Abstract
Objective:
The objective of this study was to evaluate possible influences of parenteral nutrition on growth and bone development in preterms and to search for markers of bone status.
Study Design:
Metacarpus bone transmission time (mc-BTT) was performed at birth, 21 days and 36 weeks of gestational age (GA) in preterms, receiving two different nutritional regimens, together with biochemical analysis.
Result:
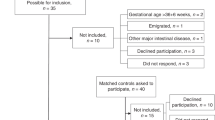
A total of 234 patients were studied. Newborns with aggressive nutrition had significantly better growth rate and higher values of mc-BTT until discharge. Mc-BTT at day 21 correlates positively with nutritional intakes and phosphatemia; lower limb length positively correlated with mc-BTT (P<0.01). Newborns with low energy intake in the first week of life (<70 kcal kg−1 per day) and low serum phosphate level (<1.4 mmol l−1) at 21 days had lower mc-BTT at 36 weeks of GA (P<0.01).
Conclusion:
Aggressive parenteral intakes in preterms improve growth and bone status in the short-medium term, suggesting that early nutrition could influence bone development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
American Academy of Pediatrics Commitee on Nutrition: Nutritional needs of low-birth-weight infants. Pediatrics 1985; 75: 976–986.
Embleton NE, Pang N, Cooke RJ . Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001; 107: 270–273.
Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK . Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117: 1253–1261.
Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ et al. Early nutrition mediates the influence of severity of illness on extremely low birth weight infants. Pediatr Res 2011; 69 (6): 522–529.
Scattolin S, Gaio P, Betto M, Patron S, De Terlizzi F, Intini F et al. Parenteral amino acid intakes: possible influences of higher intakes on growth and bone status in preterm infants. J Perinatol 2012; 69: 1–7.
Hay WW Jr . Strategies for feeding the preterm infant. Neonatology 2008; 94: 245–254.
Ziegler EE, O’Donnel AM, Nelson SE, Fomon SJ . Body composition of the reference fetus. Growth 1976; 40: 329–341.
Salle BL, Braillon P, Glorieux FH et al. Lumbar bone mineral content measured by dual energy x-ray absorptiometry in newborns and infants. Acta Paediatr 1992; 81: 953–958.
Javaid MK, Cooper C . Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab 2002; 16 (2): 49–367.
Rohana J, Hasmawati J, Zulkifli SZ . Risk factors associated with low bone mineral content in very low birth weight infants. Singapore Med J 2007; 48 (3): 191–194.
So K, Ng P . Treatment and prevention of neonatal osteopenia. Curr Paediatr 2005; 15: 106–113.
Viswanathan S, Khasawneh W, McNelis K, Dykstra C, Amstadt R, Super DM et al. Metabolic bone disease: a continued challenge in extremely low birth weight infants. J Parenter Enteral Nutr 2013; 38 (8): 1–9.
Rauch F, Schoenau E . The developing bone: slave or master of its cells and molecules? Pediatr Res 2001; 50: 309–314.
Rauch F, Schoenau E . Skeletal development in premature infants: a review of bone physiology beyond nutritional aspects. Arch Dis Child Fetal Neonatal Ed 2002; 86: F82–F85.
Bozzetti V, Tagliabue P . Metabolic bone disease in preterm newborn: an update on nutritional issue. Ital J Pediatr 2009; 35: 20.
Dabezies EJ, Warren PD . Fractures in very low birth weight infants with richets. Clin Orthop Relat Res 1997; 335: 233–239.
Visser F, Spij AJ, Brus F . The validity of biochemical markers in metabolic bone disease in preterm infants: a systematic review. Acta Paediatrica 2012; 101: 562–568.
Tomlinson C, McDevitt H, Ahmed SF, White MP . Longitudinal changes in bone health as assessed by the speed of sound in very low birth weight preterm infants. J Pediatr 2006; 148: 450–455.
Mitchell SM, Rogers SP, Hicks PD, Hawthorne KM, Parker BR, Abrams SA . High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatrics 2009; 9: 47.
Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, De Curtis M . Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr 1998; 27: 184–190.
McDevitt H, Ahmed SF . Quantitative ultrasound assessment of bone health in the neonate. Neonatology 2007; 91: 2–11.
DeTerlizzi F, Battista S, Cavani F, Canè V, Cadossi R . Influence of bone tissue density and elasticity on ultrasound propagation: an in vitro study. J Bone Mineral Res 2000; 15: 2458–2466.
Rubinacci A, Moro GE, Boehm G, De Terlizzi F, Moro GL, Cadossi R . Quantitative ultrasound for the assessment of osteopenia in preterm infants. Eur J Endocrinol 2003; 149: 307–315.
Ritschl E, Wehmeijer K, De Terlizzi F, Wipfler E, Cadossi R, Douma D et al. Assessment of skeletal development in preterm and term infants by quantitative ultrasound. Pediatr Res 2005; 58: 1–7.
