Abstract
The high burden of anaemia during pregnancy underscores the urgent need to gain a comprehensive understanding of the factors contributing to its widespread occurrence. Our study assessed the prevalence and the trends of moderate-to-severe anaemia (MSA) in late pregnancy (28 to 36 weeks) and then investigated the key determinants driving this prevalence among women in Lagos, Nigeria. We conducted a secondary data analysis involving 1216 women enrolled in the Predict-PPH study between January and March 2023. We employed a multivariate binary logistic regression model with a backward stepwise selection approach to identify significant predictors of MSA. The study revealed a 14.5% prevalence of MSA during pregnancy. Independent predictors of MSA included having given birth to two or more children (adjusted odds ratio = 1.46, 95% confidence interval: 1.03–2.07), having a maternal body mass index (BMI) of 28 kg/m2 or higher (adjusted odds ratio = 1.84, 95% confidence interval: 1.29–2.61), having less than tertiary education (adjusted odds ratio = 1.51, 95% confidence interval: 1.08–2.11), and being unemployed (adjusted odds ratio = 1.97, 95% confidence interval: 1.19–3.26). It is crucial for pregnant women, particularly those with higher parities and elevated BMI, to be monitored regularly for anaemia and its consequences during their antenatal care. Additionally, addressing the link between low education, unemployment, and anaemia necessitates comprehensive strategies that empower women in terms of education and economic status to enhance the overall well-being of individuals and communities, ultimately reducing the prevalence of anaemia and associated health issues in pregnancy.
Similar content being viewed by others
Introduction
Anaemia is an indicator of both poor nutrition and poor health. Its occurrence in pregnancy may result in women’s impaired health, and quality of life, and consequential impairment in newborn and child’s development and learning1. Anaemia is the most common medical disorder of pregnancy2 and a major contributor to maternal and perinatal morbidity and mortality worldwide3. It is a decrease in the concentration of circulating haemoglobin (Hb) in the peripheral blood, below the level that is considered sufficient for one's age, gender, and geographical location4. According to the World Health Organization (WHO), anaemia is typically characterized by Hb levels lower than 11.0 g/dL at any stage of pregnancy and less than 10.0 g/dL postpartum5.
Based on WHO estimates, globally, 41.8% of pregnant women experience anaemia, with a notably higher prevalence on the African continent, where approximately 56% of pregnant women are identified as anaemic6. This is even more staggering within the context of pregnancy due to the physiological changes in the second trimester that lead to an elevation in plasma volume, accompanied by a relatively smaller increase in red cell mass, resulting in hemodilution—manifesting as ‘physiological anaemia’7. According to the 2018 Nigerian Demographic and Health Survey (NDHS), approximately 58% of pregnant women in Nigeria grapple with this condition8.
In sub-Saharan Africa (SSA), an Hb level below 10.0 g/dL is frequently employed as a criterion for diagnosing anaemia during pregnancy4. This cut-off has been justified based on the work of Lawson et al.9, which suggested that serious harm to the mother and fetus hardly occurs above this cut-off value, which is regarded as moderate to severe levels according to the WHO classification10. Globally, 23% of maternal mortality is attributed to moderate-to-severe anaemia (MSA) during pregnancy, particularly occurring at 28 weeks and beyond11. The magnitude and burden of MSA vary globally. It is estimated that about 37% (32 million) of pregnant women aged 15–49 years were affected by anaemia, with roughly half of these cases attributed to moderate to severe levels12. This condition is a significant public health concern, especially in developing countries, and can lead to increased morbidity and mortality, particularly in pregnant women and their newborns13.
The high burden of anaemia in pregnancy, therefore, emphasizes the pressing need to comprehensively understand the determinants behind the ubiquity of MSA during pregnancy. Furthermore, the third trimester is a crucial period in pregnancy because the demand for iron increases as the fetus undergoes rapid growth and development in utero14. Therefore, in this study, we delved into the multifaceted landscape of MSA in pregnancy by determining the prevalence and the trends of MSA, and then explored the intricate web of determinants that drive this prevalence among women in the third trimester of pregnancy (28 to 36 weeks) in Lagos, Nigeria. By shedding light on these critical factors, we aim to contribute to a deeper understanding of this pressing global health issue and pave the way for effective strategies for the prevention and management of MSA in a resource-limited setting such as Nigeria.
