
Importin β1 siRNA treatment reduces RanBP2 and causes abnormal chromosomal congression and defective mitosis. (a) Schedule of fixing or collecting mitotic HeLa cells after siRNA Imp-β1 depletion. (b) Lysates of mitotic HeLa cells transfected with control or Imp-β1 siRNAs were analyzed 72 h later by IB with anti-Imp-β1 antibody. The same membrane was stripped and reprobed with anti-β-actin and anti-α-tubulin (as loading control). (c) Effects of Imp-β1 depletion on the protein levels of RanBP2-associated proteins. The same membranes as in (b) were analyzed by IB with the indicated antibodies. RanBP2 was significantly reduced in the Imp-β1-depleted lysates, whereas TopoIIα, Pom121 and Lamin A/C were not affected. (d) Quantification (relative percentage) of mitotic chromosomal defect phenotypes for the indicated siRNA and/or plasmid. Values are based on three independent experiments, counting 100 mitotic cells in each experiment. Mean values±S.D. (error bars) are shown. All cells were treated with double thymidine block, stained with 4',6-diamidino-2-phenylindole (DAPI) and visualized by confocal microscopy. (e) Confocal microscopic images of mitotic HeLa cells transfected with control or Imp-β1 siRNA and analyzed 72 h post-transfection; the cells were stained with anti-RanBP2 (green) and anti-Imp-β1 (red) antibodies and DAPI (blue). (f–h) Representative confocal microscopic images were stained with various antibodies as indicated. White boxes show the enlarged region. Bar=5 μm
To determine whether soluble full-length importin β1 could rescue the observed chromosomal defects, FLAG-importin β1-RNAi-resistant plasmids (Supplementary Tables S1 and S2) were co-transfected together with importin β1 siRNA, which partially rescued the chromosomal defects (Figure 2d). As predicted, there were significantly more defects in siRNA importin β1 cells than in cells co-transfected with siRNA+FLAG-importin β1-RNAi-resistant plasmid (14 versus 9%; n=300 mitotic cells) (Figure 2d).
To rule out the possibility that the importin-β1-depleted phenotypes are indirect consequences of interphase, RanBP2 and Pom121 were followed with confocal microscopy, and their localization in control siRNA and in importin-β1-depleted cells was determined (Supplementary Figure S4). Although 35% of the latter cells had an abnormal nuclear morphology (Supplementary Figure S4), triplicate experiments did not establish statistical significance (P>0.1; n=150) (Supplementary Figure S4). Why was the amount of RanBP2 protein controlled by importin β1 depletion? Our results suggest that importin β1 depletion leads to a reduction of RanBP2 and RanGAP1 sumoylation, such that importin β1 and RanBP2 no longer localize on the kinetochores, mitotic spindles and centrosomes, thus leading to aneuploidy (Figures 2d–h).
RanBP2 down-modulation induces chromosome missegregation and aneuploidy
The above-described results prompted us to examine the consequences of RanBP2 depletion on importin β1. In HeLa cells subjected to RanBP2 siRNA treatment for 3 days and analyzed by IB, RanBP2 levels were reduced by almost 90% compared with control cells (Figure 3a). Again, there were no significant changes in TopoIIα expression. By contrast, the level of importin β1 was reduced, and RanGAP1 sumoylation was significantly reduced (Figure 3b). With the nearly complete knockdown of RanBP2, less RanGAP1 and importin β1 were localized in the kinetochores and mitotic spindles, and the frequency of chromosomal separation defects increased compared with control siRNA (Figures 3c and d). Consistent with previous findings, we found that mitotic RanBP2 siRNA treatment caused chromosomal misalignments in 25% of the mitotic cells, whereas these defects were not seen in mock-treated cells (n=300 mitotic cells) (Figure 3e).
RanBP2 siRNA treatment reduces importin β1 and causes abnormal chromosomal congression and defective mitosis. (a) Lysates of mitotic HeLa cells transfected with control or RanBP2 siRNAs were analyzed 72 h post transfection by IB with anti-RanBP2 antibody. The same membrane was stripped and reprobed with anti-β-actin (as a loading control). (b) Effects of RanBP2 depletion on the protein levels of RanBP2-associated proteins. The same membranes as in panel (a) were analyzed by IB with the indicated antibodies. (c and d) Confocal microscopic images of mitotic HeLa cells transfected with control or RanBP2 siRNAs. At 72 h post transfection, the cells were stained with RanBP2 (green) antibody and anti-RanGAP1 (red) or Imp-β1 (red) antibodies and 4',6-diamidino-2-phenylindole (DAPI; blue). Bar=5 μm. (e) Quantification (relative percentage) of mitotic chromosomal defect phenotypes in cells transfected with control siRNA or RanBP2 siRNA. Values are based on three independent experiments, counting 100 mitotic cells in each experiment. Mean values±S.D. (error bars) are shown. All cells were treated with double thymidine block, stained with DAPI and visualized by confocal microscopy
RanBP2 down-modulation-induced metaphase catastrophe, G2/M phase arrest and mitotic cell death