Rack B, Lochmüller EM, Janni W, Lipowsky G, Engelsberger I, Friese K et al. Ultrasound for the assessment of bone quality in preterm and term infants. J Perinatol 2012; 32: 218–226.
Harrison CM, Gibson AT . Osteopenia in preterm infants. Arch Dis Child Fetal Neonatal 2013; 98: F272–F275.
Bowden LS, Jones CJ, Ryan SW . Bone mineralization in ex-preterm infants aged 8 years. Eur J Pediatr 1999; 158 (8): 658–661.
Fewtrell MS, Cole TJ, Bishop NJ, Lucas A . Neonatal factors predicting childhod height in preterm infants: evidence for a persisting effect of early metabolic bone disease? J Pediatr 2000; 137: 668–673.
Hernandez CJ, Beaupré GS, Carter DR . A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int 2003; 14 (10): 843–847.
Barker DJP . The fetal origins of adult disease. Proc R Soc Lond 1995; 262: 37–43.
Betto M, Gaio P, Ferrini I, De Terlizzi F, Zambolin M, Scattolin S et al. Assessment of bone health in preterm infants through quantitative ultrasound and biochemical markers. J Matern Fetal Neonatal Med 2013; 27 (13): 1343–1347.
Aladangady N, Coen PG, White MP, Rae MD, Beattie TJ . Urinary excretion of calcium and phosphate in preterm infants. Pediatr Nephrol 2004; 19: 1225–1231.
Clark RH, Thomas P, Peabody J . Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003; 111: 986–990.
Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999; 104: 280–289.
Van Goudoever JB, Colen T, Wattimena JLD, Huijmans JGM, Carnielli VP, Sauer PJ . Immediate commencement of amino acid supplementation in preterm infants: effect on serum amino acid concentrations and protein kinetics on the first day of life. J Pediatr 1995; 127: 458–465.
Thureen PT, Melara D, Fennessey PV, Hay WW . Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res 2003; 53: 24–32.
Ridout E, Melara D, Rotinghaus S, Thureen PJ . Blood urea nitrogen concentration as a marker of amino acid intollerance in neonates with birthweight less than 1250g. J Perinatol 2005; 25: 130–133.
Bass SL, Eser P, Daly R . The effect of exercise and nutrition on the mechanostat. J Musculoskelet Neuronal Interact 2005; 5: 239–254.
Klein GL . Metabolic bone disease of total parenteral nutrition. Nutrition 1998; 14: 149–152.
Bouchier D, Weston PJ . Metabolic acidosis in the first 14 days of life in infants of gestation less than 26 weeks. Eur J Pediatr 2014; 174 (1): 49–54.
Kalhoff H, Diekmann L, Rudloff S, Manz F . Renal excretion of calcium and phosphorus in premature infants with incipient late metabolic acidosis. J Pediatr Gastroenter Nutr 2001; 33: 565–569.
Bonsante F, Iacobelli S, Latorre G, Rigo J, De Felice C, Robillard PY et al. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants-It is time to change composition of the early parenteral nutrition. PLoS One 8 (8): e72880.
Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP . Urinary phoshate/creatinine, calcium/creatinine and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr 1997; 131: 252–257.
Figueras-Aloy J, Álvarez-Domínguez E, Pérez-Fernández JM, Moretones-Suñol G, Vidal-Sicart S, Botet-Mussons F . Metabolic bone disease and bone mineral density in very preterm infants. J Pediatr 2014; 164: 499–504.
Backstrom MC, Kouri T, Kuusela AL, Sievanen H, Koivisto AM, Ikonen RS et al. Bone isoenzyme of serum alcaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr 2000; 89 (7): 867–873.
Tinnion RJ, Embleton ND . How to use… alkaline phosphatase in neonatology. Arch Dis Child Educ Pract 2012; 97: 157–163.
Hung YL, Chen PC, Jeng SF, Hsieh CJ, Peng SS, Yen RF et al. Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J Paediatr Child Health 2011; 47: 134–139.
Faerk J, Peitersen B, Petersen S, Michaelsen KF . Bone mineralization in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch Dis Child Fetal Neonatal 2002; 87: F133–F136.
Gibson AT, Pearse RG, Wales JKH . Knemometry and the assessment of growth in premature infant. Archi Dis Child 1993; 69: 498–504.
Mercy J, Dillon B, Morris J, Emmerson AJ, Mughal MZ . Relationship of tibial speed of sound and lower limb lenght to nutrient intake in preterm infants. Arch Dis Child Fetal Neonatal Ed 2007; 92: F381–F385.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Meneghelli, M., Pasinato, A., Salvadori, S. et al. Bone status in preterm infant: influences of different nutritional regimens and possible markers of bone disease. J Perinatol 36, 394–400 (2016). https://doi.org/10.1038/jp.2015.212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.212