Abstract
Helicobacter pylori infection, a worldwide health issue, is typically treated with standard antibiotic therapies. However, these treatments often face resistance and non-compliance due to side effects. In this umbrella review, we aimed to comprehensively assess the impact of probiotics supplementation in different preparations on Helicobacter pylori standard treatment. We searched PubMed, Embase and Cochrane Central Register of Controlled Trials in the Cochrane Library from inception to June 1, 2023, to identify systematic reviews with meta-analyses that focused on eradication rates, total side effects and other outcomes of interest. The most comprehensive meta-analysis was selected for data extraction. AMSTAR 2 was used to assess quality of meta-analyses. Overall, 28 unique meta-analyses based on 534 RCTs were included. The results suggests that probiotics supplementation with pooled probiotic strains was significantly associated with improved eradication rates (RR 1.10, 95% CI 1.06–1.14) and reduced risk of total side effects (RR 0.54, 95% CI 0.42–0.70) compared with standard therapy alone. Single-strained or multi-strained preparation of probiotics supplementation showed similar results. Despite Bifidobacterium spp. showing the highest potential for eradication, the study quality was critically low for most meta-analyses, necessitating further high-quality research to explore the optimal probiotic strains or their combinations for Helicobacter pylori treatment.aq_start?>Kindly check and confirm the edit made in article title.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori), a Gram-negative and transmissible bacterium, infects the epithelial lining of the stomach in approximately half of the global population, which equates to an astonishing estimate of roughly 4.4 billion individuals, consequently imposing a significant medical and social burden1. The infection predominantly triggers a lifelong, chronically progressive gastric inflammation, potentially leading to a variety of diseases such as peptic ulcer, gastric atrophy, gastric intestinal metaplasia and, in more severe instances, gastric cancer or mucosa-associated lymphoid tissue lymphoma2. Following international consensus established since 2017, it is advised that H. pylori be eradicated promptly upon diagnosis3. Currently, the most widely recommended standard therapy involves either triple or quadruple therapeutic regimens given for 7–14 days, including a proton-pump inhibitor (PPI) and two distinct antibiotics such as clarithromycin combined with either amoxicillin or metronidazole, optionally complemented by bismuth salts3,4. However, the past two decades have witnessed a concerning global increase in antibiotic resistance of H. pylori, which has coincided with a continuous decline in the success rates of eradication therapies5,6. Moreover, common side effects of antibiotics such as diarrhea, nausea, and vomiting contribute to patient non-compliance, thereby resulting in less satisfactory eradication rates when using standard therapy7.
Probiotics, defined as live microorganisms, serve a vital function in health maintenance, particularly within the digestive system, when consumed in adequate amounts8. Numerous benefits that probiotics confer have been unveiled by researchers, including immunomodulation, protection against pathogens, and improved barrier function9. Given these properties, probiotics have garnered significant interest for their potential application in the management of H. pylori infections. Commonly used probiotics in medical treatments encompass genera like Lactobacillus, Bifidobacterium, Saccharomyces, along with various combined formulations. Evidence have found that probiotics could inhibit H. pylori colonization from both in vivo and in vitro studies10,11. Several meta-analyses have further investigated the potential of probiotics to enhance H. pylori eradication rates and reduce side effects when used in conjunction with standard treatment12,13,14. However, these findings have not gone without skepticism15, impeding the integration of probiotics into the routine clinical management of H. pylori treatment. Furthermore, the effectiveness of various probiotic strains, or their combined preparation, may vary within standard H. pylori treatment. It is crucial to understand that the efficacy of probiotics is cardinally strain-specific16, not genus-specific, which underscores the necessity for precise identification and utilization of effective strains in clinical applications.
Therefore, in this article, to systematically assess the impact of probiotic supplementation on H. pylori treatment, an umbrella review was conducted by scrutinizing meta-analyses limited to randomized controlled trials (RCTs). We aimed to tackle the state of the art in this scientific arena, providing a comprehensive and up-to-date perspective.
Methods
This umbrella review was conducted in accordance with the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement17. The study protocol was prospectively registered on PROSPERO (CRD42023444917) (https://www.crd.york.ac.uk/PROSPERO/).
Search strategy
The search strategy was crafted by a medical information specialist (H.C.) in collaboration with the review team. The literature search was conducted independently by two researchers (Z.Y. and Y.Z.) in the following electronic databases from inception to June 1, 2023, with the keywords including “helicobacter pylori”, “probiotics” for all relevant systematic reviews with meta-analyses: PubMed, Embase, Cochrane Central Register of Controlled Trials in the Cochrane Library. The search words and/or Medical Subject Heading (MeSH) terms are listed in Table S1. Additional searches regarding this topic were conducted on Google Scholar and Web of Science. There was no language restriction. The titles and abstracts retrieved from the database underwent meticulous screened, with meta-analyses that met the inclusion criteria being identified by full text reading. In the event of any discrepancy in the literature selection process between the two reviewers, a third author (K.H.) intervened for resolution. Besides, the reference lists of all included articles were manually searched to identify any additional eligible studies that might otherwise have been overlooked.
