Abstract
Study design:
Experimental laboratory investigations with paraplegic mouse models.
Objectives:
To review the most recent advances in the field of spinal cord injury research; immune system response, regeneration, and functional recovery.
Settings:
Laval University and Laval University Medical Center, Quebec, Canada.
Methods:
Assessment of regenerative processes and locomotor function recovery induced by a variety of treatments and approaches in wild-type and genetically engineered mice with complete or incomplete lesions of the spinal cord.
Results:
Recent studies have reported a number of significant observations providing additional insight into the role and mechanism of regeneration, immune system response, and functional recovery after spinal cord injury (SCI) using incomplete paraplegic mice with Nogo-A, NgR, EphA4, GFAP/vimentime, LIF, or Fas gene knock-out. A novel antibody called CXCL10 was also recently found to increase tissue sparing and angiogenesis after SCI. In an attempt to explore the possibilities of reactivating spared neurons below the injury level, researchers have found that pharmacological activation of specific subtypes of serotonin receptors (eg, 5-HT1A/2A/7) can sustain the production of basic locomotor-like movements in complete paraplegic mice.
Conclusion:
The growing availability of genetically engineered and mutant mouse strains along with molecular biology tools has led scientists to increasingly use murine models in SCI research. These new tools and models may assist scientists in understanding further the complex pathological consequences of SCI.
Similar content being viewed by others
Main
Currently, more than 500 000 individuals are living with a spinal cord injury (SCI) in North America and Europe. Despite administration of methylprednisolone (ie, an anti-inflammatory drug) or surgeries aimed at decompressing the spinal cord, damage caused by SCI (eg, car accidents, sports, and falls) are devastating and irreversible. There is no significant regenerative processes taking place naturally (ie, without intervention) in the spinal cord and no treatment has allowed the recovery of normal functions and reconnections with the brain after SCI.
Generally, the main consequences of SCI are an immediate and irreversible loss of both sensory function and voluntary motor control below the level of injury. For example, a paraplegic patient severely injured at the low-thoracic vertebral level will be incapable of feeling his body and contracting his muscles from below the waist. A tetraplegic patient (ie, high cervical injury) will have both his upper and lower body paralyzed.
In the last 30 years, animal research has provided scientists valuable information concerning the detailed cellular mechanisms and consequences of SCI (for recent reviews, see McKerracher and David1, Kwon et al2 and Park3). However, despite numerous studies that have been carried out on SCI-animals (eg, rats, cats, and monkeys), critical details of these mechanisms are still missing. More recently, the growing availability of transgenic and mutant mouse strains along with molecular biological tools has led scientists to increasingly use murine models in SCI research. The present article reports recent original research performed in paraplegic mice that may be considered as significant.
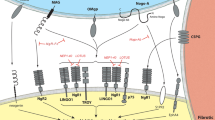
One of the mechanisms underlying the lack of spinal cord regeneration and sensory/motor function recovery following SCI concerns myelin (oligodendrocytes). Myelin is known mostly for its role in insulating axons and facilitating propagation of action potentials. A number of proteins associated with myelin are, in fact, responsible for partially inhibiting regeneration and regrowth post-SCI. Three of these proteins, the myelin-associated glycoprotein (MAG), the oligodendrocyte-myelin glycoprotein (OMgp) and NOGO have been identified in the last few years. At the injured axon level, they activate the receptor complex, NgR-p75NTR, and subsequently, the Rho signalling pathway.1 Insight into the relative importance of these proteins have been provided recently using mutant mice lacking Nogo-A4 and NgR,5 which displayed significant axonal regrowth following SCI. These studies provide clear evidence for the critical role of Nogo-A protein and NgR receptor (also called Nogo-66 receptor) in preventing, at least partially, cut axons from growing and re-establishing connections with neurons across the lesion site.
Formation of a glial scar at the injury site represents another mechanism associated with the lack of regeneration of spinal cord neurons. In turn, this scar creates a ‘physical’ barrier to regrowth of axons across the lesion site. Recent studies using paraplegic mouse models have revealed that proteins called EphA4, GFAP and vimentime may play a determinant role by aiding in the generation of glial scars. Indeed, significant axonal regrowth was observed in the absence of these endogenous molecules in EphA4 −/− and double-mutant GFAP/vimentine −/− paraplegic mice.6, 7 Therefore, it is believed that these proteins may constitute new genetic and molecular targets for spinal cord repair.
Other recently discovered factors may well lead to development of innovative therapies for SCI. These factors are associated with the complex inflammatory response triggered by injury. The latter involves secondary cell death and apoptosis which increase cavity/scar formation and, hence, the size of the barrier that must be overcome by potential regrowing axons. A novel antibody against a chemo-attractant molecule called CXCL10 was found recently to reduce the inflammatory response and to increase tissue sparing and angiogenesis in incomplete paraplegic mice.8 A protein called leukemia inhibitory factor (LIF) was found also to control the immune system reaction since LIF knockout mice display a reduced microglial/macrophage response to SCI.9 Finally, a study performed also in 2004 using Fas −/− mutant mice has established that secondary cell death following SCI involves the clear participation of the Fas/Fas ligand signal transduction system.10
These recent advances, made possible by the generation of new paraplegic mouse models, have undoubtedly contributed to increasing our understanding of the pathophysiology and direct consequences of SCI (ie, paralysis and lack of sensory functions).
