Abstract
Study design:
Medical records review.
Objective:
To assess the effectiveness of the Memokath (Engineers & Doctors A/S, Denmark) thermosensitive stent as a ‘nondestructive’ means of reducing bladder outlet resistance by treating detrusor sphincter dyssynergia (DSD) of neurogenic bladder dysfunction associated with spinal cord injury.
Setting:
Spinal Injuries Unit, Sheffield, England.
Methods:
A medical records review was performed to examine our experience of Memokaths over the last 10 years. During this time, 29 patients with spinal cord injury (17 tetraplegic and 12 paraplegic) underwent stenting of the external urethral sphincter either for prevention of dysreflexic symptoms, high residual urine volumes and subsequent urinary tract infection (UTI) or for protection of the upper tracts.
Results:
A total of 33 stents were inserted into 29 men (25–77 years) with suprasacral spinal cord injury. Initial results showed that the Memokath was effective in almost all for relief of dysreflexic symptoms and elimination of DSD on pressure flow urodynamics. However, to date, 30 of the 33 stents have been removed. The overall mean working life of the Memokath was 21 months. Four stents were removed electively and 23 for complications, which included stent migration (seven) and blockage (14). Single-ended stents were more prone to migration, which was rare after 1 year (1–13 months, median 3 months, mean 5.5 months). Stent blockage by encrustation or prostatic ingrowth did not occur before 12 months (12–45 months, median 30, mean 27.9 months).
Conclusions:
In selected patients, temporary, thermo-expandable (Memokath) stents are effective in the treatment of DSD. The ‘working life’ of a Memokath stent is 21 months; however, complications do occur which may necessitate removal. Our overall experience with Memokath stents was disappointing. In future, Memokath stents will only be inserted after careful consideration in patients with prior ‘failed’ transurethral sphincterotomy or with caution in patients suitable for reconstructive surgery.
Similar content being viewed by others
Introduction
Nationally, 58% of new spinal cord injuries result in quadriplegia. Many of these patients have neurogenic bladder dysfunction with associated detrusor sphincter dyssynergia (DSD). Up to half of these patients with DSD can develop serious complications if appropriate intervention is not instituted.1 The ideal management of a hostile bladder is the reduction of the intravesical pressure to a safe level and efficient drainage with intermittent self catheterization:2 the patient is rendered ‘safe and dry’. An alternative, particularly for those with poor dexterity, is to make the patient ‘safe and wet’ by reducing the bladder outlet resistance. Transurethral sphincterotomy (TUS) has been the standard operation to reduce lower outflow tract resistance for some time. A variety of less destructive interventions have since emerged. Permanent stents (UroLume®) have been shown to be an effective alternative to sphincterotomy, although secondary bladder neck obstruction was a frequent problem3 and removal, if needed, is difficult.
The Memokath (Engineers & Doctors A/S, Denmark) is a NiTinol (nickel–titanium) alloy tightly coiled stent that is designed to prevent urothelial ingrowth. It has a thermosensitive shape memory; the stents are deployed using warmed irrigant (50°C) upon which they expand to 34Ch (028 T) or 44Ch (028SW, 028TW and 045TW), anchoring the stent in place. When cooled irrigant (approx 5–10°C) is used the coil becomes malleable allowing easy removal, which is simple and generally atraumatic for a nonencrusted stent. Initial reports of Memokath usage (in the prostatic urethra) appeared promising in the short term.4, 5, 6 However, others have found that Memokaths are not without complication and should be used with caution.7
Patients and methods
Over 9 years we inserted 33 Memokath stents into 29 men (25–77 years, median 45 years) with suprasacral spinal cord injury. In all, 17 patients were tetraplegic, 13 high (C4–6) and four low (below C6). There were 12 paraplegics, 10 with thoracic and two with lumbar lesions. The mechanisms of cord insult were trauma (26), spinal arterio-venous malformation (2) and transverse myelitis (1). Analysis was by retrospective review of medical records.
All patients had urodynamically proven DSD. About 10 patients had undergone prior conventional TUS. Six men had undergone one prior TUS; three men had two sphincterotomies and one man had three previous sphincterotomies. Previously, 25 of this group managed their bladders by condom drainage, three with ISC and one with an indwelling catheter.
