Abstract
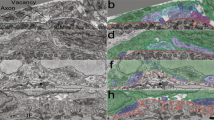
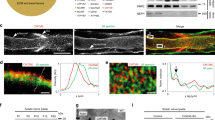
Presynaptic and postsynaptic membranes directly oppose each other at chemical synapses, minimizing the delay in transmitting information across the synaptic cleft. Extrasynaptic neuronal surfaces, in contrast, are almost entirely covered by processes from glial cells1. The exclusion of glial cells from the synaptic cleft, and the long-term stability of synapses, presumably result in large part from the tight adhesion between presynaptic and postsynaptic elements2,3. Here we show that there is another requirement for synaptic maintenance: glial cells of the skeletal neuromuscular synapse, Schwann cells, are actively inhibited from entering the synaptic cleft between the motor nerve terminal and the muscle fibre. One inhibitory component is laminin 11, a heterotrimeric glycoprotein that is concentrated in the synaptic cleft4. Regulation of an inhibitory interaction between glial cells and synaptic cleft components may contribute to synaptic rearrangements, and loss of this inhibition may underlie the loss of synapses that results from injury to the postsynaptic cell5,6,7,8,9,10,11,12.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Peters, A., Palay, S. L. & Webster, H. D. The Fine Structure of the Nervous System (Oxford Univ. Press, New York, (1991)).
Vaughn, J. E. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 3, 255–285 (1989).
Colman, D. R. Neurites, synapses, and cadherins reconciled. Mol. Cell. Neurosci. 10, 1–6 (1997).
Patton, B. L., Miner, J. H., Chiu, A. Y. & Sanes, J. R. Localization, regulation and function of laminins in the neuromuscular system of developing, adult and mutant mice. J. Cell Biol. 139, 1507–1521 (1997).
Duchen, L. W., Excell, B. J., Patel, R. & Smith, B. Changes in motor end-plates resulting from muscle fibre necrosis and regeneration. A light and electron microscopic study of the effects of the depolarizing fraction (cardiotoxin) of Dendroaspis jamesoni venom. J. Neurol. Sci. 21, 391–417 (1974).
Jirmanová, I. Ultrastructure of motor end-plates during pharmacologically-induced degeneration and subsequent regeneration of skeletal muscle. J. Neurocytol. 4, 141–155 (1975).
Matthews, M. R. & Nelson, V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J. Physiol. 245, 91–135 (1975).
Purves, D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J. Physiol. 252, 429–463 (1975).
Blinzinger, K. & Kreutzberg, G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforschung 85, 145–157 (1968).
Svensson, M. & Aldskogius, H. Synaptic density of axotomized hypoglossal motorneurons following pharmacological blockade of the microglial cell proliferation. Exp. Neurol. 120, 123–131 (1993).
Titmus, M. J. & Faber, D. S. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Progr. Neurobiol. 35, 1–51 (1990).
Kreutzberg, G. W. in The Axon: Structure, Function and Pathophysiology (eds Waxman, J. D. K. & Stys, P. K.) 355–374 (Oxford Univ. Press, New York, (1995)).
Hall, Z. W. & Sanes, J. R. Synaptic structure and development: the neuromuscular junction. Cell 72, 99–121 (1993).
Hunter, D. D., Shah, V., Merlie, J. P. & Sanes, J. R. Alaminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature 338, 229–234 (1989).
Miner, J. H. et al. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of hetrotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell. Biol. 137, 685–701 (1997).
Sanes, J. R., Engvall, E., Butkowski, R. & Hunter, D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J. Cell Biol. 111, 1685–1699 (1990).
Noakes, P. G., Gautam, M., Mudd, J., Sanes, J. R. & Merlie, J. P. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 374, 258–262 (1995).
Hunter, D. D. et al. Primary sequence of a motor neuron-selective adhesive site in the synaptic basal lamina protein S-laminin. Cell 59, 905–913 (1989).
Porter, B. E., Weis, J. & Sanes, J. R. Amotoneuron-selective stop signal in the synaptic protein s-laminin. Neuron 14, 549–559 (1995).
Miledi, R. & Slater, C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc. R. Soc. Lond. B 169, 289–306 (1968).
Winlow, W. & Usherwood, P. N. R. Ultrastructural studies of normal and degenerating mouse neuromuscular junctions. J. Neurocytol. 4, 377–394 (1975).
Covault, J., Cunningham, J. M. & Sanes, J. R. Neurite outgrowth on cryostat sections of innervated and denervated skeletal muscle. J. Cell Biol. 105, 2479–2488 (1987).
Anton, E. S., Sandrock, A. W. J & Matthew, W. D. Merosin promotes neurite growth and Schwann cell migration in vitro and nerve regeneration in vivo: evidence using an antibody to merosin, ARM-1. Dev. Biol. 164, 133–146 (1994).
Milner, R. et al. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev. Biol. 185, 215–228 (1997).
Son, Y.-J., Trachtenberg, J. T. & Thompson, W. J. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 19, 280–285 (1996).
Pfrieger, F. W. & Barres, B. A. New views on synapse–glia interactions. Curr. Opin. Neurobiol. 6, 615–621 (1996).
Pfrieger F. W. & Barres, B. A. Synaptic efficacy enhanced by glial cells in vitro. Science 277, 1684–1688 (1997).
Bargmann, C. Making memories stick? Nature 391, 435–436 (1998).
Cheng, Y.-S., Champliaud, M.-F., Burgeson, R. E., Marenkovich, M. P. & Yurchenco, P. D. Self-assembly of laminin isoforms. J. Biol. Chem. 272, 31525–31532 (1997).
Acknowledgements
We thank J. Cunningham and S. Weng for assistance, Y.-S. Cheng and P. Yurchenco for laminins, J. Lichtman for comments, and J. Liu for participation in preliminary experiments. This work was supported by grants from the NIH and the NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patton, B., Chiu, A. & Sanes, J. Synaptic laminin prevents glial entry into the synaptic cleft. Nature 393, 698–701 (1998). https://doi.org/10.1038/31502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/31502
This article is cited by
-
HERC1 Ubiquitin Ligase Is Required for Normal Axonal Myelination in the Peripheral Nervous System
Molecular Neurobiology (2018)
-
Focal segmental glomerulosclerosis: molecular genetics and targeted therapies
BMC Nephrology (2015)
-
Neuromuscular synaptogenesis: coordinating partners with multiple functions
Nature Reviews Neuroscience (2014)
-
Marked relationship between matrix metalloproteinase 7 and brain atrophy in HIV infection
Journal of NeuroVirology (2011)
-
Basement membranes and human disease
Cell and Tissue Research (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.