Abstract
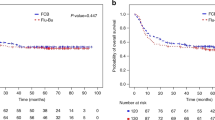
We hypothesized that IV busulfan (Bu) dosing could be safely intensified through pharmacokinetic (PK-) dose guidance to minimize the inter-patient variability in systemic exposure (SE) associated with body-sized dosing, and that this should improve outcome of AML/MDS patients undergoing allogeneic stem cell transplantation. To test this hypothesis, we treated 218 patients (median age 50.7 years, male/female 50/50%) with fludarabine 40 mg/m2 once daily x4, each dose followed by IV Bu, randomized to 130 mg/m2 (N=107) or PK-guided to average daily SE, AUC of 6000 μM min (N=111), stratified for remission status and allo-grafting from HLA-matched donors. Toxicity and GvHD rates in the groups were similar; the risk of relapse or treatment-related mortality remained higher in the fixed-dose group throughout the 80-month observation period. Further, PK-guidance yielded safer disease control, leading to improved overall and PFS, most prominently in MDS patients and in AML patients not in remission at allogeneic stem cell transplantation. We conclude that AML/MDS patients receiving pretransplant conditioning treatment with our 4-day regimen may benefit significantly from PK-guided Bu dosing. This could be considered an alternative to fixed-dose delivery since it provides the benefit of precise dose delivery to a predetermined SE without increasing risk(s) of serious toxicity and/or GvHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas ED. A history of allogeneic hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG (eds). Thomas’ Hematopoietic Cell Transplantation, 4th edn. Wiley-Blackwell: Oxford, UK, 2009, pp 3–7.
Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Elurnoy N et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977; 49: 511–533.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Russell JA, Savoie ML, Balogh A, Turner AR, Larratt L, Chaudhry MA et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400cGy total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant 2007; 13: 814–821.
Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclosphosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant 2008; 14: 672–684.
Pidala J, Kim J, Anasetti C, Kharfan-Dabaja MA, Field T, Perkins J et al. Targeted i.v. BU and fludarabine (t-i.v. BU/Flu) provides effective control of AML in adults with reduced toxicity. Bone Marrow Transplant 2011; 46: 641–649.
Perkins JB, Kim J, Anasetti C, Fernandez HF, Perez LE, Ayala E et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1099–1107.
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol 2013; 6: 15.
Rambaldi A, Grassi A, Masciulli A, Boschini C, Micò MC, Busca A et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2015; 16: 1525–1536.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant 2008; 14: 220–228.
Russell JA, Kangarloo SB, Williamson T, Chaudhry MA, Savoie ML, Turner AR et al. Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant 2013; 19: 1381–1386.
Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D et al. Pharmacokinetics of once daily IV busulfan as part of pretransplant preparative regimens; a comparison with an every 6 hour dosing schedule. Biol Blood Marrow Transplant 2007; 13: 56–64.
Popat UR, Bassett R, Chen J, Alousi AM, Anderlini P, Ciurea SO et al. Allogeneic transplantation for myelofibrosis: benefit of dose intensity. J Clin Oncol 2013; 31 (suppl): (abstract 7011).
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International Scoring System for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 2002; 8: 145–154.
Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL et al. Clofarabine±fludarabine with once daily iv busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant 2011; 17: 893–900.
Kebriaei P, Madden T, Wang X, Thall PF, Ledesma C, de Lima M et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant 2013; 48: 26–31.
Kebriaei P, Madden T, Kazerooni R, Wang X, Thall PF, Ledesma C et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant 2011; 17: 412–420.
Nieto Y, Thall P, Valdez B, Andersson B, Popat U, Anderlini P et al. High-dose infusional gemcitabine combined with busulfan and melphalan with autologous stem-cell transplant in patients with refractory lymphoid malignancies. Biol Blood Marrow Transplant 2012; 18: 1677–1686.
D'Argenio DZ, Schumitzky A. ADAPT II User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource: Los Angeles, CA, USA, 1997.
Przepiorka D, Khouri I, Ippoliti C, Ueno NT, Mehra R, Körbling M et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transplant 1999; 24: 763–768.
Tang X, Alatrash G, Ning J, Jakher H, Stafford P, Zope M et al. Increasing Chimerism after allogeneic stem cell transplantation is associated with longer survival time. Biol Blood Marrow Transplant 2014; 20: 1139–1144.