Eligibility criteria
We aimed to amalgamate data from systematic reviews and meta-analyses to provide a more expansive perspective on the impact of probiotic supplementation on H. pylori eradication therapy. Therefore, we selected H. pylori eradication rates, total side effects, specific gastrointestinal (GI) side effects, and other nonspecific side effects as outcomes of interest. Hereinto, specific GI side effects range from diarrhea, nausea, vomiting, nausea/vomiting, loss of appetite, constipation, abdominal distension, bloating, epigastric pain, abdominal pain, taste disturbance, metallic taste, to flatus, while other nonspecific side effects include skin rash, stomatitis, dizziness, palpitation, and blurred vision.
Studies fulfilling the following criteria were eligible in our umbrella review: (a) studies should be systematic reviews with meta-analyses of RCTs published in any language; (b) study populations should have undergone systematic and standardized eradication treatment including triple therapy, bismuth-containing quadruple therapy, or sequential therapy for H. pylori infection, irrespective of the number of times of infection and age; (c) patients in the control group should have received standard therapy with or without a placebo, while patients in the experimental group should have received standard therapy with probiotics; (d) availability of relative information on successful H. pylori eradication rates. Exclusion criteria were as follows: (a) irrelevant studies, duplicate publications, animal studies, systematic reviews without meta-analyses, comments, letters, case reports, conference abstracts, or editorials; (b) study populations were restricted to children.
When only a single meta-analysis investigated the outcome of interest, it was directly chosen for result presentation and discussion. However, when several meta-analyses probed the same outcome of interest, the most comprehensive study bearing the most complete outcome data was selected. This strategy, employed to prevent data duplication, reinforce the rigor of the assessment, and streamline the interpretation of results, has been used in other published umbrella reviews when overlapping meta-analyses exist18,19,20,21. Selection was primarily determined by the following key factors: (a) the methodological quality as gauged by the AMSTAR 2 assessment; (b) the date of publication; (c) the number of included RCT studies and the number of patients. Generally, when multiple meta-analyses investigated the same outcome of interest, we selected the one with the highest AMSTAR 2 assessment as a first priority for data extraction. If multiple meta-analyses with the identical AMSTAR 2 assessment investigating the same outcome of interest were published more than three years apart, we selected the most recent one for data extraction, which typically features the largest sample size. If multiple meta-analyses were conducted within the same three-year span, we prioritized the meta-analysis that incorporated the greatest number of RCT studies or patients. By using aforementioned filtering strategies, two reviewers (Z.Y. and Z.H.) independently selected the most comprehensive study for each outcome of interest. In all cases, rationale for selection was recorded. For conflicting evaluations, the third reviewer (H.C.) was consulted to solve the dispute and a final decision was made by the majority of the votes.
Data extraction
Two reviewers (Z.Y. and Y.Z.) independently screened all records and resolved conflicts by consensus. Relevant data were extracted from included articles by one reviewer (Z.Y.) and peer reviewed by a second reviewer (Y.Z.). Extracted details included study characteristics (e.g., title, publication year, number of RCT studies, number of patients), estimated summary effects (odds ratio or risk ratio with 95% confidence intervals) in the intention-to-treat (ITT) analysis, study quality assessments and study limitations. Furthermore, the model of effect (random or fixed), heterogeneity (I2 statistic and Cochran’s Q test P value), and publication bias assessment (P value of Egger’s test or funnel plot) for each outcome of interest were extracted.
Quality assessment
Two independent reviewers (Z.Y. and Y.Z.) conducted quality assessment of all included studies, and any discrepancies were solved via discussion to reach the consensus. First, the reviewers evaluated the methodological quality of the included meta-analyses by using The Measurement Tool to Assess systematic Reviews (AMSTAR) 2, a critical appraisal tool in assessing the quality of systematic reviews and meta-analyses22. The AMSTAR 2 assessment contains 16 items and generates an overall quality rating based on weaknesses in critical domains. The quality was determined to be “high,” “moderate,” “low,” or “critically low.” Additionally, the reviewers categorized the evidence of outcomes into five classes, guided by the evidence classification criteria: Class I (convincing evidence), Class II (highly suggestive evidence), Class III (suggestive evidence), Class IV (weak evidence), and NS (non-significant)23.
Patient and public involvement
Patients and the public were not involved in the planning, design, and implementation of the study, as this study used secondary data. No patients were asked to advise on interpretation or writing up of the manuscript.