Participants and methods
Study design and settings
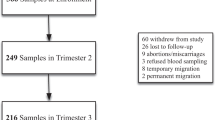
This was a descriptive cross-sectional study of pregnant women enrolled at baseline in the recently conducted “Predict-PPH” study15. “Predict-PPH” was a prospective cohort study of consecutively consenting healthy pregnant women aged 15–49 years and at 28–36 weeks’ gestation enrolled at the antenatal clinics of five hospitals in Lagos, Nigeria from January to June 202315. These were the Lagos University Teaching Hospital (LUTH) in Idi-Araba, Federal Medical Center Ebute Meta (FMC-Eb), 68 Nigerian Army Reference Hospital (68-NARHY) in Yaba, Lagos Island Maternity Hospital (LIMH) in Lagos Island, and Lagos State University Teaching Hospital (LASUTH) in Ikeja. These hospitals are the foremost public health institutions in Lagos State, Southwest Nigeria, with a population of over 20 million inhabitants, and they act mainly as referral centres for other government-owned and private hospitals in Lagos and its surrounding States. They all have established obstetrics and gynaecology departments with comprehensive maternity units that account for a cumulative annual delivery of 15,70015. Over 90% of payments for health services in these hospitals are usually made directly out-of-pocket without insurance while approximately 10% of the remaining clients pay through some form of social health insurance including the Lagos State Ilera Eko Scheme (LSIES) and the National Health Insurance Scheme (NHIS)16.
Eligibility criteria
We included and analyzed the data of n = 1216 women with complete datasets collected at baseline enrollment from the primary study15. Excluded at enrolment were women with a major medical condition such as sickle cell anaemia, significant renal and hepatic impairment, coagulation disorders and antepartum fetal demise. Further exclusion in this current study were women whose haemoglobin (Hb) concentration was not recorded at enrollment.
Extracted variables of interest
Variables extracted for analyses in the dataset included site and type of enrolment facility, participant’s age in years, gestational age at enrolment in weeks, number of previous childbirths, body mass index (BMI) in kg/m2, marital, educational and employment status, mode of conception, type of pregnancy, antepartum bleeding in index pregnancy, ultrasound diagnosis of uterine fibroids and Hb concentration at enrolment in g/dL determined using HemoCue® B-Hemoglobin system. Body mass index (BMI; calculated as maternal weight [using the actual pre-gestational or first-trimester measurement] in kilograms divided by the square of height in meters)17. As recommended by the WHO10, participants were categorized based on their Hb concentration as non-anaemic (≥ 11 g/dL), mild anaemia (10 to < 11 g/dL), moderate anaemia (7 to < 10 g/dL) and severe anaemia (< 7 g/dL).
Operational definitions of study outcomes
We assessed two study outcomes: the prevalence of moderate-to-severe anaemia (MSA), defined as the proportion of study participants with Hb concentration less than 10 g/dL10, and determinants of MSA, which are the baseline variables from the Predict-PPH study15 that are significantly associated with MSA.
Sample size calculation
Using Fisher's formula18, we estimated that a sample size of n = 308 would be required to determine the prevalence of MSA based on a type I error rate of 5% at a 95% confidence level of 1.96 and a derived proportion of 27.6%4. In addition, to identify predictors of MSA, we estimated the sample size using the maximum modelling principle proposed by Peduzzi et al.19. This required that a minimum of 10 events (women with MSA in pregnancy) would occur for each incorporated prediction variable given that the prevalence of MSA is 27.6%4 and that model building included 12 potential predictor variables from the dataset. A sample size of n = 458 women was required to identify independent predictors of MSA in pregnancy assuming a non-response or data recording error rate of 5%. We, therefore, included all the n = 1216 women who had their complete datasets at baseline in the primary study15 in the statistical analyses. In the original study15, there was no predetermined allocation of participants to the study sites. The number (and proportion) of participants from each study site was based on the volume of pregnant women seen and who consented to enrolment at each site during the study period.
