« Prev Next »
Epistasis and the Shifting Balance Theory
Throughout his career, Wright made many contributions to the study of the relationship between genotype and phenotype using guinea pigs as a model system. In particular, he wondered how multiple genes interacted to produce specific phenotypes, and he explored several possible mechanisms by which this process occurred. One possibility was that the combined effect of the alleles at two different loci was equal to the sum of these alleles' individual effects; this idea was known as the additive model. Another possibility was that that the combined effect of the alleles at two different loci was either greater or less than the sum of the alleles' individual effects; this idea was known as the nonadditive model or epistasis. Wright was struck by the strong and pervasive nonadditive genetic interactions that he saw in his guinea pigs. Thus, in his views of the evolutionary process, Wright continually emphasized the importance of considering the effects of combinations of genes rather than just genes' individual effects (Wright, 1968, 1977, 1978).
In particular, Wright correctly recognized that epistasis could complicate the evolutionary process. Consider the haploid situation in which individuals with genotype ab have a fitness of 1, and individuals with genotype AB have a fitness of 1.1. If Ab individuals and aB individuals have fitnesses somewhere between 1 and 1.1, then natural selection permits the evolutionary transition of a population from the ancestral genotype ab to the derived genotype AB, because both of the intermediate genotypes (Ab and aB) have higher fitnesses than the ancestral genotype. Similarly, if the fitness of Ab individuals is between 1 and 1.1 but the fitness of aB individuals is less than 1 (or vice versa), AB could still evolve in the population because one transition step is possible; however, the population would be constrained to evolve via the intermediate genotype that had a higher fitness than the ancestral genotype ab. But what happens in the case of strong epistasis in which the fitnesses of the Ab and aB genotypes are both less than 1? In this case, how could a population consisting of only ab individuals evolve to one consisting of only AB individuals? Remember, natural selection does not "think ahead"; thus, it seems logical to conclude that the two transitional genotypes (Ab and aB) with lower fitness would be selected against. In this situation, epistasis would act as an evolutionary constraint, forestalling the evolution of the most-fit genotype.
Tables 1 and 2 illustrate how the additive and epistatic models of gene interaction have different effects on haploid fitness levels. In these two tables, the italicized letters represent genotypes, the fitnesses of which are presented in parentheses. As you can see, in the additive case (Table 1), the change from allele a to allele A increases overall fitness by 0.06 regardless of the allele present at locus B, while the change from allele b to allele B increases overall fitness by 0.04 regardless of the allele present at locus A. On the other hand, in the epistasis case (Table 2), the fitness effect of changing from allele a to allele A depends on the allele at locus B. Specifically, if allele B is present, changing from allele a to allele A increases fitness by 0.15, but if allele b is present, changing from allele a to allele A decreases fitness by 0.10. Likewise, the fitness effect of changing from allele b to allele B depends upon the allele present at locus A.
Table 1: Example Fitness Levels in an Additive Model of Gene Interaction
| B | b | |
| A | AB (1.10) | Ab (1.06) |
| a | aB (1.04) | ab (1.00) |
Table 2: Example Fitness Levels in an Epistatic Model of Gene Interaction
| B | b | |
| A | AB (1.10) | Ab (0.90) |
| a | aB (0.95) | ab (1.00) |
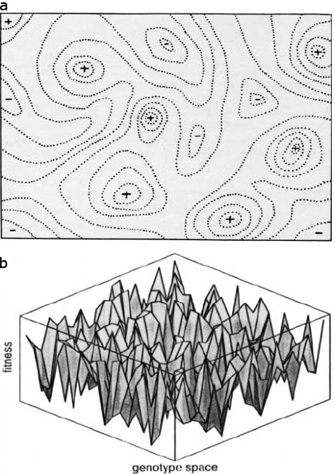
When interactions involve more than two genetic loci, the limiting effects of epistasis tend to increase. These more complicated systems are also more difficult to visualize and analyze. To assist in the visualization of such systems, Wright invented the metaphor of the adaptive landscape, which has been used by countless biologists since (Skipper, 2004). In figures of adaptive landscapes, the height represents the average fitness of a population with the underlying allelic frequencies. Following Wright, biologists have often displayed adaptive landscapes with contour lines connecting points of equal fitness, much like the lines drawn on elevation maps that connect points of equal altitude or isotherms on weather maps that connect lines of equal temperature (Figure 1).
Most forms of natural selection will push populations upward on the adaptive landscape toward genotypes of higher fitness. In contrast, random genetic drift and mutation can cause populations to spread around peaks, and possibly even to go into and through adaptive valleys. But what happens if a population gets stuck on one adaptive peak that is higher than surrounding regions yet is not the highest peak possible? Wright sought to answer this very question, and in doing so, he developed the shifting balance theory.