Results
Literature search results and study selection process
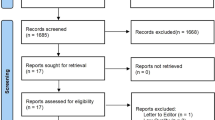
Our systematic literature search yielded a total of 191 distinct articles. Subsequently, we discerned 28 systematic reviews with meta-analyses of RCTs that satisfied the eligibility criteria12,13,14,15,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47. Details of the selection protocol are presented as a diagram in Fig. 1. Agreement between the two reviewers (Z.Y. and Y.Z.) for study selection was almost perfect (κ = 0.88, 95% confidence interval 0.67–1.08; P < 0.001).
Preferred reporting items for systematic reviews and meta-analyses flow chart for study inclusion. The initial search retrieved 191 records (identification), among which 58 were removed as duplicates. After the screening of title and abstract, 133 records were chosen for full-article reading (screening). Finally, 28 meta-analyses met the inclusion criteria of the umbrella review.
Our preliminary review on the impact of probiotic supplementation alongside standard H. pylori treatment reveals that single-strained probiotics, particularly those containing Lactobacillus spp., Bifidobacterium spp., and Saccharomyces spp. are more commonly used. These probiotics have shown promise in increasing the success rate of H. pylori eradication and in mitigating side effects. While recent research points to varying levels of effectiveness among probiotics47,48, consensus is still lacking regarding the optimal probiotic choice for H. pylori eradication. Therefore, in this umbrella review, we examine the effectiveness of probiotics in different preparations that might lead to distinct outcomes of interest.
Among the included meta-analyses, 20 unique outcomes of interest classified into the following four categories were extracted: eradication rates (marked in orange), total side effects (marked in orange), specific GI side effects (marked in pink), and other nonspecific side effects (marked in green), as shown in Figs. 2, 3, 4, 5 and 6. We scrutinized every single outcome of interest from single-strained preparation (Lactobacillus spp., Bifidobacterium spp., Saccharomyces spp.), combined preparation (multi-strained), and the pooled probiotic strains. Most of the included meta-analyses reported on eradication rates and total side effects, but fewer studies have concurrently analyzed these outcomes for either single-strained or multi-strained preparations. Besides, sample sizes varied widely across the included articles, from dozens to thousands. Table S2 summaries details of the included studies. After quality assessment of evidence through AMSTAR 2, only one study was assessed “moderate” quality35, and three studies were assessed “low” quality38,39,44, with the remaining classified as “critically low” quality. Table S3 delineates the comprehensive details of the AMSTAR 2 assessment for each meta-analysis included in our study. Our findings indicate a prevalent issue with the declaration of methods prior to the conduct of the review. Out of 28 meta-analyses assessed, a significant majority (24 meta-analyses) failed to affirmatively state their methods before the review launched. Only 4 meta-analyses complied with this criterion35,38,39,44. This lack of upfront methodological transparency raises concerns about the potential for post hoc decision-making, which could introduce bias and undermine the reliability of the meta-analysis findings. Another area of concern pertains to the listing of excluded reviews. Our assessment revealed that 26 out of 28 meta-analyses did not provide a satisfactory explanation for the literature they excluded from their analysis. Conversely, only 2 meta-analyses managed to meet this standard by adequately justifying their selection and exclusion criteria24,35. This discrepancy underscores the need for more rigorous documentation and transparency in the review process, ensuring that readers and future researchers can fully understand the scope of the literature considered and the rationale behind exclusions.
Outcome of interest in pooled probiotic strains
A meta-analysis including 25 primary RCT studies conducted by McFarland et al.35 was selected as the most comprehensive study bearing the highest AMSTAR 2 rating among all the other meta-analyses. The research team found that probiotics supplementation with pooled probiotic strains was significantly associated with increased eradication rates versus the control group (RR 1.10, 95% CI 1.06–1.14) (moderate; IV)35. At the first mention of this result, it is evaluated under the AMSTAR 2 assessment tool22, which includes 16 items and generates an overall quality rating from “high,” “moderate,” “low,” to “critically low” based on deficiencies in critical domains. The quality of this study was determined to be “moderate.” Furthermore, the evidence of outcomes was categorized into one of five levels according to evidence classification criteria23, with this study’s evidence being classified as Class IV, indicating “weak evidence.” This means that, although an association is observed, more research is needed to establish this conclusively.
The comparison of the probiotic supplementation with pooled probiotic strains and the control indicated a notable decreased risk of total side effects (RR 0.54, 95% CI 0.42–0.70) (moderate; IV)35. Specifically, probiotics supplementation were found significantly associated with lower risk of diarrhea (low; IV)35, nausea/vomiting (low; IV)38, nausea (critically low; III)14, vomiting (critically low; IV)14, constipation (low; IV)38, abdominal pain (critically low; IV)13, and taste disturbance (critically low; IV)14 compared to the control group. However, probiotics supplementation with pooled probiotic strains was not linked to lower risk of loss of appetite38, abdominal distension38, bloating13, epigastric pain38, metallic taste13, flatus13, skin rash13, headache13, and dizziness13, as compared to the control group (Fig. 2) (Table S4).