Statistical analysis
We tested continuous variables for normality using the Kolmogorov–Smirnov test with Lilliefors’ significance correction and descriptive statistics were then computed for the participants’ clinical and obstetric characteristics. Categorical variables were expressed as frequencies and percentages, whereas continuous variables were displayed as mean (± standard deviation) for normally distributed data or median (interquartile range) for skewed distributions. Bivariate analyses of variables that are potential predictors of MSA in pregnancy were performed using Pearson’s Chi-square test. A multivariate binary logistic regression model was then developed using a stepwise selection approach to identify significant predictors of MSA in pregnancy. Variables that were associated with MSA in pregnancy (P < 0.10) in the bivariate analyses were included in the pool of variables for the backward stepwise regression model. An Akaike’s Information Criterion was generated constantly and the last model step with the smallest AIC was selected as the best-fit model. Associations in the final model were regarded as significant if P < 0.05. Data analyses were performed with IBM SPSS Statistics for Windows, Version 28.0 (IBM Corporation, Armonk, NY, USA).
Ethical considerations
Approval for the primary study15 was granted by the Health Research Ethics Committees of the Lagos University Teaching Hospital (ADM/DSCST/HREC/APP/5443), Lagos State University Teaching Hospital (LREC/06/10/2000) and Institute of Tropical Medicine, Antwerp (IRB/RR/AC/002). The research was conducted ethically according to the World Medical Association Declaration of Helsinki. Before enrollment, all study participants provided written informed consent, and a rigorous commitment to maintaining the privacy and confidentiality of participant information was upheld throughout and after the conduct of the study.
Results
A total of n = 1216 women who had their complete datasets out of the 1222 women enrolled at baseline in the primary study15 were included in the data analyses. Out of these, n = 176 (14.5%) had MSA. The characteristics of the enrolled cohorts are presented in Table 1.
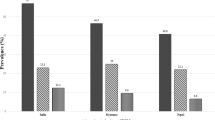
Figure 1 showed a progressive reduction in the prevalence of MSA from 22.5% at 28 weeks to 12.5% at 36 weeks’ gestation.
In the bivariable analyses, factors that were significantly associated with the MSA were: enrolment gestational age, number of previous childbirths, increased BMI, and educational and employment status. In the final multivariable analysis, the following predictor variables were independently associated with MSA: previous childbirths ≥ 2 (adjusted odds ratio = 1.46, 95% confidence interval: 1.03–2.07), maternal BMI ≥ 28 kg/m2 (adjusted odds ratio = 1.84, 95% confidence interval: 1.29–2.61), having less than tertiary education (adjusted odds ratio = 1.51, 95% confidence interval: 1.08–2.11), and being unemployed (adjusted odds ratio = 1.97, 95% confidence interval: 1.19–3.26) (Table 2).
Discussion
In this descriptive cross-sectional study of healthy pregnant women enrolled at baseline in the Predict-PPH study, one in seven of the women were reported as having moderate-to-severe anaemia (MSA) during pregnancy. The prevalence of MSA reduced progressively between 28- and 36-weeks’ gestation. Independent predictors of MSA included having given birth to two or more children, having a high pre- or early-pregnancy body mass index (BMI), having less than tertiary education, and being unemployed.
The prevalence of 14.5% for MSA reported in our study is similar to the 15.3% found by Uche-Nwachi et al.20 in a study of 2287 pregnant women attending 40 public healthcare centers in Trinidad and Tobago between January 2000 and December 2005 and the global prevalence of 16.5% reported by the World Health Organization (WHO)12 based on antenatal Hb assessments done at the end of the second trimester. However, this is notably lower than the 25% seen among pregnant women in Kakamega county of Kenya21 and the 27.6% reported in another Lagos in 20144. This discrepancy may suggest a gradual reduction in pregnancy-related anaemia due to increased awareness and compliance with antenatal iron supplementation, particularly among women enrolled in our clinics. This variation could also be attributed to the study’s location in an urban area of Lagos metropolis with predominantly higher socioeconomic status participants22, contributing to the remarkably low anaemia prevalence.
Furthermore, we observed a trend towards decreasing MSA prevalence from 22.5% at 28 weeks’ gestation to 12.5% at 36 weeks. This contrasts with the findings from a previous study by Okunade et al. in Lagos4 where anaemia was less common in the 2nd trimester. Similarly, another Lagos study by Anorlu et al.23 and an East African study conducted by Chrispinus et al. in Kakamega county in Kenya21 reported significantly higher anaemia rates of anaemia in the second and third trimesters, particularly among pregnant women who had recently registered for antenatal care. Previous research has validated that anaemia tends to be exacerbated by the physiological haemodilution characteristic of pregnancy, with a more noticeable impact in the last two trimesters24. However, the reduced prevalence with advancing gestational age in our study may suggest better compliance with antenatal care, supplements, and nutrition among pregnant women as their pregnancies approach full term.