Elements of the Shifting Balance Theory
The shifting balance theory explains how populations that are caught on suboptimal peaks in adaptive landscapes can move across regions of low fitness (adaptive valleys) and subsequently up higher fitness peaks. This theory is synthetic because it depends on several evolutionary forces—natural selection, random genetic drift, and migration—acting together, and it requires both gene interactions and genetic differentiation among populations.
At its core, the shifting balance theory is a process that permits populations to hold onto selective gains (i.e., local fitness peaks in the adaptive landscape) while still being able to randomly explore neighboring genotype space for possibly superior peaks. Wright's solution to these conflicting aims was population subdivision. He thought that many, if not most, species were subdivided into small populations that exchanged only a few migrants with each other but were not completely isolated (Wright, 1931, 1932, 1977, 1978). Because of the small size of each of these populations, genetic drift would have a significant effect on the genetic composition of each, thus allowing the populations to differentiate genetically by an appreciable amount. In this way, each of the populations would act as a small experiment in evolution (Wade & Goodnight, 1998).
The shifting balance theory consists of three distinct phases (Wright, 1977):
- Phase 1, the exploratory phase, is characterized by the action of genetic drift. As a result, the small populations move around in genotypic space. Most stay on the suboptimal fitness peak, but some get caught in adaptive valleys.
- In phase 2, natural selection causes the populations that are in the adaptive valleys to move through genotypic space toward new, higher-fitness peaks.
- Finally, in phase 3, those populations that are at higher fitness peaks send off migrants to the other populations, and these migrants cause the other populations to move to the higher fitness peaks. Eventually, all of the populations (the metapopulation) move to the higher fitness peak as a result of this process.
Wright's theory was met with skepticism by a number of other researchers, including Ronald Fisher. Although Fisher recognized that nonadditive gene interactions were indeed common, he did not see these interactions as being important in the evolutionary process. Furthermore, because Fisher thought that most populations were large and more or less uniform in their genetic composition, what mattered most to his view of evolution was the effect on fitness of an allele averaged over all genetic backgrounds. Hence, the existence of nonadditive genetic interactions was almost irrelevant in Fisher's theory of evolution. Fisher also argued that ecological factors would change the adaptive landscape faster than populations could shift between peaks via the shifting balance theory. In other words, according to Fisher, hills would become valleys and valleys would become hills faster than populations could move from one hill to the next (Fisher, 1958).
Testing the Shifting Balance Theory
Wright's shifting balance theory continues to be controversial among evolutionary biologists, and the concerns first raised by Fisher in the 1930s still linger today. Moreover, due to the complexities of Wright's model, it is exceedingly difficult to test all aspects of the shifting balance theory at once, especially in natural populations. Individual aspects of this theory have been tested, however.
For instance, in the early 1990s, investigators Michael Wade and Charles Goodnight performed an experimental study that mimicked some aspects of phase 3 (migration) in the shifting balance process using laboratory populations of the flour beetle Tribolium castaneum (Figure 2). For each experimental treatment, Wade and Goodnight (1991) set up 50 subpopulations that each consisted of 20 beetles from the same founding population. They next scored the total number of offspring that resulted from each of these subpopulations as the subpopulation's productivity. Then, Wade and Goodnight took beetles from the individual subpopulations that had productivities above the average productivity of all subpopulations, and they placed these beetles in a migrant pool. This was done in relation to the subpopulation's productivity, such that the subpopulations with the highest productivity contributed the most to the migrant pool, while those with productivities just above average contributed little to the pool. Similarly, subpopulations with below-average productivity received migrants from the migrant pool in accordance with their productivity levels; those subpopulations with the lowest productivity received the most beetles from the migrant pool, while those with productivities just below the average received few insects from the pool. This process was repeated every generation. In addition, Wade and Goodnight set up controls wherein subpopulations contributed to the migrant pool and received beetles from the pool, but their contributions and receipt amounts were random with respect to the productivity of the subpopulations. Thus, the controls had exactly the same number of migrants as the experimental groups, but this migration was random with respect to the productivity of the subpopulations. Finally, Wade and Goodnight also performed the same procedure with migration occurring either every other generation or every third generation.
In conducting these experiments, Wade and Goodnight observed that the average productivity of the subpopulations under experimental conditions (those whose contributions to the migrant pool were based on productivity) increased relative to that of the control subpopulations (those whose contributions to the migrant pool were random with respect to productivity). These responses were observed no matter whether migration occurred every generation, every other generation, or every third generation; however, it was most pronounced when migration occurred every other generation. From these results, Wade and Goodnight argued that selection among the subpopulations was in fact driving higher productivity of the entire population, much as Wright envisioned in his description of phase 3.