To firmly establish that mitosis is disturbed (at different stages) in down-modulated RanBP2 cells, HeLa cells stably expressing histone H2B fused to green fluorescent protein (H2B-GFP) were examined using live cell imaging. This assay was under real-time, simultaneous detection of mitosis, ploidy and mitotic catastrophe.43 Down-modulation of RanBP2 resulted in a prolonged metaphase (>40 min). We found that chromosomal segregation defects and impaired chromosome alignment were rarely observed in control cells (Figure 4a Supplementary Video S3). By contrast, in the live cell images, which showed chromosomal segregation defect phenotypes (Figure 4b and Supplementary Video S1) together with impaired chromosome alignment (Figure 4c and Supplementary Video S2) in RanBP2 siRNA H2B-GFP cells. In response to RanBP2 depletion, cell division was halted during metaphase, a phenomenon we refer to as metaphase catastrophe. Insufficient mitotic arrest was previously shown to result in abnormal cell-cycle progression via chromosomal abnormalities.44, 45, 46 We also investigated the rate of cell death under these conditions. In cells transfected with siRNAs against RanBP2 but not control, the death cells gradually increased (Figure 4d). Next we plan to confirm whether this cell death is accompanied by apoptotic features. We monitored the percentage of Annexin V-positive cells. Forty-eight hours after transfection, we stained siRNA-transfected HeLa cells with FITC-labeled Annexin V and propidium iodide (PI).47 We found that the percentage of Annexin V-positive and Annexin V/PI double-positive cells considerably increased following transfection with RanBP2 depletion (Figures 4e and f, *P<0.05; n=300 cells). To examine the effects of RanBP2 depletion on the cell-cycle profile, cells transfected with siRNA and incubated for 3 days thereafter were analyzed by flow cytometry (Figure 4g), which showed a twofold increase in mitotic cell death/apoptotic cells in the transfectants compared with the controls (Figures 4g and h, apoptotic cells were indicated as **P<0.01). Moreover, depletion of RanBP2 resulted in an increase in the percentage of cells at the G2/M transition compared with control siRNA-transfected cells in triplicate experiments (32 versus 18%, respectively) (Figures 4g and h). These data indicate that cells were arrested in G2/M phase after RanBP2 depletion. Together, these data suggested that RanBP2 depletion induces metaphase catastrophe, impaired chromosome alignment, G2/M phase arrested and mitotic cell death, indicating a crucial role for RanBP2 in faithful chromosomal segregation.
RanBP2 down-modulation induces G2/M phase arrest, metaphase catastrophe and cell death. (a–c) Time-lapse microscopy of HeLa cells stably expressing histone H2B-GFP. At 72 h post transfection with (a) control or (b and c) RanBP2 siRNAs, the cells were processed for time-lapse microscopic analysis. Images were acquired every 3 min. Arrow indicates a fragmented chromosome. White arrowhead indicates an apoptotic bleb. (d) siRNAs against RanBP2 and control were transfected, respectively. Cell images were acquired using a phase-contrast microscope 24 or 48 h after siRNA transfection. White arrowhead indicates an apoptotic cell. (e) Cell death induced by knockdown of RanBP2. HeLa cells 48 h after siRNA transfection were stained with FITC-labeled Annexin V and PI. (f) Annexin V and PI-positive HeLa cells 48 h after siRNA transfection. HeLa cells randomly selected (n=300) were counted. Values are an average of three independent experiments. Error bars represent S.D. (g) At 48 h post transfection with control or RanBP2 siRNAs, HeLa cells were examined for their cell-cycle profiles by flow cytometry. (h) Percentage of cell-cycle phases in cells from panel (g). Asterisks indicates the significant P values (*P<0.05; **P<0.01)
RanBP2-depletion-induced mitotic catastrophe is not a side effect of nuclear import
Next, to rule out the possibility that the observed RanBP2-depletion phenotypes were secondary effects or byproducts of disrupted nuclear import during interphase, we generated a fluorescent import construct using a nuclear protein fragment, NuMA2/NuMA325–829,8 fused to a nuclear localization signal and GFP, yielding GFP-NuMA2-NLS. This construct is imported into the nucleus whereas a mutant version of the same protein, GFP-NuMA2-ΔNLS, in which the NLS signal is mutated, is not. HeLa cells were transfected with either of the two constructs 24 h after treatment with RanBP2 siRNA. A significant reduction in RanBP2 localization at the nuclear membrane (nuclear rim) was observed 72 h after siRNA treatment, as was cytoplasmic staining of RanBP2 during interphase (Supplementary Figure S5). GFP-NuMA2-NLS protein accumulated in the nucleus of RanBP2-depleted cells, in contrast to GFP-NuMA2-ΔNLS, which remained cytoplasmic (Supplementary Figure S5). Consistent with these results, RanBP2 was previously shown to have little, if any, effect on NPC numbers, which during interphase remained substantially intact after RanBP2 depletion.35 Together, these data strongly suggest that RanBP2 depletion does not cause a major defect in nuclear import but is instead needed for chromosomal alignment and segregation.