In addition to these direct consequences, there are also a number of equally serious clinical concerns occurring within a few weeks to a few months after SCI. These are mostly associated with patient immobilization and lack of physical activity. Generally, these lead to overall deterioration of the body, impairment of organ function and, in some cases death. The long lists of indirect consequences include: cardiovascular problems, obesity, immune system deficiency, infections (skin, kidneys, and lungs), hormonal deregulation, osteoporosis, muscular atrophy, chronic pain, and spasticity. Increasing evidence from the literature suggests, however, that muscular contractions and intense locomotor activity-based therapy may prevent, and even partially reverse, some of the clinical concerns of SCI patients.
To examine this possibility, a series of experiments using paraplegic mice has been undertaken recently to assess the ‘therapeutic’ potential of remaining and intact neurons located below the level of injury. These experiments have focused more particularly on neurons of the Central Pattern Generator (CPG) localized in the lumbar segments of the spinal cord. It appears that these CPG neurons can be activated potently with specific pharmacological agents. Indeed, administration of agonists selectively binding serotonergic receptors 2A/2C (5-HTR2A/2C) was found to acutely induce locomotor-like movements in hindlimbs of adult mice completely transected at the low-thoracic level.11 This effect was shown to be blocked by selective 5-HT2 or N-methyl-D-aspartate receptor antagonists suggesting a combined role for these subtypes of serotonergic and glutamatergic receptors in CPG activation. Using other 5-HT receptor agonists, additional experiments have provided evidence of a more specific role for the 5-HTR2A rather than for the 5-HTR2C receptors in spinal stepping generation.12 Moreover, the combined use of 5-HT1A/7 receptor ligands with 5-HTR7−/− mutant mice has revealed a highly specific role for these two additional receptor subtypes in locomotor rhythmogenesis after SCI.13 This new line of research has revealed the existence of a close-relationship among specific subtypes of 5-HT receptors in the spinal cord (ie 5-HT1A, 5-HT2A, and 5-HT7) and the CPG network. 5-HT agonists selective for these receptor subtypes were shown to potently activate CPG neurons, and thus, induce basic stepping movements on a treadmill in complete paraplegic animals. Several studies are currently examining the long-term benefits of chronic treatments with these agonists for preventing or reversing the health concerns associated with SCI.14, 15
In brief, the use of wild-type and mutant paraplegic mouse models may assist scientists in understanding further the complex pathology of SCI. Recent development of less invasive approaches for surgery on mice (ie, with no laminectomy)11, 12, 13, 14, 15, 16 and of functional assessment methods adapted for small-size animals17, 18 will undoubtedly further facilitate the use of murine models in SCI research. This relatively new direction in SCI research will hopefully increase the number of significant findings in supporting the development of more effective therapies for SCI patients.
References
McKerracher L, David S . Easing the brakes on spinal cord repair. Nat Med 2004; 10: 1052–1053.
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR . Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004; 4: 451–464.
Park E . The role of excitatoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 2004; 21: 754–774.
Simonen M et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 2003; 38: 201–211.
Kim JE, Liu BP, Park JH, Strittmatter SM . Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004; 44: 439–451.
Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM . Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neuroscience. 2004; 24: 10064–10073.
Menet V, Prieto M, Privat A, Gimenez Y Ribotta M . Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA 2003; 100: 8999–9004.
Glaser J, Gonzalez R, Perreau VM, Cotman CW, Keirstead HS . Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J Neurosci Res 2004; 77: 701–708.
Kerr BJ, Patterson PH . Potent pro-inflammatory actions of leukemia inhibitory factor in the spinal cord of the adult mouse. Exp Neurol 2004; 188: 391–407.
Yoshino O et al. The role of Fas-mediated apoptosis after traumatic spinal cord injury. Spine 2004; 29: 1394–1404.
Guertin PA . Role of NMDA receptor activation in serotonin agonist-induced air-stepping in paraplegic mice. Spinal Cord 2004; 42: 185–190.
Landry ES, Guertin PA . Differential effects of 5-HT1 and 5-HT2 receptor agonists on hindlimb movements in paraplegic mice. Prog Neuro-Psychopharmacol Biol Psychiatr 2004; 28: 1053–1060.
Guertin PA, Rouillard C, Lévesque D, Sawchuk M, Hochman S, Landry E . Evidence of a specific role for 5-HT2A and 5-HT7 receptors in hindlimb stepping generation in paraplegic mice. Soc Neurosci 34th Ann Meeting 2004; October 23–27, San Diego, CA, USA.
Landry E, Frenette J, Guertin PA . Body weight, limb size, and muscular properties of early paraplegic mice. J Neurotrauma 2004; 21: 1008–1016.
Picard S, Brown J, Guertin PA . Caractérisation de la perte osseuse chez la souris adulte paraplégique: Étude histomorphométrique et biomécanique. Ann Pathol 2005; 25 (in press).
Sheng H et al. A no-laminectomy spinal cord compression injury model in mice. J Neurotrauma 2004; 21: 595–603.
Guertin PA . Semi-quantitative assessment of hindlimb movement recovery without intervention in adult paraplegic mice. Spinal Cord 2004; Epub ahead of print: Nov 30.
Anderson KD, Abdul M, Steward O . Quantitative assessment of deficits and recovery of forelimb motor function after cervical spinal cord injury in mice. Exp Neurol 2004; 190: 184–191.
Acknowledgements
We thank Drs Paul Khan and Marie-Claude Perreault for corrections. To the memory of Mr Christopher Reeve who passed away on October 10th, 2004.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guertin, P. Paraplegic mice are leading to new advances in spinal cord injury research. Spinal Cord 43, 459–461 (2005). https://doi.org/10.1038/sj.sc.3101754
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101754