A History of Scientific Scrutiny and Skepticism
Despite the results of studies such as that conducted by Wade and Goodnight, debate continues regarding Wright's theory. For instance, in 1997, Jerry Coyne and colleagues wrote a perspective for the journal Evolution criticizing the shifting balance theory on both theoretical and empirical grounds. Coyne et al. noted that the conditions under which the shifting balance process appears to operate via Wright's phases are restrictive. Indeed, even advocates of the importance of the shifting balance theory recognize the conflict between the different phases of the theory. For instance, Wade and Goodnight (1991) noted that the conditions that favor phase 1 restrict the likelihood that phase 2 will operate as described by Wright.
Of course, the main empirical criticism of the shifting balance theory raised by Coyne et al. (1997) relates to the absence of a demonstration of all three phases at work in a natural population. Wade and Goodnight (1998) countered this objective with a perspective, also published in the journal Evolution, contrasting the Fisherian and Wrightian modes of evolution. In their article, Wade and Goodnight contended that although the shifting balance process may not work exactly as Wright had envisioned, a great deal of evidence supports each of the components of Wright's theory. In fact, there is considerable and growing evidence for the existence of multiple fitness peaks arising from epistasis in natural populations (Whitlock et al., 1995; Weinreich et al., 2005), and populations of species are often genetically differentiated (Wade & Goodnight, 1998; Connor & Hartl, 2004).
Extensions and refinements of the shifting balance model continue yet today. Very recently, Burton and Travis (2008) presented a different version of the shifting balance process wherein they suggested range expansion as the cause of shifts in adaptive peaks. It is well known that deleterious mutations accumulate in populations at the front of a range expansion due to high levels of random genetic drift. Such deleterious mutations are said to "surf" on the advancing wave of a population expansion, and they can often persist at reasonably high frequencies (Excoffier & Ray, 2008). Burton and Travis (2008) reasoned that the deleterious mutations at the expanding fronts of populations would present opportunities for new advantageous alleles that worked well with the deleterious mutations to also increase in frequency via selection, and thus cause a peak shift. Computer simulation results by Burton and Travis support this idea.
Debates regarding Fisherian and Wrightian views of the evolutionary process are likely to continue for quite some time. However, it is probable that both views are correct in different contexts. As Wade and Goodnight (1998) point out, Fisher and Wright were trying to explain different facets of the evolutionary process. In Wade and Goodnight's words, "[t]he central problem of evolution for Wright was explaining the origins of adaptive novelty, whereas for Fisher it was explaining the refinement of existing adaptations."
References and Recommended Reading
Connor, J. K., & Hartl, D. L. A Primer of Ecological Genetics (Sunderland, MA, Sinauer Associates, 2004)
Coyne, J. A., et al. Perspective: A critique of Sewall Wright's shifting balance theory of evolution. Evolution 51, 643–671 (1997)
Excoffier, L., & Ray, N. Surfing during population expansions promotes genetic revolutions and structuration. Trends in Ecology and Evolution 23, 347–351 (2008)
Fisher, R. A. The Genetical Theory of Natural Selection, 2nd ed.(New York, Dover, 1958)
Kauffman, S. A., & Levin, S. Towards a general theory of adaptive walks on rugged landscapes. Journal of Theoretical Biology 128, 11–45 (1987)
Skipper, R. A., Jr. The heuristic role of Sewall Wright's 1932 adaptive landscape diagram. Philosophy of Science 71, 1176–1188 (2004)
Wade, M. J., & Goodnight, C. J. Wright's shifting balance theory: An experimental study. Science 253, 1015–1018 (1991)
———. Perspective: The theories of Fisher and Wright in the context of metapopulations: When nature does many small experiments. Evolution 52, 1537–1553 (1998)
Weinreich, D. M., et al. Perspective: Sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59, 1165–1174 (2005)
Whitlock, M. C., & Phillips, P. C. The exquisite corpse: A shifting view of the shifting balance. Trends in Ecology and Evolution 15, 347–348 (2000)
Whitlock, M. C., et al. Multiple fitness peaks and epistasis. Annual Review of Ecology and Systematics 26, 601–629 (1995)
Wright, S. Evolution in Mendelian populations. Genetics 16, 97–159 (1931)
———. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proceedings of the Sixth International Congress on Genetics 1, 356–366 (1932)
———. Evolution and the Genetics of Populations. Vol. 1: Genetic and Biometric Foundations (Chicago, University of Chicago Press, 1968)
———. Evolution and the Genetics of Populations. Vol. 2: The Theory of Gene Frequencies (Chicago, University of Chicago Press, 1969)
———. Evolution and the Genetics of Populations. Vol. 3: Experimental Results and Evolutionary Deductions (Chicago, University of Chicago Press, 1977)
———. Evolution and the Genetics of Populations. Vol.4: Variability Within and Among Natural Populations (Chicago, University of Chicago Press, 1978)



 Figure 1: Wright's adaptive landscapes, then and now.
Figure 1: Wright's adaptive landscapes, then and now.