(A) Number of systematic reviews with meta-analyses included which investigated outcomes of interest between H. pylori standard treatment supplemented with pooled probiotic strains and the control. (B) Simplified forest plot summarizing evidence for the association of H. pylori standard treatment supplemented with pooled probiotic strains with outcomes of interest in systematic reviews with meta-analyses categorized as the most comprehensive for each outcome.
Outcome of interest in single-strained preparation
Despite all meta-analyses on Lactobacillus-supplemented probiotics for H. pylori treatment receiving critically low AMSTAR 2 ratings, the analysis conducted by Wang et al.47 stood out as the most comprehensive study. This selection was based on its recency and the inclusion of the highest number of RCTs. The Lactobacillus-supplemented standard eradication therapy versus control was found associated with slightly higher eradication rates (79.8% vs. 72.8%, RR 1.09, 95% CI 1.03–1.15) (critically low; IV)47 with significant difference, and with lower risk of total side effects (20.7% vs. 40.3%, RR 0.58, 95% CI 0.45–0.76) (critically low; IV)47. In terms of specific GI side effects, the most comprehensive meta-analysis showed that supplementation of Lactobacillus spp. in H. pylori standard therapy was conducive to lower the risk of diarrhea by 69% (RR 0.31, 95% CI 0.19–0.52) (critically low; IV)45. The risk estimate of constipation45, bloating26, abdominal pain13, and taste disturbance14 in Lactobacillus-supplemented group reported by several meta-analyses (critically low; IV) showed varying degrees of decline, as compared to the control group (Fig. 3) (Table S4).
(A) Number of systematic reviews with meta-analyses included which investigated outcomes of interest between H. pylori standard treatment supplemented with Lactobacillus spp. and the control. (B) Simplified forest plot summarizing evidence for the association of H. pylori standard treatment supplemented with Lactobacillus spp. with outcomes of interest in systematic reviews with meta-analyses categorized as the most comprehensive for each outcome.
The meta-analysis conducted by Jiang et al.46 was selected as the most comprehensive study for single-strained preparation of Bifidobacterium spp. due to its recency and the inclusion of the highest number of RCTs. Notably, among different single-strained preparations, supplementation of Bifidobacterium spp. in H. pylori standard treatment exhibited great potential to eradicate pathogens, demonstrating a significant higher odds of curative outcome (OR 3.73, 95% CI 2.79–5.00) (critically low; III)46 as compared to the control. A significant lower odds of total side effects (OR 0.37, 95% CI 0.27–0.50) (critically low; III)46 was also found. Specifically, supplementation of Bifidobacterium spp. was significantly associated with lower odds of diarrhea (critically low; IV)46, nausea/vomiting (critically low; IV)46, constipation (critically low; IV)46, and abdominal distension (critically low; IV)46, while the odds of nausea (critically low; NS)46 and loss of appetite (critically low; NS)46 did not significantly decline between the experimental group and the control group (Fig. 4) (Table S4). Collectively, there was no other nonspecific side effect reported in Lactobacillus-supplemented or Bifidobacterium-supplemented standard therapy versus control.
(A) Number of systematic reviews with meta-analyses included which investigated outcomes of interest between H. pylori standard treatment supplemented with Bifidobacterium spp. and the control. (B) Simplified forest plot summarizing evidence for the association of H. pylori standard treatment supplemented with Bifidobacterium spp. with outcomes of interest in systematic reviews with meta-analyses categorized as the most comprehensive for each outcome.
Despite two meta-analyses presented by Lv et al.34 and Wang et al.47 suggesting that Saccharomyces spp. supplementation during H. pylori treatment was not associated with enhanced eradication rates, the most comprehensive meta-analysis conducted by Zhou et al.44 contradicted the hypothesis. The meta-analysis, despite lacking the most recent data, but possessing the highest AMSTAR 2 rating and the greatest number of RCTs, revealed that the eradication rates were significantly higher in the Saccharomyces spp. supplementation group versus the control group (81.8% vs. 74.3%, RR 1.09, 95% CI 1.05–1.13) (low; III)44. Besides, Zhou et al.44 reported lower risk of total side effects (RR 0.47, 95% CI 0.36–0.61) (low; III) using standard treatment supplemented with Saccharomyces spp. Although the risk of most side effects occurred during Saccharomyces-supplemented H. pylori eradication was not significantly reduced27,36,44, the risk of specific GI side effects such as diarrhea (critically low; III)44, nausea (low; III)44, constipation (low; IV)44, abdominal distension (low; IV)44, and stomatitis (low; IV)44 as a nonspecific side effect was decreased in varying degrees, as compared to the control (Fig. 5) (Table S4).
