« Prev Next »
Coronary artery disease is increasing in prevalence worldwide, and it is currently the leading cause of mortality in industrialized nations. This disease is partially explained by genetics, and it is known to run in families (Watkins & Farrall, 2006). However, lifestyle and environmental factors, such as diet, also contribute to coronary artery disease and heart failure. Thus, this disorder shows multifactorial inheritance, because both genetic and environmental factors trigger its onset. But what are the distinguishing factors of this and other multifactorial disorders? Over time, the answer to this question has become increasingly clear.
Early Research in Multifactorial Inheritance
The first scientist to study multifactorial inheritance was Francis Galton, Charles Darwin's cousin. Like his contemporary, Gregor Mendel, Galton studied the inheritance of traits. However, unlike Mendel, Galton observed what he called "blending" characters (Galton, 1897). Blending is now known as continuous variation, describing a gradation in expression in which phenotypes (such as human height) do not fall into distinct categories. When a trait that exhibits continuous variation is plotted on a graph, the phenotypic distribution forms a bell-shaped curve. Accordingly, most individuals have an intermediate phenotype, and the majority of individuals group at the mean (Mossey, 1999). Traits with continuous variation are also called quantitative traits. With these traits, involvement of a wide range of genetic and environmental factors results in the production of wide-ranging genotypes.
In contrast, for some traits with multifactorial inheritance, there is no gradation. Mendel studied these so-called "nonblending" traits, placing them in distinct categories (Olby, 2000). These traits show discontinuous variation, which occurs when there is an abrupt change from one phenotype to another, as in Mendel's round and wrinkled peas. Here, there was no intermediate variation between the round and wrinkled peas; rather, the peas fell into either one category or the other. Another example of a trait that shows discontinuous variation is the human ABO blood antigen system.
For years, followers of Mendel and Galton argued over whether the Mendelian theory or the Galtonian theory correctly described inheritance in humans. Although Mendelian inheritance correctly described some human diseases and traits, it did not describe all of them. Likewise, the Galtonian theory did not fit all observations. Today, we know that both theories correctly describe inheritance patterns for different traits, including various human diseases. The question of whether a disease exhibits Mendelian or Galtonian patterns simply depends on which disease we are studying.
What Are the Characteristics of a Multifactorial Disease?
A multifactorial disease has a combination of distinctive characteristics that can be differentiated from clear-cut Mendelian or sex-limited conditions. These traits include the following:
- The disease can occur in isolation, with affected children born to unaffected parents. Although familial aggregation is also common (i.e., there may be multiple cases in the same family), there is no clear Mendelian pattern of inheritance.
- Environmental influences can increase or decrease the risk of the disease.
- The disease occurs more frequently in one gender than in the other, but it is not a sex-limited trait. In addition, first-degree relatives of individuals belonging to the more rarely affected gender have a higher risk of bearing the disease (International Commission on Radiological Protection, 2000).
- The concordance rates in monozygotic and dizygotic twins contradict Mendelian proportions. A concordance rate is a measure of the rate at which both twins bear a specific disease (Mossey, 1999; Griffiths et al., 1999).
- The disease occurs more frequently in a specific ethnic group (i.e., Caucasians, Africans, Asians, Hispanics, etc.).
Going back to the example of coronary artery disease, we know that there are a number of factors that increase the risk of disease onset, including obesity, type II diabetes, high blood pressure, high levels of low-density lipoprotein cholesterol, and even gum disease (Myers et al., 1990; Watkins & Farrall, 2006; Williams et al., 2008; Wilson et al., 1998). Although coronary artery disease runs in families, it does not show Mendelian inheritance patterns and can occur in isolation (Yusuf et al., 2004). Coronary artery disease also occurs more often in men than women (Zdravkovic et al., 2002), and its risk is higher among African Americans than among Caucasians or Asians (Clark & Emerole, 1995). All of these characteristics are consistent with the classification of coronary artery disease as a multifactorial disorder.
Are Multifactorial Diseases Continuous or Discontinuous?
Recall that traits that fall into discrete categories are referred to as discontinuous, while those that display a gradient of phenotypes are classified as continuous. Interestingly, there are many diseases that result from discontinuous variation that show complex phenotypes resembling continuous variation (Griffiths et al., 1999). Scientists propose that this is because there is a base of continuous variation on which the susceptibility to a disease develops. According to this theory, a disease develops and is expressed only after a certain critical liability threshold is reached. The further the liability threshold is surpassed, the more severe the disease phenotype is (Mossey, 1999). In contrast, an individual who does not reach the liability threshold will never develop the disease. Therefore, an individual either has the disease or does not, and the disease shows discontinuous variation.