Overexpression of RanBP2 does not rescue importin β1 mitotic defects
To map the RanBP2 domain that physically binds to importin β1, full-length GFP-RanBP2-FL (1–3224 aa), two deletion mutants, GFP-RanBP2-N1 (1–900 aa) and GFP-RanBP2-N2 (1–1826 aa), and the GFP net vector (Figure 5a) were transiently transfected into HeLa cells, followed by immunoprecipitation (IP) of the cell extracts with an anti-GFP antibody. As shown in Figure 5b, importin β1 interacted with the RanBP2-FL and RanBP2-N2 fragments but not with RanBP2-N1 or the GFP net vector (Figure 5b) during mitosis, which is consistent with another recent report of this interaction during interphase.27 To rule out that increased RanBP2 expression mimics the importin β1 siRNA phenotypes through sequestration of endogenous importin β1, different GFP-RanBP2 fragments were transiently overexpressed in HeLa cells. The results showed that chromosomal missegregation defects were not significantly altered by any of the GFP-RanBP2 constructs compared with GFP net vector in mitotic cells, even when transfected by various methods (Supplementary Figure S6). A careful examination of the relative intensity of RanBP2 in those GFP-RanBP2-transfected cells showed that total RanBP2 was only slightly increased through our various transient transfection methods (Supplementary Figure S6), which might explain why we did not observe enhancement of the phenotypes resulting from the chromosomal defects.
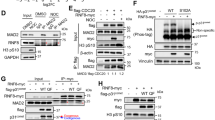
Ran binding is important for the interaction between the N-terminal of RanBP2 and importin β1. (a) Scheme of full-length RanBP2 and two C-terminal deletion mutants with GFP tags. Numbers on the right refer to amino acids. LRD, leucine-rich domain; Zinc, zinc finger motifs; E3, SUMO E3 ligase domain; CY, cyclophilin homology domain. (b) HeLa cells were transfected with a GFP, GFP-RanBP2-FL, RanBP2-N2 or RanBP2-N1 expression plasmid. After double thymidine block, lysates prepared from the cells were immunoprecipitated with anti-GFP antibody and then analyzed by IB with anti-Imp-β1 or anti-GFP antibodies. Asterisks indicate the GFP fusion proteins. Numbers indicate molecular mass markers in kilodaltons. HC, IgG heavy chain; LC, IgG light chain. In lanes marked ‘Input,’ 20 μl of the 500 μl of extract used per IP were analyzed directly. (c) Scheme of full-length RanBP2 and the N- and C-terminal deletion mutant (M1) with the GST tag. Numbers on the right refer to amino acids. (d) In vitro expressed and purified GST-RanBP2-M1, Imp-β1-N2-His and His-Ran-GTP were mixed and pulled-down with glutathione-Sepharose beads. Pulled-down lysates were separated by SDS-PAGE and then either stained with Coomassie brilliant blue (CBB) or subjected to IB with anti-6 × His antibody. Numbers indicate molecular mass markers in kilodaltons. (e) Scheme of GFP-tagged full-length RanBP2 carrying the W1211R/K1212M mutation and FLAG-tagged Imp-β1-FL carrying the I178D/Y255A mutation. (f and g) HeLa cells were transfected with a GFP-RanBP2-FL (wt), GFP-RanBP2-FL_W1211R/K1212M (mt), FLAG-Imp-β1-FL (wt) or FLAG-Imp-β1-FL_ I178D/Y255A (mt) expression plasmid. After double thymidine block, lysates prepared from the cells were immunoprecipitated with anti-GFP or anti-FLAG antibody and then analyzed by IB with anti-Imp-β1, anti-GFP, anti-RanBP2 or anti-FLAG antibodies. In lanes marked ‘Input,’ 20 μl of the 500 μl of extract used per IP were analyzed directly
Ran is also required for the mitotic interaction between RanBP2 and importin β1
A thorough understanding of the RanBP2–importin β1 interaction must take into account the possible role of the GTPase Ran, as both RanBP2 and importin β1 contain Ran-interacting regions. Ran might be part of the complex formed by RanBP2 and importin β1, perhaps having a stabilizing role in their interaction. To test this hypothesis, GST-RanBP2-M1900–1826 and His-Imp-β1-N21–650 were expressed and the two proteins were then mixed in vitro with or without Ran-GTP (Figures 5c and d). The addition of His-Ran-GTP proteins significantly enhanced the binding between GST-RanBP2-M1900–1826 and His-Imp-β1-N21–650 (Figure 5d). Next, we generated constructs containing mutagenized Ran-binding domains of RanBP226 and importin β148 (RanBP2-mt and Imp-β1-mt, respectively) to determine whether the lack of Ran binding in one partner affected the mitotic localization of the other partner (Figure 5d). The absence of Ran-binding domains in RanBP2-mt and Imp-β1-mt significantly reduced the interaction between RanBP2 and importin β1 compared with the wild type (Figures 5e–g). Taken together, these data suggest that Ran regulates the binding between RanBP2 and importin β1.