(A) Number of systematic reviews with meta-analyses included which investigated outcomes of interest between H. pylori standard treatment supplemented with Saccharomyces spp. and the control. (B) Simplified forest plot summarizing evidence for the association of H. pylori standard treatment supplemented with Saccharomyces spp. with outcomes of interest in systematic reviews with meta-analyses categorized as the most comprehensive for each outcome.
Outcome of interest in multi-strained preparation
Risk estimates regarding the effects of multi-strained probiotics supplementation on H. pylori eradication rates were available for eight meta-analyses. The meta-analyses conducted by McFarland et al.39 bearing the highest AMSTAR 2 rating was selected as the most comprehensive study. The results showed that eradication rates improved by 12% in the experimental group (85.8%) compared with the control group (76.8%) and that the RR was 1.12 (95% CI 1.08–1.17) (low; IV)39. Also, the risk of total side effects decreased by 55% in the multi-strained probiotics supplementation group compared with the control group. The RR for the experimental group was 0.45 (95% CI 0.30–0.65) (low; IV)39. In terms of specific side effects, they noted a significant decrease in the risk of diarrhea (RR 0.44, 95% CI 0.25–0.77) (low, IV)39 in the multi-strained probiotics supplementation group compared with the control group. No other nonspecific side effects were reported in association with the multi-strained preparation during the standard treatment of H. pylori as compared to the control (Fig. 6) (Table S4).
(A) Number of systematic reviews with meta-analyses included which investigated outcomes of interest between H. pylori standard treatment supplemented with multi-strained preparation and the control. (B) Simplified forest plot summarizing evidence for the association of H. pylori standard treatment supplemented with multi-strained preparation with outcomes of interest in systematic reviews with meta-analyses categorized as the most comprehensive for each outcome.
Discussion
Our comprehensive umbrella review collates evidence from 28 unique meta-analyses based on 534 RCTs, highlighting an association between probiotics supplementation and improved eradication rates and reduced total side effects during H. pylori standard treatment. These findings, in alignment with previous systematic reviews with meta-analyses, further solidify the growing body of evidence indicating the beneficial use of probiotics in enhancing the eradication rates of H. pylori and reducing the incidence of related side effects.
The evidence demonstrates a significant improvement in H. pylori eradication rates with probiotics supplementation when used alongside standard therapy. It aligns with the conclusions of another umbrella review, which exclusively examined the effect of probiotics supplementation on H. pylori eradication without considering its impact on reducing side effects49. This improvement is especially crucial considering the prevailing issues with the standard triple or quadruple regimens12,13,14, which confront with reduced effectiveness due to rising antibiotic resistance5,6. Probiotics supplementation, by providing a means of modulating the gastrointestinal microbiota, may offer a new approach to combatting such resistance9. Furthermore, their capacity to alleviate common antibiotic-associated side effects, such as diarrhea, nausea, and vomiting, may improve patient adherence to therapy7.
The observed variation in outcomes between different probiotic genera suggests that specific genera or combinations thereof may be more effective in treating H. pylori. Notably, supplementation with Bifidobacterium spp. showed greater effectiveness as a single-strained preparation at the genus level for H. pylori eradication, while Lactobacillus spp. and Saccharomyces spp. also exhibited beneficial effects. This underscores the possibility of tailoring probiotic supplementation based on genus-specific effects, which could optimize H. pylori eradication rates and minimize side effects. It is imperative to acknowledge that the efficacy of probiotics is highly strain-specific, and not all strains within a genus may offer the same health benefits16. This strain-specificity is critical for understanding the mechanisms of action and for guiding the selection of probiotic strains in clinical practice. Future research should further investigate the mechanisms underlying these genus-specific effects and the potential synergistic effects of multi-strained probiotics.
The utilization of probiotics could be considered as supplementary therapy for H. pylori eradication primarily due to three distinct capabilities: (1) they foster mucin production, which, in turn, restricts the pathogen's adhesion to the gut surface; (2) they generate short-chain fatty acids and other antimicrobial substances, potentially decreasing the density of H. pylori; and (3) they offer protection against human pathogens through host receptor competition and immune modulation mechanisms50. Recent decades have witnessed an abundance of in vitro studies demonstrating that Bifidobacterium spp. could inhibit pathogens using a variety of mechanisms such as production of organic acids, antibacterial peptides, and quorum-sensing inhibitors, as well as immune stimulation51,52,53,54. These methods collectively offer molecular evidence highlighting their inherent potential to prevent H. pylori infections. For instance, certain strains of Bifidobacterium spp. are known to produce acetate, which has been demonstrated to play a key protective role against some infectious diseases52. Adding to this, a recent study found that zinc acetate can increase the sensitivity of H. pylori to levofloxacin, an antibiotic commonly used in H. pylori eradication regimens55. This provides a possible mechanism for enhancing the treatment of H. pylori by increasing the acetate produced by Bifidobacterium spp., which probably could explain why supplementation of Bifidobacterium spp. might show greater capacity for pathogen eradication compared to other probiotic strains. However, these potential mechanisms, based on in vitro studies and animal models, require further confirmation in human clinical trials.