An example of how the liability threshold works can be seen in individuals with cleft lip and palate. Cleft lip and palate is a birth defect in which an infant is born with unfused lip and palate tissues. An individual with cleft lip and palate can have unaffected parents who do not seem to have a family history of the disorder. Despite the fact that the child's parents may not have the disorder, they may have contributed some underactive genes that are required for lip and palate formation. Indeed, there seems to be a genetic component to this defect, because the incidence of cleft lip and palate is higher in families with an affected child (Mossey, 1999). Additionally, some nutritional deficiencies and maternal cigarette smoking are associated with this birth defect, so environmental factors are also involved (Ericson et al., 1979; Wilcox et al., 2007). When an individual is born with cleft lip and palate, the contributing factors for this condition have surpassed the liability threshold. If the threshold is exceeded by a fair amount, the birth defect increases in severity. In these cases, it becomes more likely that other family members are also affected (Mossey, 1999). Cleft lip and palate is thus a multifactorial disorder with discontinuous variation.
Continuing Studies in Multifactorial Inheritance
In humans, there are many other disorders that show multifactorial inheritance patterns, such as multiple sclerosis, diabetes, asthma, cancer, and numerous birth defects. All of these diseases are due to a complex interaction of genetic factors, such as copy number variation, epistatic interactions, and modifier effects, as well as various environmental factors. In cases with discontinuous trait variation, this multitude of factors may or may not exceed the liability threshold, making it a challenge to predict whether disease will result.
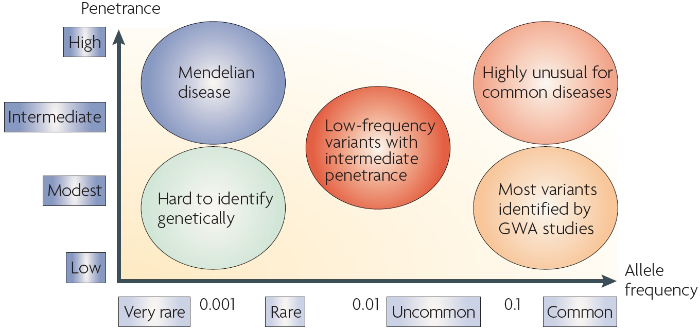
Numerous methods have been developed to study complex disorders. One of the more promising methods is the use of genome-wide association studies (GWAS) that identify the common genetic factors that underlie major complex disorders (Figure 1). Nonetheless, there is still much to be learned about the causes and nature of various multifactorial conditions.
References and Recommended Reading
Clark, L. T., & Emerole, O. Coronary heart disease in African Americans: Primary and secondary prevention. Cleveland Clinic Journal of Medicine 62, 285–292 (1995)
Ericson, A., et al. Cigarette smoking as an etiologic factor in cleft lip and palate. American Journal of Obstetrics and Gynecology 135, 348–351 (1979)
Galton, F. The average contribution of each several ancestor to the total heritage of the offspring. Proceedings of the Royal Society 61, 401–413 (1897)
Griffiths, A. J. F., et al. Modern Genetic Analysis. E-book available online (New York, W. H. Freeman, 1999)
International Commission on Radiological Protection. Publication 83: Risk Estimation for Multifactorial Diseases (Stockholm, International Commission on Radiological Protection, 2000)
McCarthy, M. I., et al. Genome-wide association studies for complex traits: Consensus, uncertainty and challenges. Nature Reviews Genetics 9, 356–369 (2008) (link to article)
Mossey, P. A. The heritability of malocclusion: Part 1—Genetics, principles and terminology. British Journal of Orthodontics 26, 103–113 (1999)
Mossey, P. A., & Little, J. Epidemiology of oral clefts: An international perspective. In Cleft Lip and Palate: From Origin to Treatment, ed. D. F. Wyszynski (Oxford, Oxford University Press, 2002), 127–158
Myers, R. H., et al. Parental history is an independent risk factor for coronary artery disease: The Framingham study. American Heart Journal 120, 963–969 (1990)
Olby, R. C. Horticulture: The font for the baptism of genetics. Nature Reviews Genetics 1, 65–70 (2000) doi:10.1038/35049583 (link to article)
Watkins, H., & Farrall, M. Genetic susceptibility to coronary artery disease: From promise to progress. Nature Reviews Genetics 7, 163–173 (2006) doi:10.1038/nrg1805 (link to article)
Wilcox, A. J., et al. Folic acid supplements and risk of facial clefts: National population based case-control study. British Medical Journal 334, 464 (2007)
Williams, R. C., et al. The potential impact of periodontal disease on general health: A consensus view. Current Medical Research and Opinion 24, 1635–1643 (2008).
Wilson, P. W., et al. Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847 (1998)
Yusuf, S., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364, 937–952.
Zdravkovic, S., et al. Heritability of death from coronary heart disease: A 36-year follow-up of 20,966 Swedish twins. Journal of Internal Medicine 252, 247–254 (2002)






