The N-terminal of importin β1 interacts with RanBP2 by sequestering endogenous RanBP2 and thereby induces mitotic catastrophe defects
Finally, we biochemically mapped the in vivo interaction domain between RanBP2 and importin β1. Five cDNA fragments covering the entire length of importin β1 (including the full length and four fragments of the protein), named Imp-β1-FL (1–876 aa), Imp-β1-N1 (1–250 aa), Imp-β1-N2 (1–650 aa), Imp-β1-M (250–650 aa) and Imp-β1-C (650–876 aa), were generated. Each cDNA was coupled to a 6 × His tag and then expressed in an Escherichia coli system (Figure 6a). Pull-down assays with mitotic HeLa cell lysates showed that RanBP2 interacted with Imp-β1-N2 (1–650 aa) but not with the other fragments nor with the vector alone. Consistent with this result, Imp-β1-N2 (1–650 aa) also localized to the nuclear envelope, but other fragments did not (Supplementary Figure S7). Additionally, we found that Imp-β1-N1 (1–250 aa), which contained HEAT repeats 1–6, and Imp-β1-M (250–650aa), which contained HEAT repeats 7–14, were also required for binding; Imp-β1-N1, Imp-β1-M and Imp-β1-C failed to bind to RanBP2 (Figure 6b). These mapping results suggested that overexpression of FLAG-Imp-β1-N2 (1–650 aa) mimics the RanBP2 siRNA phenotype through the sequestration of endogenous RanBP2, making it unavailable to interact productively with full-length importin β1, thus causing the re-appearance of the enhanced chromosome segregation abnormalities. This scenario is analogous to the mitotic catastrophe phenotype observed after the reduction of RanBP2 levels by RNAi. Indeed, we observed chromosomal segregation defects and mitotic catastrophe in 30% of FLAG-Imp-β1-N2 (1–650 aa) mitotic cells but not in cells transfected with the other fragments (Figure 6c and Supplementary Figure S8). Taken together, our findings indicate that the N-terminal of importin β11–650 interacts with RanBP2 by sequestering endogenous RanBP2 and thereby induces mitotic catastrophe defects.
N-terminal of importin β1 interacts with RanBP2. (a) Scheme of full-length Imp-β1 and four mutants with 6 × His tags. Numbers on the right refer to amino acids (left). An illustration of an interaction model between importin β1 and RanBP2 (right). (b) In vitro expressed and purified 6 × His tagged Imp-β1 fragments were mixed with mitotic HeLa lysates and pulled-down with Ni-NTA beads. Pulled-down lysates were analyzed by IB with anti-RanBP2 or anti-6 × His antibodies. Asterisks indicate the 6 × His fusion proteins. Numbers indicate molecular mass markers in kilodaltons. In lanes marked ‘Input,’ 20 μl of the 500 μl of lysate used per pull-down were analyzed directly. (c) Quantification (relative percentage) of mitotic chromosomal defect phenotypes in FLAG-net-vector, FLAG-Imp-β1-FL, FLAG-Imp-β1-N1 and FLAG-Imp-β1-N2 transfected cells. Values are based on three independent experiments, counting 100 mitotic cells in each experiment. Mean values±S.D. (error bars) are shown. All cells were treated with double thymidine block, stained with DAPI (4',6-diamidino-2-phenylindole) and visualized by confocal microscopy
Discussion
During mitosis, chromosomal segregation is carried out by a complex cellular machinery45 in which abnormalities in the cell cycle and mitosis eventually lead to cell death. There is increasing evidence that nucleoporins control chromosomal instability and tumor development.1, 4, 49 In the present work, we demonstrated that down-modulation of RanBP2 induces abnormal chromosomal segregation, improper mitotic progression and mitotic cell death, evidence of the crucial role of RanBP2 in proper mitotic progression and faithful chromosomal segregation. Mitotic defects following RanBP2 siRNA treatment were previously described only in fixed cells.35, 36, 38, 39 Here, through live cell imaging, chromosomal segregation defects and metaphase catastrophe could, for the first time, be recorded in live cells undergoing mitosis (especially during metaphase–anaphase transitions) following RanBP2 siRNA treatment (Figure 4 and Supplementary Videos S1 and S2).
Mitotic catastrophe is defined in morphological terms as a mechanism of cell death occurring during or after aberrant mitosis.45, 50 In this study, we also identified a novel link between the RanBP2–importin β1 complex and the Ran GTPase that is required for proper chromosomal segregation. Consistent with this finding, Roscioli et al.51 reported the interaction of importin β1 with RanGAP1 (the bona fide binding partner of RanBP2) in mitotic cells. In addition, previous findings suggested that importin β1, RanBP2 and SUMO-RanGAP1 co-localize along microtubules.38, 39 Our immunofluorescence results provide the first evidence that at least a small portion of mitotic RanBP2 interacts with γ-tubulin at the centrosome region (Figure 1 and Supplementary Figure S2). They are also consistent with recent proteomic studies of the mitotic drosophila centrosome, in which RanBP2 was localized.40 It is unclear exactly how RanBP2 is recruited to the centrosome/spindle pole, but the mechanism seems to involve a cascade of events. Our results indicated that RanBP2 is not the only factor involved in the regulation of chromosomal segregation and that it has other roles in mitotic progression in addition to mediating metaphase catastrophe (Figure 7). Thus, several issues, including the exact mechanism by which RanBP2 contributes to mitotic cell death and the roles of importin β1 and Ran in mitotic catastrophe, are subjects for future research.
In mammals, aneuploidy has been linked to cancer progression,52 which, like cancer development, is a complex process involving functional and genetic abnormalities. Our recent findings of the interaction of nucleoporin Nup98 with Galectin-353 and the association of nucleoporin Tpr with p5354 further strengthen the relationship between nucleoporins and carcinogenesis.49
Collectively, this study sheds light on a novel physiological function of RanBP2 in the regulation of mitotic progression and chromosomal segregation, which may facilitate the discovery of new treatment regimens for cancer by targeting mitosis. It may also provide mechanistic insights into previously reported phenotypes associated with altered RanBP2 expression, including tumor progression and the embryonic lethality of RanBP2-null mice.36 Future clinical investigations should also explore how RanBP2 contributes to carcinogenesis.