Although the results of this umbrella review are promising, they should be interpreted with caution due to several limitations. The quality of the included systematic reviews with meta-analyses was critically low for most studies, as determined by the AMSTAR 2 criteria. Also, the considerable heterogeneity among the included meta-analyses may have influenced the outcomes. Plus, there are still unanswered questions regarding the optimal genus/strains, dosages, and duration of probiotics for H. pylori treatment, and the specific mechanisms through which they impact the potential probiotics-gut microbiota cross talk and immune system remain unclear. Moreover, further exploration in high-quality design is necessary to determine whether certain subgroups of patients might benefit more from probiotic supplementation, taking into account factors such as age, disease severity, recurrence rate and antibiotic resistance profiles.
To enhance the quality of systematic reviews with meta-analyses in future research, it is essential to address these limitations. First, adopting rigorous methodologies that adhere closely to established guidelines, such as those outlined in the AMSTAR 2, can significantly improve study quality. As shown in Table S3, most of the meta-analyses failed to meet the requirements in question 2 (24/28), 7 (26/28), and 10 (26/28), which ultimately leads to poor AMSTAR 2 rating. Efforts should be made to minimize heterogeneity among studies by clearly defining inclusion criteria, intervention protocols, and outcome measures. Additionally, there is a pressing need for more comprehensive data on probiotics’ specific strains, dosages, and treatment durations, necessitating targeted research in these areas. Furthermore, future reviews should place a greater emphasis on subgroup analyses to identify patient populations that may derive the most benefit from probiotic supplementation. This involves a more detailed examination of factors such as age, disease severity, recurrence rates, and antibiotic resistance profiles. Incorporating such analyses can help in tailoring probiotic supplementation strategies to individual patient needs, potentially leading to more effective management of H. pylori infection.
In conclusion, probiotics supplementation is a plausible adjunctive strategy in the management of H. pylori, with potential benefits in terms of eradication rates improvement and side effects reduction. Until rigorous evidence is available, clinicians should consider probiotics as a promising, albeit not definitively proven, adjunct to H. pylori eradication therapy. Besides, our umbrella review underscores the need for well-designed, high-quality RCTs and systematic reviews with meta-analyses that will further validate the effectiveness of probiotics, identify optimal genus/strains and combinations, and determine their best therapeutic application within the clinical setting. This may also pave the way for personalized probiotics supplementation strategies in the management of H. pylori infection in future.
Data availability
The datasets generated or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Hooi, J. K. Y. et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429. https://doi.org/10.1053/j.gastro.2017.04.022 (2017).
Tshibangu-Kabamba, E. & Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 18, 613–629. https://doi.org/10.1038/s41575-021-00449-x (2021).
Malfertheiner, P. et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66, 6–30. https://doi.org/10.1136/gutjnl-2016-312288 (2017).
Chey, W. D., Leontiadis, G. I., Howden, C. W. & Moss, S. F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 112, 212–239. https://doi.org/10.1038/ajg.2016.563 (2017).
Savoldi, A., Carrara, E., Graham, D. Y., Conti, M. & Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 155, 1372–1382. https://doi.org/10.1053/j.gastro.2018.07.007 (2018).
De Francesco, V. et al. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver Dis. 19, 409–414 (2010).
Dascalu, R. I. et al. Multidrug resistance in Helicobacter pylori infection. Front. Microbiol. 14, 1128497. https://doi.org/10.3389/fmicb.2023.1128497 (2023).
Senok, A. C., Ismaeel, A. Y. & Botta, G. A. Probiotics: Facts and myths. Clin. Microbiol. Infect. 11, 958–966. https://doi.org/10.1111/j.1469-0691.2005.01228.x (2005).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. https://doi.org/10.1038/s41591-019-0439-x (2019).
Aiba, Y., Suzuki, N., Kabir, A. M., Takagi, A. & Koga, Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am. J. Gastroenterol. 93, 2097–2101. https://doi.org/10.1111/j.1572-0241.1998.00600.x (1998).
Pinchuk, I. V. et al. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 45, 3156–3161. https://doi.org/10.1128/AAC.45.11.3156-3161.2001 (2001).
Zhang, M. et al. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microb. Pathog. 147, 104403. https://doi.org/10.1016/j.micpath.2020.104403 (2020).
Zhang, M. M., Qian, W., Qin, Y. Y., He, J. & Zhou, Y. H. Probiotics in Helicobacter pylori eradication therapy: A systematic review and meta-analysis. World J. Gastroenterol. 21, 4345–4357. https://doi.org/10.3748/wjg.v21.i14.4345 (2015).