All Memokath stents were acquired from the company of Engineers & Doctors (Denmark). The indications for insertion (Figure 2) were for dysreflexic symptoms (15), high residual urine volumes and subsequent recurrent or persistent urinary tract infection (UTI) (17), for protection of the upper tracts (6) or to predict outcome following TUS (1). All stents (Figure 1) were inserted under direct vision using a rigid (028T, 028TW, 045TW) or a flexible cystoscope (028SW).
Results
In all, 33 stents were inserted into 29 men; four men required stent removal and the insertion of new stents. 17 stents were single ended. Three were 028T–34Ch stents; two 028SW 44Ch stents (deployed with a flexible cystoscope) and twelve 028TW–44Ch stents (deployed with a rigid cystoscope). In total, 16 stents were double ended (045TW–44Ch). Stent length varied from 30 to 60 mm, although the majority (16) were 40 mm (Table 1 and Figure 1). The stents were positioned with the proximal end at the level of the veru montanum and the distal end beyond the external urethral sphincter mechanism, thereby preventing sphincteric closure during DSD without causing dribbling incontinence by traversing the bladder neck (see Figure 2).
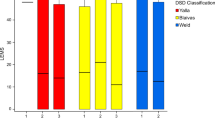
The interval from injury to stent insertion varied from six months to 32 years (median 10.5 years, mean 12.8 years). All patients had pressure flow urodynamic (UDS) evidence of DSD prior to stent insertion. However, repeat UDS was not always possible with the stent in situ (Figure 3). At follow-up, no UDS were performed in seven men as the upper tracts had improved on ultrasound and symptomatic dysreflexia had resolved. We were unable to pass the catheters in five patients. In all, 15 patients showed improvement in their UDS. Six showed evidence of persistent DSD proximal or distal to the stent.
A total of 21 patients have been seen in the last year and all are under regular review. Minimum follow-up time was 14 months. Three patients have died of nonurological disorders, one of whom died with his stent in situ. One patient has been discharged from our unit with his stent in situ; he was transferred to another unit after domestic relocation.
To date, 30 of the 33 stents have been removed; the reasons for this are summarised in Table 2. The age of the stent at removal or at time of this review ranged from 0 to 47 months (median 22 months, mean 21 months). Four stents were removed electively; three prior to cystoplasty and one for fertility reasons, to enable an antegrade ejaculate. The man whose stent was removed for fertility subsequently underwent TUS and is doing well on condom drainage.
In all, 23 stents were removed for complications. Initial complications were often minor and included persistent haematuria (3), UTI (4), acute retention (2) and migration (3). Complications that did not present until later often contributed to removal and included significant stent migration (7), encrustation or blockage (14) and severe or complicated UTI (6).
All seven of the stents that migrated were single-ended stents (028). Stents that migrated early presented with retention of urine and a recurrence of dysreflexic symptoms. One of these patients completely extruded his stent per urethra. All stents that did migrate did so before 13 months (1–13 months, median 3, mean 5.5 months). One stent was dislodged with an ISC catheter, of the remainder, three stents migrated proximally and three distally.
Stent blockage by encrustation (Figure 4) or prostatic ingrowth was also a problem and was the reason for removal in 14 patients. It did not, however, occur before 12 months (12–45 months, median 30, mean 27.9 months).
In cases of sepsis attributed to the stent, stone formation was always present and was often the main reason for removal. In two, a re-emergence of symptoms of autonomic dysreflexia warranted alternative treatment. The final outcome of the stent episodes is summarised in Table 2 and the final outcome of the bladder management of our cohort of patients is summarised in Table 3. Those patients in whom the Memokath failed and are now on condom drainage either underwent transurethral sphincterotomy to reduce bladder outlet resistance or remain under close review.
During this review, three patients died of pneumonia (unrelated to their stent). Two had high cervical injuries (C4 and C5) and one low thoracic injury (T12). Their bladder management had been one ileal diversion; one underwent a second TUS after removal of his Memokath, and one died with his functioning stent in situ.
Discussion
The commonest spinal cord injury, the suprasacral cord injury, causes spastic paraplegia or tetraplegia with reflex bladder activity. In almost all, the final result is a mal-coordinated void with DSD. If appropriate measures are not taken, complications may ensue,1 which may become life threatening (ie, hydronephrosis, vesico-ureteric reflux and urosepsis leading to renal damage).