Materials and Methods
Plasmids
HeLa cell cDNA was synthesized using a SuperScript III CellsDirect cDNA synthesis system kit (Life Technologies Corporation, Carlsbad, CA, USA). The full-length RanBP2/Nup358 coding region was PCR-amplified from cDNA and subcloned into pEGFP-C3 (Clontech Laboratories, Moutain View, CA, USA) by introducing a NotI site using a site-directed mutagenesis kit (TaKaRa, Bio Inc., Shiga, Japan). Two RanBP2 fragments (N1 and N2) were subcloned by PCR from pEGFP-C3-RanBP2 into pEGFP-N2. The RanBP2-M1 fragment was subcloned by PCR from pEGFP-C3-RanBP2 into pET28a-GST. Both a plasmid encoding the full-length human importin β1 and RanQ69L, tagged with 6 × His, in the pQE60 vector, were kind gifts from Dr. Ulrike Kutay (ETH Zurich, Switzerland). Four importin β1 fragments (N1, N2, M and C) were subcloned by PCR from pQE60-importin β1 into pQE60 or into pCMV-3 × -FLAG. pEGFP-C2-NuMA2/NuMA325–829 and pEGFP-C2-NuMA325–829ΔNLS plasmids encoding the NLS oligonucleotide were inserted into pEGFP-C2 (Clontech Laboratories). GFP-RanBP2-FL_W1211R/K1212M, FLAG-importin β1_I178D/Y255A, wild-type Ran-6 × His and importin β1 (full-length) siRNA-resistant plasmids were synthesized from each full-length construct using a site-directed mutagenesis kit. The GFP-H2B plasmid was obtained from Addgene (Cambridge, MA, USA). Details of the expression constructs and cloning primers are listed in Supplementary Tables S1 and S2. All constructs were confirmed by DNA sequencing.
Mammalian cell culture, transfections and cell synchronization
HeLa cells were obtained from the American Type Culture Collection (ATCC) and were synchronized in S phase by double thymidine block using 2 mM thymidine.13, 14, 15, 16 The cells were transfected with GFP-RanBP2-fragment plasmids using Lipofectamine 2000 (Life Technologies Corporation), TurboFect (Thermo Fishes Scientific Inc., Waltham, MA, USA) and Neon (Life Technologies Corporation) following the manufacturers’ protocols. The HeLa EGFP-H2B cell line, used in the analysis of mitotic progression, was generated by transfecting HeLa cells with EGFP-H2B cDNA and maintained in medium containing G418 (600 μg/ml) as previously described.10
RNA interference
siRNA duplexes targeting RanBP2/Nup358 (sequence 5′-CCGUUUUGGUGAGUCAACAtt-3′, siRNA ID s11773, cat no. 4390824) were purchased from Life Technologies Corporation. Importin β1 (sc-35736) and control siRNAs (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Another independent siRNA duplexes targeting Importin β1 #2 (sequence 5′-GAAAAUUUGGGGAAUGAGAACdTdT-3′)55 were purchased from Sigma-Aldrich (St. Louis, MO, USA) for phenotypes double check. siRNA transfections were performed using Lipofectamine 2000 (Life Technologies Corporation) as previously described.10
Antibodies, immunocytochemistry (ICC) and confocal microscopy
Anti-Nup358/RanBP2 rabbit polyclonal antibody (for ICC) was a kind gift from Dr. Elias Coutavas and Dr. Blobel (The Rockefeller University). Anti-Nup358/RanBP2 (sc-74518) (IB, IP), anti-importin/Karyopherin β1 (sc-137016) (IB, IP) and anti-β-actin (sc-47778) mouse monoclonal antibodies and anti-RanGAP1 (sc-25630) (ICC, IP) rabbit polyclonal antibody were from Santa Cruz Biotechnology. Anti-importin/Karyopherin β1 mouse monoclonal antibody (ab2811) (ICC) and anti-Pom121 rabbit polyclonal antibody (ab111610) were from Abcam (Cambridge, England). Anti-SUMO1 and anti-RanGAP1 (33-0800) (ICC, IB) mouse monoclonal antibodies were from Life Technologies Corporation. Anti-TopoIIα mouse monoclonal antibody (MO42-3S) was from MBL (Nagoya, Japan). Anti-Lamin A/C rabbit polyclonal antibody (2032) was from Cell Signaling (Danvers, MA, USA). Anti-GFP (A6455) (IP, IB, ICC) rabbit polyclonal antibody was from Life Technologies Corporation. Anti-GFP (012-20461) (ICC) mouse monoclonal antibody was from Wako (Osaka, Japan). Anti-α-tubulin (DM1A), anti-γ-tubulin (GTU-88) and anti-FLAG (M2) monoclonal antibodies were from Sigma-Aldrich. Anti-BubR1 (612502) mouse monoclonal antibody was from BD Transduction Laboratories (Franklin Lakes, NJ, USA). Anti-6 × His mouse monoclonal antibody was from Qiagen (KJ Venlo, Netherlands). Secondary antibodies were from Molecular Probes (Life Technologies Corporation). For immunofluorescence, synchronized HeLa cells were washed in phosphate-buffered saline (PBS) and fixed for 10 min in 4% paraformaldehyde in PBS. Cells were then permeabilized with 0.3% Triton X-100 in PBS for 10 min at room temperature. Annexin V FLUOS staining kit (Roche, Rotkreuz, Switzerland) was used for phosphatidylserine detection. Samples were mounted onto coverslips using Pro-Long Gold Antifade reagent (Life Technologies Corporation) and were examined on a Zeiss Carl Zeiss Microscopy GmbH (Jena, Germany) LSM5 EXCITER confocal microscope from Carl Zeiss Microscopy GmbH (Jena, Germany) with four laser beams, and all images were acquired using a plan-Apochromat from Carl Zeiss Microscopy GmbH at × 63 with a 1.4-N.A. objective or at × 100 with a 1.4-N.A. objective.