Shi, X. et al. Efficacy and safety of probiotics in eradicating Helicobacter pylori: A network meta-analysis. Med. Baltim. 98, e15180. https://doi.org/10.1097/MD.0000000000015180 (2019).
Lu, C. et al. Probiotic supplementation does not improve eradication rate of Helicobacter pylori infection compared to placebo based on standard therapy: A meta-analysis. Sci. Rep. 6, 23522. https://doi.org/10.1038/srep23522 (2016).
Hill, C. et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. https://doi.org/10.1038/nrgastro.2014.66 (2014).
Gates, M. et al. Reporting guideline for overviews of reviews of healthcare interventions: Development of the PRIOR statement. BMJ 378, e070849. https://doi.org/10.1136/bmj-2022-070849 (2022).
McGowan, J. et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J. Clin. Epidemiol. 75, 40–46. https://doi.org/10.1016/j.jclinepi.2016.01.021 (2016).
Farrah, K., Mierzwinski-Urban, M. & Cimon, K. Effectiveness of adverse effects search filters: Drugs versus medical devices. J. Med. Libr. Assoc. 104, 221–225. https://doi.org/10.3163/1536-5050.104.3.007 (2016).
Salvo, E. M., Ferko, N. C., Cash, S. B., Gonzalez, A. & Kahrilas, P. J. Umbrella review of 42 systematic reviews with meta-analyses: The safety of proton pump inhibitors. Aliment Pharmacol. Ther. 54, 129–143. https://doi.org/10.1111/apt.16407 (2021).
Huang, Y. et al. Dietary sugar consumption and health: Umbrella review. BMJ 381, e071609. https://doi.org/10.1136/bmj-2022-071609 (2023).
Shea, B. J. et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. https://doi.org/10.1136/bmj.j4008 (2017).
Ioannidis, J. P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 181, 488–493. https://doi.org/10.1503/cmaj.081086 (2009).
Tong, J. L., Ran, Z. H., Shen, J., Zhang, C. X. & Xiao, S. D. Meta-analysis: The effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol. Ther. 25, 155–168. https://doi.org/10.1111/j.1365-2036.2006.03179.x (2007).
Sachdeva, A. & Nagpal, J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: A systematic review and meta-analysis of randomized-controlled trials. Eur. J. Gastroenterol. Hepatol. 21, 45–53. https://doi.org/10.1097/MEG.0b013e32830d0eff (2009).
Zou, J., Dong, J. & Yu, X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 14, 97–107. https://doi.org/10.1111/j.1523-5378.2009.00716.x (2009).
Szajewska, H., Horvath, A. & Piwowarczyk, A. Meta-analysis: The effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol. Ther. 32, 1069–1079. https://doi.org/10.1111/j.1365-2036.2010.04457.x (2010).
Wang, Z. H., Gao, Q. Y. & Fang, J. Y. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J. Clin. Gastroenterol. 47, 25–32. https://doi.org/10.1097/MCG.0b013e318266f6cf (2013).
Zheng, X., Lyu, L. & Mei, Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: Evidence from a meta-analysis. Rev. Esp. Enferm. Dig. 105, 445–453. https://doi.org/10.4321/s1130-01082013000800002 (2013).
Dang, Y., Reinhardt, J. D., Zhou, X. & Zhang, G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: A meta-analysis. PLoS One 9, e111030. https://doi.org/10.1371/journal.pone.0111030 (2014).
Zhu, R. et al. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J. Gastroenterol. 20, 18013–18021. https://doi.org/10.3748/wjg.v20.i47.18013 (2014).
Gong, Y., Li, Y. & Sun, Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 8, 6530–6543 (2015).
Li, B. Z. et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: Systematic review and network meta-analysis. BMJ 351, h4052. https://doi.org/10.1136/bmj.h4052 (2015).
Lv, Z. et al. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: A meta-analysis. Exp. Ther. Med. 9, 707–716. https://doi.org/10.3892/etm.2015.2174 (2015).
McFarland, L. V., Malfertheiner, P., Huang, Y. & Wang, L. Meta-analysis of single strain probiotics for the eradication of Helicobacter pylori and prevention of adverse events. World J. Meta-anal. 3, 97–117 (2015).
Szajewska, H., Horvath, A. & Kolodziej, M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol. Ther. 41, 1237–1245. https://doi.org/10.1111/apt.13214 (2015).
Lau, C. S., Ward, A. & Chamberlain, R. S. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: A meta-analysis. Infect. Drug Resist. 9, 275–289. https://doi.org/10.2147/IDR.S117886 (2016).
Lu, M. et al. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: A meta-analysis of randomized controlled trials. PLoS One 11, e0163743. https://doi.org/10.1371/journal.pone.0163743 (2016).