The ideal management of a hostile bladder is the reduction of the intravesical pressure to a safe level and efficient drainage with ISC,2 the patient is rendered ‘safe and dry’. An alternative is to make the patient ‘safe and wet’ by reducing the bladder outlet resistance either by TUS, stent, or botulinum toxin A.8, 9 External spincterotomy has been the treatment of choice for over 30 years.10
However, potential complications of erectile dysfunctions (2.8–64%), haemorrhage (5–23%), technical failure and re-operation rate (12–26%) have led to alternatives.11 Perkash and Rivas have used neodymium:YAG laser for division of the external sphincter with apparent reduction in haemorrhagic complications.12, 13 The more minimally invasive balloon dilatation has been investigated and found to have only short-lived effects.14 Centrally acting pharmacological therapy is ineffective for DSD15 and agents such as dantrolene and baclofen not only have little effect but also potentially serious side effects (eg, fatal hepatotoxicity).16, 17
The use of endoluminal permanent wire mesh stents (UroLume®) was first reported by Milroy et al,18 in the treatment of bulbar urethral strictures. A 12-year review of wall stents for DSD in 12 patients by Hamid et al,19 found them to be an effective alternative to sphincterotomy; however, in some, subsequent bladder neck dyssynergia emerged requiring further intervention. It has been suggested that it is possible to electro-ejaculate a patient with a distally placed stent that does not obstruct the ejaculatory ducts; however, more recently this approach has fallen out of favour.6, 20 Although a feasible option in selected patients, wall stents are difficult to remove, running the risk of urethral damage, and therefore they are not an ideal temporary measure.
A ‘second-generation’ thermo-expandable ‘temporary’ or removable stent (Memokath) emerged5 providing an option for those patients in whom an irreversible destructive intervention (ie, external urethral sphincterotomy) is not desired. This especially applies to patients who have yet to decide their preferred clinical course or in those in whom fertility is an issue, where the stent can be readily removed. The Memokath is deployed in heated irrigant and easily removed by cooling with minimal trauma to the urethra so long as the stent is nonencrusted with stone.
Initial reports have varied in their appraisal of use in the treatment of DSD. Soni et al6 reported on the short-term follow-up of 10 patients and observed good bladder emptying with minimal complication. Low and McRae,7 however, describes a cautionary experience of 26 stents inserted into 24 patients. Although initial results appeared promising, almost all the stents required removal for complications of urinary infection, migration and encrustation. Initial experience by Shah et al21 at 2 years identified the versatility of stent insertion and removal as well as its effectiveness at reducing intravesical pressures. However, migration was a problem and this was more apparent with smaller stents. Their long-term experience at 7 years22 identified that migration (20.4 months) and encrustation (17.6 months) were persistent problems and 19 of the inserted 25 stents had required removal. The mean time to removal of stent for complication was 20.3 months, which concurs with our findings. However, migration occurred early and encrustation later in our series.
All our spinal cord injured patients are followed up on an annual basis with clinical review and upper urinary tract ultrasound. In our Memokath population UDS investigation was not always possible post-stent insertion. In 12 men no UDS was performed as the upper tracts had improved and dysreflexia resolved. Post-stent insertion urodynamics were performed on 21 of the 33 stents. In five men we were unable to negotiate the catheters past the stent and the procedure was abandoned. For this group, we recommend regular symptomatic review and upper tract surveillance with ultrasound. In the remaining 16 men, 15 showed an improvement and one showed no change in bladder emptying. Six of these men had persistent ‘DSD’ either proximal or distal to the stent; it was, however, insufficient to cause problems with high residuals or autonomic dysreflexia.
In our series of 33 stents, migration was a significant problem necessitating removal in seven stents. Migration was often early with nearly all occurring within 1 year (1–13, mean 5.5, median 3 months). It can be hypothesised that early migration was as a result of technical failure in accurate deployment. Alternatively, it has been suggested that delayed proximal migration could be as a result of minor but significant direct perineal trauma during transfer (from bed to wheelchair, etc). In this spinal cord-injured population, the stents we inserted were for the treatment of DSD, and consequently the stents lie distal to the prostatic fossa, where they may be prone to external trauma. It is not surprising that migration of this scale is rarely seen in the nonspinal cord-injured patient, whose stent was inserted for prostatic obstruction. It has also been suggested that manual evacuation of hard faecal matter might contribute to migration in the spinal cord injured. If the stent is still indicated after migration has been noted, the Memokath can be repositioned.6, 12 In our series only single-ended stents were prone to migrate. This may be because the addition of the proximal funnel on a double-ended model anchors the stent more securely. Low and McRae7 found that a proximal funnel alone (044 model) had a greater tendency to migrate; the double-ended stent does appear to overcome this problem. In all, 10 of the 29 patients had a previous TUS. Four of the seven migrated stents were in patients with prior sphincterotomy.