Immunoprecipitation
The IP procedures were described previously.10, 53 Briefly, mitotic HeLa cells were collected, washed with PBS, spun at 400 × g for 10 min and lysed in 1 ml of cold lysis buffer (50 mM Tris-HCl (pH 7.2), 250 mM NaCl, 0.1% Nonidet P-40, 2 mM EDTA, 10% glycerol) containing 1 × protease inhibitor mixture (Roche) and 1 mM phenylmethylsulfonyl fluoride. The lysates were centrifuged for 30 min at 4 °C at 14 000 × g. The resulting supernatants were pre-cleared with 50 μl of protein A/G bead slurry (Santa Cruz Biotechnology), mixed with 10 μl of various antibodies as specified and incubated for 1 h at 4 °C with rocking. The beads were then washed five times with 500 μl of lysis buffer. After the last wash, 50 μl of 1 × sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) blue loading buffer (New England Biolabs, Ipswich, MA, USA) was added to the bead pellet before loading. Signals were detected with an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, England) and quantified using an LAS-4000 image analyzer (Fuji Film, Tokyo, Japan) according to the manufacturer’s specifications.
Expression of recombinant proteins
To express 6 × His-tagged importin β1 fragments and Ran in E. coli XL-1 Blue cells (Agelent Technologies, Inc., Santa Clara, CA, USA) and GST-tagged RanBP2-M1 in E. coli BL21(DE3) Codon Plus (Agelent Technologies, Inc.) cells, both strains of bacteria were grown at 37 °C to an absorbance at 600 nm (A600) of 0.6 and induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside at 18 °C overnight. The cells were harvested by centrifugation and lysed in buffer containing 50 mM Tris-HCl (pH 7.7), 150 mM KCl, 0.1% Triton-X100 and Complete EDTA-free protease inhibitor mixture tablets (Roche Applied Science). The cells were lysed using a cell sonicator (SMT), and the lysate was clarified by centrifugation at 15 000 × g for 60 min. Proteins containing the 6 × His tag were purified by nickel-affinity chromatography (Qiagen). The GST-tagged protein was purified by glutathione-Sepharose and Superdex 200 10/300 GL size exclusion chromatography (GE Healthcare).
In vitro binding assays
In vitro binding assays were performed as previously described.19 Briefly, 10 μl of glutathione-Sepharose beads were suspended in Tween 20 binding buffer (20 mM HEPES, pH 7.4, 110 mM potassium acetate, 2 mM magnesium chloride and 0.1% Tween 20) containing 1 × protease inhibitor mixture (Roche), 0.25 mM GST-RanBP2-M1, 0.25 mM Kap-β1-His and 0.8 mM Ran-GTP in a total reaction volume of 40 μl. The reactions were allowed to proceed for 2 h at 4 °C with rotation, after which the beads were washed twice in cold TB-0.1% Tween 20. After the last wash, 20 μl of 1 × SDS-PAGE blue loading buffer was added to the bead pellet before loading.
Time-lapse microscopy
Time-lapse analysis of cellular histone dynamics during metaphase and anaphase transitions in live cells was recorded in GFP-H2B stable HeLa cell lines as described previously.18 The cells were placed in a microincubation chamber (7136; Corning, Corning, NY, USA) on a Zeiss LSM5 confocal microscope stage, which was heated to 37 °C and equipped with a CO2 supply (electric CO2 microscope stage incubator; Okolab, Ottaviano, Italy). Time-lapse series were generated by collecting photographs every 3 min; the photographs were then converted to eight-bit images and processed using Adobe Photoshop CS5 from Adobe Systems Incorporated (San Jose, CA, USA) and NIH Image J from National Institutes of Health (Bethesda, MD, USA).
Flow cytometry
HeLa cells transfected with siRNA were trypsinized, washed twice with PBS and fixed in 70% ethanol at −20 °C overnight. The fixed cells were resuspended in PBS containing 50 μg RNase A (Nacalai Tesque, Kyoto, Japan)/ml and 50 μg PI (Sigma-Aldrich)/ml. Cellular DNA content was analyzed using a FACSCanto II (BD Biosciences, Franklin Lakes, NJ, USA) with FACS Diva software (BD Biosciences).56
Abbreviations
- NPC:
-
nuclear pore complex
- GFP:
-
green fluorescent protein
References
Nakano H, Wang W, Hashizume C, Funasaka T, Sato H, Wong RW . Unexpected role of nucleoporins in coordination of cell cycle progression. Cell Cycle 2011; 10: 425–433.
Guttinger S, Laurell E, Kutay U . Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 2009; 10: 178–191.
Raices M, D’Angelo MA . Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol 2012; 13: 687–699.
Bukata L, Parker SL, D’Angelo MA . Nuclear pore complexes in the maintenance of genome integrity. Curr Opin Cell Biol 2013; 25: 378–386.