The association we identified between higher parity and MSA in pregnancy aligns with the findings by Al-Farsi et al. in Oman25 and a study by Gedefaw et al. in Ethiopia26, which demonstrated that women with high-parity pregnancies had a higher risk of anaemia, often showing a dose–response relationship across multiple categories of parity. This association may be due to the shorter intervals between pregnancies commonly observed in our setting, limiting the body's ability to replenish nutrient stores, including iron, depleted from previous pregnancies. Additionally, it may be explained by the increased demand for nutrients to support a growing fetus with each successive pregnancy.
Several studies have reported that lower BMI in pre- or early pregnancy is associated with anaemia in pregnancy27,28 due to the possible link of low BMI with poor nutritional intake, involving the consumption of diverse micronutrients crucial for hematopoiesis29 and chronic illness, such as tuberculosis or parasitic infections, ultimately resulting in anaemia26. However, we reported an independent association between maternal BMI ≥ 28 kg/m2 and anaemia. This link may be attributed to factors such as hypoferremia due to dilution, insufficient dietary iron intake, increased iron requirements, diminished iron absorption30, and elevated inflammation resulting from increased hepcidin levels. This, in turn, diminishes iron absorption from the intestines in obese individuals31.
The high prevalence of anaemia has been associated with low socio‐economic status21,32. Therefore, as highlighted in our study, two major indices of social status including having less than tertiary education and being unemployed were independently predictive of MSA. This underscores the interconnected relationship between low education, unemployment, poverty, and poor nutritional outcomes, including anaemia, especially in resource-limited settings such as Nigeria33. Women living in poverty often face financial limitations, limiting their access to safe, sufficient, and nutritious food, as well as quality and affordable antenatal care32,33. Therefore, our study reinforces the belief and findings from previous research that nutritional deficiency particularly that of iron is the leading cause of anaemia in pregnancy34, particularly in resource-limited countries35, where it is closely linked to poverty and malnutrition36.
The findings of this study have significant implications for clinical practice and policy. The study underscores the importance of routine screening for anaemia during antenatal visits, especially for pregnant women with identified risk factors. Clinicians should also be vigilant in assessing these risk factors and should consider individualized management strategies for at-risk pregnant women including dietary interventions, iron supplementation, and referral to specialized care if necessary. In addition, policymakers should allocate resources to ensure access to adequate antenatal care services, including routine screening for anaemia, particularly among vulnerable populations. This may involve strengthening healthcare infrastructure, providing training for healthcare professionals, and ensuring the availability of affordable and accessible iron supplementation. The study has a substantial sample size which provided robust evidence to support the findings and the multi-center settings of the study ensured that the findings are representative of the broader population of pregnant women who attend antenatal care in the Lagos metropolis.
Despite its numerous strengths, the study has some limitations. The study findings can only be generalized primarily to the urban secondary and tertiary health institutional settings in Lagos, as the participating pregnant women were enrolled with the exclusion of those attending primary health centres as well as those living in the slums and suburban areas of Lagos. Secondly, the lack of random allocation in participants’ recruitment from the study sites could increase the risk of selection bias with certain study sites disproportionately enrolling participants with specific characteristics or risk factors which could thus compromise the generalizability of the study findings to the broader population. Furthermore, the cross-sectional nature of the study prevents us from making causal inferences regarding the observed relationships between the identified risk factors and MSA. We, therefore, recommend that future longitudinal studies should be designed to employ a more comprehensive sampling strategy that includes women from a wider range of clinical and community settings, as well as those residing in slums and suburban areas to allow a trajectory tracking of anaemia during pregnancy and assess the temporal relationship between potential risk factors and the development of MSA. These studies should also conduct more in-depth analyses of potential risk factors for MSA, including socio-demographic factors, dietary habits, and underlying health conditions as a way of informing targeted interventions to address individual women-specific risk profiles, especially before conception.