Keating MJ, Smith TL, Gehan EA, McCredie KB, Bodey GP, Spitzer G et al. Factors related to length of complete remission in adult acute leukemia. Cancer 1980; 45: 2017–2029.
Common Terminology Criteria v3.0. NIH Publication No. 03-5410, June 2003.
Fisher RA . On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Stat Soc 1922; 85: 87–94.
Randles R, Wolfe D . Introduction to the Theory of Nonparametric Statistics. John Wiley, Hoboken, NJ, USA, 1979.
Freeman GH, Halton JH . Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951; 38: 141–149.
Kaplan E, Meier P . Nonparametric estimation from incomplete observations. J Amer Statist Assoc 1958; 53: 457–481.
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170.
Ibrahim JG, Chen M-Hui Sinha D . Bayesian Survival Analysis. Springer: New York, NY, USA, 2004.
Rosenbaum PR, Rubin DB . Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Amer Stat 1985; 39: 33–38.
Ho D, Imai K, King G, Stuart E . Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Pol Anal 2007; 15: 199–236.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Abu-Dahier FM, Goodeve AC, Wilson GA, Gari MA, Peake IR, Rees DC et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol 2000; 111: 190–195.
Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 2010; 95: 1857–1864.
Santos GW, Tutschka PJ, Brookmayer R, Saral R, Beschorner WE, Bias WB et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Tutschka PJ, Copelan EA, Klein JP . Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 1987; 70: 1382–1388.
Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen—a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol 2013; 31: 3549–3556.
Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood 2013; 122: 3863–7380.
Bredeson C, LeRademacher J, Kato K, Dipersio JF, Agura E, Devine SM et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood 2013; 122: 3871–3878.
Yamauchi T, Nowak BJ, Keating MJ, Plunkett W . DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res 2001; 7: 3580–3589.
Valdez BC, Andersson BS . Interstrand crosslink inducing agents in pretransplantconditioning therapy for hematologic malignancies. Environ Mol Mutagen 2010; 51: 659–668.
Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Avigdor A, Ben-Bassat I et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
De Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood 2004; 104: 865–872.
Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Socie G, Kroeger N, Blaise D et al. Higher busulfan dose intensity appears to improve leukemia-free and overall survival in AML allografted in CR2: an analysis from the acute leukemia working party of the european group for blood and marrow transplantation. Leuk Res 2015; 39: 933–937.
Hassan M, Andersson BS . Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics 2013; 14: 75–87.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 2014; 32: 3249–3257.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 2014; 32: 3497–3505.
Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Hamladji RM, Blaise D, Socie G et al. Comparing i.v. BU dose intensity between two regimens (FB2 vs FB4) for allogeneic HCT for AML in CR1: a report from the Acute Leukemia Working Party of EBMT. Bone Marrow Transplant 2014; 49: 1170–1175.
Acknowledgements
This work was funded by the National Cancer Institute grants P01-CA49639 and 2P30-CA16672, and by support from Various Donors’ Fund and the Stephen and Lavinia Boyd Fund for Leukemia Research. The authors acknowledge the excellent work of the clinical staff, research nurses and nursing and pharmacy staff of the blood and marrow transplantation unit, without whom this project would not have been possible. The assistance of Mr. Matthew Hernandez in preparation of this manuscript is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
BSA was previously a consultant for Otsuka America Pharmaceuticals, Inc., and REC has received research funding from Otsuka America Pharmaceuticals, Inc.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Andersson, B., Thall, P., Valdez, B. et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transplant 52, 580–587 (2017). https://doi.org/10.1038/bmt.2016.322
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.322
This article is cited by
-
Intravenous Busulfan, Dimethylacetamide and neurotoxicity after high-dose pretransplant conditioning chemotherapy
Bone Marrow Transplantation (2023)
-
Effects of combined test dose and therapeutic drug monitoring strategy in exposure-directed busulfan
Annals of Hematology (2023)
-
Evaluation of different pharmacokinetically guided IV busulfan exposure ranges on adult patient outcomes after hematopoietic stem cell transplantation
Annals of Hematology (2023)
-
Fixed-dose administration and pharmacokinetically guided adjustment of busulfan dose for patients undergoing hematopoietic stem cell transplantation: a meta-analysis and cost-effectiveness analysis
Annals of Hematology (2022)
-
A randomized phase III study of pretransplant conditioning for AML/MDS with fludarabine and once daily IV busulfan ± clofarabine in allogeneic stem cell transplantation
Bone Marrow Transplantation (2022)