McFarland, L. V., Huang, Y., Wang, L. & Malfertheiner, P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur. Gastroenterol. J. 4, 546–561. https://doi.org/10.1177/2050640615617358 (2016).
Zhou, B. G. et al. Probiotics-containing rescue regimen for the eradication of Helicobacter pylori infection: A systematic review. Chin. J. Evid.-based Med. 16, 550–556 (2016).
Si, X., Lan, Y. & Qiao, L. A meta-analysis of randomized controlled trials of bismuth-containing quadruple therapy combined with probiotic supplement for eradication of Helicobacter pylori. Chin. J. Intern. Med. 56, 752–759. https://doi.org/10.3760/cma.j.issn.0578-1426.2017.10.009 (2017).
Wang, F. et al. Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin. Res. Hepatol. Gastroenterol. 41, 466–475. https://doi.org/10.1016/j.clinre.2017.04.004 (2017).
Yu, M., Zhang, R., Ni, P., Chen, S. & Duan, G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: A meta-analysis of randomized controlled trials. PLoS One 14, e0223309. https://doi.org/10.1371/journal.pone.0223309 (2019).
Zhou, B. G., Chen, L. X., Li, B., Wan, L. Y. & Ai, Y. W. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter 24, e12651. https://doi.org/10.1111/hel.12651 (2019).
Yang, C., Liu, L., Majaw, J. K., Liang, L. & Chen, Y. Efficacy of Lactobacillus reuteri supplementation therapy for Helicobacter pylori eradication: A meta-analysis of randomised controlled trials. Med. Microecol. 8, 100036. https://doi.org/10.1016/j.medmic.2021.100036 (2021).
Jiang, X. et al. Efficacy and safety of bifidobacterium quadruple viable tablets in the treatment of Helicobacter pylori-infected peptic ulcer or gastritis patients: A systematic review and meta-analysis. BMC Infect Dis. 23, 313. https://doi.org/10.1186/s12879-023-08211-1 (2023).
Wang, Y., Wang, X., Cao, X. Y., Zhu, H. L. & Miao, L. Comparative effectiveness of different probiotics supplements for triple Helicobacter pylori eradication: A network meta-analysis. Front. Cell Infect. Microbiol. 13, 1120789. https://doi.org/10.3389/fcimb.2023.1120789 (2023).
Fakhry, S. M. et al. Can probiotics play a role in Helicobacter pylori (H. pylori) eradication?. Egypt. Liver J. 13, 64. https://doi.org/10.1186/s43066-023-00294-4 (2023).
Musazadeh, V. et al. The effectiveness of treatment with probiotics in Helicobacter pylori eradication: Results from an umbrella meta-analysis on meta-analyses of randomized controlled trials. Food Funct. 14, 7654–7662. https://doi.org/10.1039/d3fo00300k (2023).
Liang, B. et al. Current and future perspectives for Helicobacter pylori treatment and management: From antibiotics to probiotics. Front. Cell Infect. Microbiol. 12, 1042070. https://doi.org/10.3389/fcimb.2022.1042070 (2022).
Moroni, O., Kheadr, E., Boutin, Y., Lacroix, C. & Fliss, I. Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl. Environ. Microbiol. 72, 6894–6901. https://doi.org/10.1128/AEM.00928-06 (2006).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547. https://doi.org/10.1038/nature09646 (2011).
Kim, Y., Lee, J. W., Kang, S. G., Oh, S. & Griffiths, M. W. Bifidobacterium spp. influences the production of autoinducer-2 and biofilm formation by Escherichia coli O157:H7. Anaerobe 18, 539–545. https://doi.org/10.1016/j.anaerobe.2012.08.006 (2012).
Fanning, S. et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. U. S. A. 109, 2108–2113. https://doi.org/10.1073/pnas.1115621109 (2012).
Tao, H. et al. Transcriptomic and functional approaches unveil the role of tmRNA in zinc acetate mediated levofloxacin sensitivity in Helicobacter pylori. Microbiol. Spectr. 10, e0115222. https://doi.org/10.1128/spectrum.01152-22 (2022).
Acknowledgements
This work was supported by the National Clinical Key Specialty Construction Project [Grant Number ZK108000] and the Fundamental Research Funds for the Central Universities [Grant Number 3332023115].
Author information
Authors and Affiliations
Contributions
Study conception and design: H.C., and D.W. Data collection: Z.Y., Y.Z., and Z.H. Data analysis and interpretation: Z.Y. and Y.Z. Drafting of the manuscript: Z.Y. Critical revision: K.H. and Y.Z. All authors reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Z., Zhou, Y., Han, Z. et al. The effects of probiotics supplementation on Helicobacter pylori standard treatment: an umbrella review of systematic reviews with meta-analyses. Sci Rep 14, 10069 (2024). https://doi.org/10.1038/s41598-024-59399-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59399-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.