Conclusions
We reported that one in seven women had MSA in late pregnancy and identified independent predictors of MSA as having had two or more previous childbirths, a high maternal BMI, having less than a tertiary education, and being unemployed. We also recorded a progressive reduction in the prevalence of MSA between 28- and 36-weeks’ gestation. It is, therefore, crucial for pregnant women, particularly those with higher parities and elevated BMI, to be regularly monitored for anaemia during their antenatal care. Additionally, addressing the link between low education, unemployment, and anaemia necessitates comprehensive strategies that empower women in terms of education and economic status to enhance the overall well-being of individuals and communities, ultimately reducing the prevalence of anaemia and associated health issues in pregnancy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (KSO) upon reasonable request.
References
WHO Global nutrition targets 2025: anaemia policy brief (World Health Organization, 2014) http://www.who.int/publications/i/item/WHO-NMH-NHD-14.4. Accessed 18 Jan 2023.
Milman, N. Anemia—still a major health problem in many parts of the world!. Ann. Hematol. 90(4), 369–377 (2011).
WHO Recommendations for the Prevention of Postpartum Haemorrhage (World Health Organization, 2014) https://www.who.int/publications/i/item/9789241548502. Accessed 18 Jan 2023.
Okunade, K. S. & Adegbesan-Omilabu, M. A. Anaemia among pregnant women at the booking clinic of a teaching hospital in south-western Nigeria. Int. J. Med. Biomed. Res. 3, 114–120 (2024).
Frayne, J. & Pinchon, D. Anaemia in pregnancy. Aust. J. Gen. Pract. 48(3), 125–129 (2019).
Reveiz, L., Gyte, G. M., Cuervo, L. G. & Casasbuenas, A. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst. Rev. 10, CD003094 (2011).
Afolabi, B. B. et al. Volume regulatory hormones and plasma volume in pregnant women with sickle cell disorder. J. Renin Angiotensin Aldosterone Syst. 17(3), 1470320316670444 (2016).
NDHS. Nigeria Demographic and Health Survey 2018 Key Indicators Report (The Federal Republic of Nigeria, 2019) http://www.population.gov.ng.
Bergsjø, P. Maternity care in developing countries. Acta Obstet. Gynecol. Scand. 81(3), 276–276 (2002).
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (World Health Organization, 2011) http://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11. Accessed 27 Nov 2023.
Branca, F., Mahy, L. & Mustafa, T. S. The lack of progress in reducing anaemia among women: The inconvenient truth. Bull World Health Organ. 92(4), 231 (2014).
Pasricha, S. R. & Moir-Meyer, G. Measuring the global burden of anaemia. Lancet Haematol. 10(9), e696–e697 (2023).
Stevens, G. A. et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–19: A pooled analysis of population-representative data. Lancet Glob. Health. 10(5), e627–e639 (2022).
Georgieff, M. K., Krebs, N. F. & Cusick, S. E. The benefits and risks of iron supplementation in pregnancy and childhood. Annu. Rev. Nutr. 21(39), 121–146 (2019).
Okunade, K. S. et al. Development of antepartum risk prediction model for postpartum hemorrhage in Lagos, Nigeria: A prospective cohort study (Predict-PPH study). Int. J. Gynaecol. Obstet. https://doi.org/10.1002/ijgo.15364 (2024) (Epub ahead of print).
Onwujekwe, O. et al. Exploring effectiveness of different health financing mechanisms in Nigeria; what needs to change and how can it happen?. BMC Health Serv. Res. 19(1), 661 (2019).
Okunade, K. S. et al. Effects of selenium supplementation on pregnancy outcomes and disease progression in HIV-infected pregnant women in Lagos: A randomized controlled trial. Int. J. Gynecol. Obstet. 153(3), 533–541 (2021).
Fisher, L. D. Self-designing clinical trials. Stat. Med. 17(14), 1551–1562 (1998).
Peduzzi, P., Concato, J., Kemper, E., Holford, T. R. & Feinstein, A. R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49(12), 1373–1379 (1996).
Uche-Nwachi, E. O. et al. Anaemia in pregnancy: Associations with parity, abortions and child spacing in primary healthcare clinic attendees in Trinidad and Tobago. Afr. Health Sci. 10(1), 66–70 (2010).
Chrispinus, S. M. Anaemia in pregnancy: Prevalence and possible risk factors in Kakamega county, Kenya. Sci. J. Public Health. 2(3), 216 (2014).