Wong RW . Interaction between Rae1 and cohesin subunit SMC1 is required for proper spindle formation. Cell Cycle 2010; 9: 198–200.
Wong RW . An update on cohesin function as a ‘molecular glue’ on chromosomes and spindles. Cell Cycle 2010; 9: 1754–1758.
Wong RW, Blobel G . Cohesin subunit SMC1 associates with mitotic microtubules at the spindle pole. Proc Natl Acad Sci USA 2008; 105: 15441–15445.
Wong RW, Blobel G, Coutavas E . Rae1 interaction with NuMA is required for bipolar spindle formation. Proc Natl Acad Sci USA 2006; 103: 19783–19787.
Funasaka T, Nakano H, Wu Y, Hashizume C, Gu L, Nakamura T et al. RNA export factor RAE1 contributes to NUP98-HOXA9-mediated leukemogenesis. Cell Cycle 2011; 10: 1456–1467.
Nakano H, Funasaka T, Hashizume C, Wong RW . Nucleoporin translocated promoter region (Tpr) associates with dynein complex, preventing chromosome lagging formation during mitosis. J Biol Chem 2010; 285: 10841–10849.
Hashizume C, Nakano H, Yoshida K, Wong RW . Characterization of the role of the tumor marker Nup88 in mitosis. Mol Cancer 2010; 9: 119.
Chatel G, Fahrenkrog B . Nucleoporins: leaving the nuclear pore complex for a successful mitosis. Cell Signal 2011; 23: 1555–1562.
Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E . Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol Chem 1995; 270: 14209–14213.
Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K et al. A giant nucleopore protein that binds Ran/TC4. Nature 1995; 376: 184–188.
Kassube SA, Stuwe T, Lin DH, Antonuk CD, Napetschnig J, Blobel G et al. Crystal structure of the N-terminal domain of Nup358/RanBP2. J Mol Biol 2012; 423: 752–765.
Lin DH, Zimmermann S, Stuwe T, Stuwe E, Hoelz A . Structural and functional analysis of the C-terminal domain of Nup358/RanBP2. J Mol Biol 2013; 425: 1318–1329.
Melchior F, Guan T, Yokoyama N, Nishimoto T, Gerace L . GTP hydrolysis by Ran occurs at the nuclear pore complex in an early step of protein import. J Cell Biol 1995; 131: 571–581.
Singh BB, Patel HH, Roepman R, Schick D, Ferreira PA . The zinc finger cluster domain of RanBP2 is a specific docking site for the nuclear export factor, exportin-1. J Biol Chem 1999; 274: 37370–37378.
Yaseen NR, Blobel G . GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin nup358. J Biol Chem 1999; 274: 26493–26502.
Ferreira PA, Nakayama TA, Pak WL, Travis GH . Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature 1996; 383: 637–640.
Aslanukov A, Bhowmick R, Guruju M, Oswald J, Raz D, Bush RA et al. RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism. PLoS Genet 2006; 2: e177.
Pichler A, Gast A, Seeler JS, Dejean A, Melchior F . The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 2002; 108: 109–120.
Asally M, Yasuda Y, Oka M, Otsuka S, Yoshimura SH, Takeyasu K et al. Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. FEBS J 2011; 278: 610–621.
Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL et al. Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol 2010; 8: e1000350.
Gloerich M, Vliem MJ, Prummel E, Meijer LA, Rensen MG, Rehmann H et al. The nucleoporin RanBP2 tethers the cAMP effector Epac1 and inhibits its catalytic activity. J Cell Biol 2011; 193: 1009–1020.
Hamada M, Haeger A, Jeganathan KB, van Ree JH, Malureanu L, Walde S et al. Ran-dependent docking of importin-beta to RanBP2/Nup358 filaments is essential for protein import and cell viability. J Cell Biol 2011; 194: 597–612.
Walde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U et al. The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo- and transport receptor-specific manner. Traffic 2012; 13: 218–233.
Werner A, Flotho A, Melchior F . The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol Cell 2012; 46: 287–298.
Ocwieja KE, Brady TL, Ronen K, Huegel A, Roth SL, Schaller T et al. HIV integration targeting: a pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog 2011; 7: e1001313.
Zhang R, Mehla R, Chauhan A . Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus-1 preintegration complex (DNA). PLoS One 2010; 5: e15620.
Schaller T, Ocwieja KE, Rasaiyaah J, Price AJ, Brady TL, Roth SL et al. HIV-1 capsid-cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog 2011; 7: e1002439.
Bichel K, Price AJ, Schaller T, Towers GJ, Freund SM, James LC . HIV-1 capsid undergoes coupled binding and isomerization by the nuclear pore protein NUP358. Retrovirology 2013; 10: 81.
Mahadevan K, Zhang H, Akef A, Cui XA, Gueroussov S, Cenik C et al. RanBP2/Nup358 potentiates the translation of a subset of mRNAs encoding secretory proteins. PLoS Biol 2013; 11: e1001545.
Prunuske AJ, Liu J, Elgort S, Joseph J, Dasso M, Ullman KS . Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules. Mol Biol Cell 2006; 17: 760–769.
Salina D, Enarson P, Rattner JB, Burke B . Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol 2003; 162: 991–1001.
Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell 2008; 133: 103–115.
