Abstract
Noise effects in technological applications, far from being a nuisance, can be exploited with advantage — for example, unavoidable thermal fluctuations have found application in the transport and sorting of colloidal particles1,2,3 and biomolecules4,5,6. Here we use a microfluidic system to demonstrate a paradoxical migration mechanism in which particles always move in a direction opposite to the net acting force (‘absolute negative mobility’) as a result of an interplay between thermal noise, a periodic and symmetric microstructure, and a biased alternating-current electric field. This counterintuitive phenomenon could be used for bioanalytical purposes, for example in the separation and fractionation of colloids, biological molecules and cells.
Similar content being viewed by others
Main
Newton's second law would seem to exclude absolute negative mobility as a response by a system at rest to a static force. However, such paradoxical behaviour has been experimentally observed in semiconductor devices7 and theoretically predicted in simplified stochastic model systems8,9. Nonlinear dynamics are necessary to reconcile absolute negative mobility with Newton's law, and the system needs to operate far from equilibrium to avoid conflict with the second law of thermodynamics.
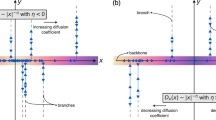
To provide a proof-of-principle for absolute negative mobility in a lab-on-a-chip, we designed a simple microfluidic device consisting of periodically arranged posts with alternating small and large gaps (Fig. 1; for methods, see supplementary information). The device is filled with negatively charged beads in an aqueous buffer. Electric fields are generated by applying a voltage along the x-axis, so that a positive voltage generates a positive force on the beads along the x-axis. Applying an alternating voltage, UAC(t), that switches periodically between ±U0, there is no net motion of the beads for symmetry reasons. This set-up represents our unperturbed non-equilibrium system at rest. But what will be the average migration velocity v in the x direction in response to a static perturbation voltage, UDC, superimposed on UAC(t)?
Movement of 2-µm polystyrene beads in response to a static voltage, UDC, superimposed to an alternating-current voltage that switches between ±30 V every 25 s. Red dots: experimentally measured velocities, averaged over 40 beads and 200 s. Error bars are of statistical origin, but variabilities in the beads and microstructures also contribute. The line shows the theoretical response characteristics obtained from numerical simulations. Inset: partial view (optical micrograph image) of the poly(dimethylsiloxane) microstructure showing the rectangular posts and migrating beads (dark dots). In both the x and y directions, the gaps between the posts are alternately smaller and larger than the bead diameter. For movie, see supplementary information.
To answer this question, we computed the electric field in the microstructure and, including thermal-noise effects, simulated the bead motion by stochastic differential equations, adapted quantitatively to the microstructure geometry and the experimentally determined free diffusion and mobility of the microbeads (see supplementary information). The key feature of the resulting response curve (Fig. 1) is the negative slope that is symmetrical around the origin — a distinct and unambiguous signature of absolute negative mobility. We also measured the average bead velocity by tracking the beads using real-time video microscopy for different values of UDC (see supplementary information) and found that the measured experimental response was in good agreement with theory (Fig. 1).
To explain how absolute negative mobility occurs, we consider the small gaps in the microstructure as ‘traps’ — electrical field lines can pass through them but the beads cannot. For 0<UDC<U0, the alternating total voltages ±U0+UDC yield a back-and-forth motion of the beads along the x direction. Whenever a bead succeeds in passing through a large gap, it is trapped by the adjacent small gap in the x direction (Fig. 1, inset), unless it thermally diffuses sufficiently far in the y direction to proceed through another large gap. The smaller the voltage, the more time it has to do so and the farther it gets before being trapped. As |+U0+UDC|>|−U0+UDC|, the bead becomes trapped after moving forwards a short distance when +U0+UDC>0, whereas it travels backwards for a longer distance when −U0+UDC<0, resulting in absolute negative mobility.
We find that this response by a particle is very sensitive to particle size, even to the extent of allowing particles of different sizes to be steered in opposite directions (our unpublished results). Absolute negative mobility therefore holds promise for bioanalytical applications, for example in the sorting of cells, organelles and biochemical compounds.
References
Rousselet, J., Salome, L., Ajdari, A. & Prost, J. Nature 370, 446–448 (1994).
Faucheux, L. P. & Libchaber, A. Faraday Trans. 91, 3163–3166 (1995).
Marquet, C., Buguin, A., Talini, L. & Silberzan, P. Phys. Rev. Lett. 88, 168301 (2002).
Han, J. & Craighead, H. G. Science 288, 1026–1029 (2000).
Bader, J. S. et al. Proc. Natl Acad. Sci. USA 96, 13165–13169 (1999).
Huang, L. R., Cox, E. C., Austin, R. H. & Sturm, J. C. Analyt. Chem. 75, 6963–6967 (2003).
Keay, B. J. et al. Phys. Rev. Lett. 75, 4102–4105 (1995).
Eichhorn, R., Reimann, P. & Hänggi, P. Phys. Rev. Lett. 88, 190601 (2002).
Cleuren, B. & Van den Broeck, C. Phys. Rev. E 67, 055101 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
Describes methods and materials of microdevice fabrication, detailed conditions of the ANM experiment, and details of the theoretical model and numerical simulations. (doc-file, 26,5 KB)
Supplementary Figure
Describes the experimental setup, including a microdevice scheme, an enlargement of the structured part of the microdevice and an illustration of the time-dependence of the applied voltages (UAC(t) and static perturbation UDC). (doc-file, 108 KB)
Supplementary Movie
This movie demonstrates ANM of colloidal particles in the microstructure. (avi-file, 2,62 MB)
Rights and permissions
About this article
Cite this article
Ros, A., Eichhorn, R., Regtmeier, J. et al. Absolute negative particle mobility. Nature 436, 928 (2005). https://doi.org/10.1038/436928a
Published:
Issue Date:
DOI: https://doi.org/10.1038/436928a
This article is cited by
-
Overcrowding induces fast colloidal solitons in a slowly rotating potential landscape
Nature Communications (2023)
-
Non-Fourier heat transport in nanosystems
La Rivista del Nuovo Cimento (2023)
-
Tunable particle separation via deterministic absolute negative mobility
Scientific Reports (2020)
-
3D metamaterials
Nature Reviews Physics (2019)
-
Boosting migration of large particles by solute contrasts
Nature Materials (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.