Wang, J. & Maduako, I. N. Spatio-temporal urban growth dynamics of Lagos Metropolitan Region of Nigeria based on Hybrid methods for LULC modeling and prediction. Eur. J. Remote Sens. 51(1), 251–265 (2018).
Anorlu, R. I., Oluwole, A. A. & Abudu, O. O. Sociodemographic factors in anaemia in pregnancy at booking in Lagos, Nigeria. J. Obstet. Gynaecol. 26(8), 773–776 (2006).
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H. & Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 27(2), 89–94 (2016).
Al-Farsi, Y. M. et al. Effect of high parity on occurrence of anemia in pregnancy: A cohort study. BMC Pregnancy Childbirth. 20(11), 7 (2011).
Gedefaw, L., Ayele, A., Asres, Y. & Mossie, A. Anemia and associated factors among pregnant women attending antenatal care clinic in Wolayita Sodo Town, Southern Ethiopia. Ethiop. J. Health Sci. 25(2), 155–162 (2015).
Agrawal, S. & Singh, A. Obesity or underweight—what is worse in pregnancy?. J. Obstet. Gynaecol. India. 66(6), 448–452 (2016).
Liu, X. et al. Effect of pre-pregnancy body mass index on adverse pregnancy outcome in north of China. Arch. Gynecol. Obstet. 283(1), 65–70 (2011).
Balarajan, Y., Ramakrishnan, U., Özaltin, E., Shankar, A. H. & Subramanian, S. Anaemia in low-income and middle-income countries. Lancet. 378(9809), 2123–2135 (2011).
Cepeda-Lopez, A. C., Aeberli, I. & Zimmermann, M. B. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int. J. Vitam. Nutr. Res. 80(4–5), 263–270 (2010).
McClung, J. P. & Karl, J. P. Iron deficiency and obesity: The contribution of inflammation and diminished iron absorption. Nutr. Rev. 67(2), 100–104 (2009).
Noronha, J. A., Bhaduri, A., Bhat, H. V. & Kamath, A. Maternal risk factors and anaemia in pregnancy: A prospective retrospective cohort study. J. Obstet. Gynaecol. 30(2), 132–136 (2010).
Siddiqui, F., Salam, R. A., Lassi, Z. S. & Das, J. K. The intertwined relationship between malnutrition and poverty. Front. Public Health. 8, 453 (2020).
Breymann, C. & Auerbach, M. Iron deficiency in gynecology and obstetrics: Clinical implications and management. Hematol. Am. Soc. Hematol. Educ. Program. 2017(1), 152–159 (2017).
Daru, J. et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. Lancet Glob. Health. 6(5), e548–e554 (2018).
Abd Rahman, R., Idris, I. B., Isa, Z. M., Rahman, R. A. & Mahdy, Z. A. The prevalence and risk factors of iron deficiency anemia among pregnant women in Malaysia: A systematic review. Front. Nutr. 15(9), 847693 (2022).
Acknowledgements
Our deepest gratitude to the Johnson & Johnson Global Public Health R&D Capacity Development Team for their contribution to the development of the Predict-PPH study protocol. We would also like to express our appreciation to the personnel at the Research Management Office of the College of Medicine, University of Lagos, for their support in obtaining the funding for this study.
Funding
This study was funded through an Education Grant by the Johnson and Johnson Foundation (Scotland) and a seed grant by the Bill and Melinda Gates Foundation. The content of this paper is solely the responsibility of the author and does not necessarily represent the official views of the Johnson and Johnson Foundation (Scotland) and the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, or preparation and the decision to publish the manuscript.
Author information
Authors and Affiliations
Contributions
K.S.O., F.O.O., and A.A.O. contributed to the study's conception and design. Material preparation, data collection and analysis were performed by K.S.O., F.O.O., O.A.O., Y.A.O., A.O.U., A.O., A.A.A., M.A.A., T.O., I.Y.A., V.A., A.C.O., A.O.O., H.A., and O.O.A. The first draft of the manuscript was written by K.S.O., O.A.O., A.A.A. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okunade, K.S., Olowoselu, F.O., Oyedeji, O.A. et al. Prevalence and determinants of moderate-to-severe anaemia in the third trimester of pregnancy: a multicenter cross-sectional study in Lagos, Nigeria. Sci Rep 14, 11411 (2024). https://doi.org/10.1038/s41598-024-61487-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61487-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.