Klein UR, Haindl M, Nigg EA, Muller S . RanBP2 and SENP3 function in a mitotic SUMO2/3 conjugation-deconjugation cycle on Borealin. Mol Biol Cell 2009; 20: 410–418.
Joseph J, Tan SH, Karpova TS, McNally JG, Dasso M . SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J Cell Biol 2002; 156: 595–602.
Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M . The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr Biol 2004; 14: 611–617.
Muller H, Schmidt D, Steinbrink S, Mirgorodskaya E, Lehmann V, Habermann K et al. Proteomic and functional analysis of the mitotic Drosophila centrosome. EMBO J 2010; 29: 3344–3357.
Kalab P, Pralle A, Isacoff EY, Heald R, Weis K . Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 2006; 440: 697–701.
Ciciarello M, Mangiacasale R, Thibier C, Guarguaglini G, Marchetti E, Di Fiore B et al. Importin beta is transported to spindle poles during mitosis and regulates Ran-dependent spindle assembly factors in mammalian cells. J Cell Sci 2004; 117: 6511–6522.
Rello-Varona S, Kepp O, Vitale I, Michaud M, Senovilla L, Jemaa M et al. An automated fluorescence videomicroscopy assay for the detection of mitotic catastrophe. Cell Death Dis 2010; 1: e25.
Rao CV, Yamada HY, Yao Y, Dai W . Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice. Carcinogenesis 2009; 30: 1469–1474.
Vakifahmetoglu H, Olsson M, Zhivotovsky B . Death through a tragedy: mitotic catastrophe. Cell Death Differ 2008; 15: 1153–1162.
Stevens JB, Abdallah BY, Liu G, Ye CJ, Horne SD, Wang G et al. Diverse system stresses: common mechanisms of chromosome fragmentation. Cell Death Dis 2011; 2: e178.
Kimura M, Yoshioka T, Saio M, Banno Y, Nagaoka H, Okano Y . Mitotic catastrophe and cell death induced by depletion of centrosomal proteins. Cell Death Dis 2013; 4: e603.
Bednenko J, Cingolani G, Gerace L . Importin beta contains a COOH-terminal nucleoporin binding region important for nuclear transport. J Cell Biol 2003; 162: 391–401.
Funasaka T, Wong RW . The role of nuclear pore complex in tumor microenvironment and metastasis. Cancer Metastasis Rev 2011; 30: 239–251.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012 19: 107–120.
Roscioli E, Di Francesco L, Bolognesi A, Giubettini M, Orlando S, Harel A et al. Importin-beta negatively regulates multiple aspects of mitosis including RANGAP1 recruitment to kinetochores. J Cell Biol 2012; 196: 435–450.
Chandhok NS, Pellman D . A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr Opin Genet Dev 2009; 19: 74–81.
Funasaka T, Balan V, Raz A, Wong RW . Nucleoporin Nup98 mediates galectin-3 nuclear-cytoplasmic trafficking. Biochem Biophys Res Commun 2013; 434: 155–161.
Funasaka T, Tsuka E, Wong RW . Regulation of autophagy by nucleoporin Tpr. Sci Rep 2012; 2: 878.
Miki T, Okawa K, Sekimoto T, Yoneda Y, Watanabe S, Ishizaki T et al. mDia2 shuttles between the nucleus and the cytoplasm through the importin-{alpha}/{beta}- and CRM1-mediated nuclear transport mechanism. J Biol Chem 2009; 284: 5753–5762.
Endo A, Moyori A, Kobayashi A, Wong RW . Nuclear mitotic apparatus protein, NuMA, modulates p53-mediated transcription in cancer cells. Cell Death Dis 2013; 4: e713.
Acknowledgements
We thank Elias Coutavas and Gunter Blobel (The Rockefeller University) for Anti-Nup358/RanBP2 rabbit polyclonal antibody. We also thank the members of our lab for technical support and helpful discussions. This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas, Grants-in-Aid for Challenging Exploratory Research and Grants-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan, and by grants from the Asahi Glass Foundation, the Suzuken Memorial Foundation, the Sumitomo Foundation, the Kowa Life Science Foundation, the Mochida Memorial Foundation, the Sagawa Foundation, the Inamori Foundation, the Uehara Memorial Foundation and the Takeda Science Foundation (to RW). CH was supported by the Hayashi Memorial Foundation for Women Natural Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Supplementary Information accompanies this paper on Cell Death and Disease website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Hashizume, C., Kobayashi, A. & Wong, R. Down-modulation of nucleoporin RanBP2/Nup358 impaired chromosomal alignment and induced mitotic catastrophe. Cell Death Dis 4, e854 (2013). https://doi.org/10.1038/cddis.2013.370
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.370
Keywords
This article is cited by
-
The RanBP2/RanGAP1-SUMO complex gates β-arrestin2 nuclear entry to regulate the Mdm2-p53 signaling axis
Oncogene (2021)
-
Pulchelloid A, a sesquiterpene lactone from the Canadian prairie plant Gaillardia aristata inhibits mitosis in human cells
Molecular Biology Reports (2021)
-
Nucleoporin Nup58 localizes to centrosomes and mid-bodies during mitosis
Cell Division (2019)
-
Visualization of human karyopherin beta-1/importin beta-1 interactions with protein partners in mitotic cells by co-immunoprecipitation and proximity ligation assays
Scientific Reports (2018)
-
The GTPase RAN regulates multiple steps of the centrosome life cycle
Chromosome Research (2016)