Stent blockage occurred in 14, resulting either from the ingrowth of granulation tissue at the ends of the stent, or due to stent encrustation with calculus formation. Encrustation appears to be more of a problem in the spinal cord-injured patient.4 This may be because most have infected urine and high residuals, which would ordinarily predispose to calculus formation. As expected, stent blockage occurred ‘late’, not seen before 1 year, with a mean time of 27.9 months (median 30 months). In the population stented for benign bladder outflow obstruction, encrustation was rare. This was attributed by the authors to particular characteristics of the Memokath alloy or the gentle massaging action of subtle movement within the prostatic urethra.4 All patients in our series with stent calculus formation had a preceding history of UTI; this may well be a causal relationship.
Persistent and recurrent UTI, where the stent was thought to be the focus, contributed to the removal of six stents. Four cases presented with suprapubic pain and pyrexia, two of which had significant perigenital cellulitis and associated orchitis; one other presented with local pain and another with discharge per urethra. On stent removal, symptoms resolved in all patients. Urine cultures were all positive. However, it is worthy of note that intermittent urine cultures performed on this group are invariably positive. Therefore, although the higher rate of positive urine cultures may not be clinically relevant in the majority,23 in those with persistent or recurrent symptomatic UTI, one should consider stent explantation. We, as have others, would also advocate prophylactic antibiotics before stent implantation.7
The technique of ‘permanent’ stent removal with minimal trauma is described by Gajewski et al.24 Our experience and that of others have found that removal of UroLume® stents is fraught with difficulty and can result in significant urethral injury and consequent stricture formation.3, 7, 19 In contrast, the Memokath stent, is readily removable with minimal trauma. However, in situations where the stent is encrusted, urethral damage may occur during explantation. In our series, a young T5 paraplegic sustained significant urethral scarring following removal of stent for ingrowth of granulation tissue. The result was a dense urethral stricture, which will complicate future management.
In our experience Memokath stents do have a place in the management of the spinal cord-injured patient. If stents do require removal, then the options available are to simply observe and monitor the patient, to perform a transurethral sphincterotomy, a permanent indwelling catheter or stent replacement. The choice of the above would depend upon the clinical situation. The final issue is one of cost. Each Memokath costs approximately £600. This must be weighed against the time spent in hospital following TUS and the ensuing complications. It also gives the patient an option of a temporary measure, which should not interfere with subsequent management.
Conclusion
We conclude that in selected patients, temporary, thermo-expandable (Memokath) stents are effective for the treatment of DSD in the setting of a hostile bladder. This particularly applies to patients in whom final definitive management has yet to be decided, in those in whom fertility is an issue or following a previous TUS. Post-stent urodynamic studies are not always possible for technical reasons. We found that the working life of a Memokath stent was 21 months. Complications do occur and may necessitate removal of the stent. Our overall experience with Memokath stents was disappointing and presently we are not inserting any new stents. In patients with tetraplegia, or those with poor dexterity unable to perform ISC, we recommend a transurethral sphincterotomy. In future, Memokath stents will only be inserted after careful consideration in patients with prior ‘failed’ sphincterotomy or, with caution, in patients suitable for reconstructive surgery.
References
Alander DH, Parker J, Stauffer ES . Intermediate-term outcome of cervical spinal cord-injured patients older than 50 years of age. Spine 1997; 22: 1189–1192.
Shaw PJR, Milroy EJG, Timoney AG, El Din A, Mitchell N . Permanent external striated sphincter stents in patients with spinal injuries. BJU Int 1990; 66: 297–302.
McFarlane JP et al. Long term outcome of permanent urethral stents in the treatment of DSD. BJU Int 1996; 78: 729–732.
Perry MJA, Roodhouse AJ, Gidlow AB, Spicer TG, Ellis BW . Thermo-expandable intraprostatic stents in bladder outlet obstruction: an 8 year study. BJU Int 2002; 90: 216–223.
Poulsen AL, Schou J, Ovesen H, Nordling J . Memokath: a second generation of intraprostatic Spirals. BJU Int 1993; 72: 331–334.
Soni BM, Vaidyanatham S, Krishnan KR . Use of Memokath, a second generation urethral stent for the relief of urinary retention in male spinal cord injured patients. Paraplegia 1994; 32: 480–488.
Low Al, McRae PJ . Use of Memokath for DSD after spinal cord injury – a cautionary tale. Spinal Cord 1998; 36: 39–44.
Juma S, Niku SD, Brodak PP, Joseph AC . Urolume urethral wallstent in the treatment of DSD. Paraplegia 1994; 32: 616–621.
Dykstra DD, Sidi AA . Treatment of DSD with Botulinum A toxin: a double blind study. Arch Phys Med Rehab 1990; 71: 24.
Schellhammer PF, Hackler RH, Bunts RC . External sphinctertomy: an evaluation of 150 patients with neurogenic bladder. J Urol 1973; 110: 199–202.
Lockhart JL . Indications and problems with external urethral sphincterotomy. Probl Urology 1989; 3: 44–50.
Perkash I . Laser sphincterotomy and ablation of the prostate using a sapphire chisel contact tip firing Nd. YAG laser. J Urol 1994; 52: 2020.
Rivas DA, Chancellor BM, Staas Jr EW, Gomella GL . Contact Nd-Yt-AI-garnet laser ablation of the external sphincter in spinal cord injured men with DSD. Urology 1995; 45: 1028–1031.
Chancellor MB, Hirsch IH, Kiilholma P, Staas WE . Technique of external sphincter balloon dilatation. Urology 1992; 40: 308.
Bianchine J . Drugs for Parkinsons disease: centrally acting muscle relaxants. In: Gilman AG, Goodman LS, Gilman A (eds). The Pharmacological Basis of Therapeutics. New York: MacMillan Publishig Co., Inc. 1980, pp 475–495.
Hacken HJ, Krucker V . Clinical Laboratory assessment of the efficacy of baclofen on urethral sphincter spasticity in patients with traumatic paraplegia. Urology 1977; 3: 237.
Hackler RH, Braecker BH, Klein FA, Brady SM . A clinical experience with dantrolene sodium for external urinary sphincter hypertonidty in spinal cord injured patients. J Urol 1980; 124: 78.
Milroy EJ, Chapple CR, Cooper JE . A new treatment of urethral strictures. Lancet 1988; 1: 1424–1427.
Hamid R et al. The mesh wallstent in the treatment of DSD in men with spinal cord injury: a 12 year follow up. BJU Int 2003; 91: 51–53.
Chancellor MB et al. Placement of a wire mesh prosthesis in the external sphincter of men with spinal cord injuries. Radiology 1993; 187: 551–555.
Shah NC, Foley SJ, Edham I, Shah PJR . Use of Memokath temporary urethral stent in treatment of detrusor-sphincter dyssynergia. J Endourol 1997; 11: 485–488.
Hamid R, Arya M, Wood S, Patel HRH, Shah PJR . The use of the Memokath™ stent in the treatment of detrusor sphincter dyssynergia in spinal cord injury patients: a single centre seven year experience. Eur Urol 2003; 43: 539–543.
Gilmore DS, Schick DS, Young MN, Montgomerie JZ . Effect of external urinary collection system on colonisation & UTI with pseudomonas and klebsiella in men with spinal cord injury. J Am Paraplegic Soc 1992; 15: 155.
Gajewski JB et al. Removal of UroLume endoprosthesis; experience of the North American Study Group for DSD application. J Urol 2000; 163: 773–776.
Acknowledgements
We acknowledge Mr DG Thomas for his contribution to this work.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehta, S., Tophill, P. Memokath® stents for the treatment of detrusor sphincter dyssynergia (DSD) in men with spinal cord injury: The Princess Royal Spinal Injuries Unit 10-year experience. Spinal Cord 44, 1–6 (2006). https://doi.org/10.1038/sj.sc.3101800
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101800
Keywords
This article is cited by
-
Contemporary Treatment of Detrusor Sphincter Dyssynergia: a Systematic Review
Current Bladder Dysfunction Reports (2018)
-
Outcome after treatment of detrusor–sphincter dyssynergia by temporary stent
Spinal Cord (2008)
-
Long-term follow-up study of intraurethral stents in spinal cord injured patients with detrusor-sphincter dyssynergia
Spinal Cord (2007)
-
Management of detrusor–external sphincter dyssynergia
Nature Clinical Practice Urology (2006)