Abstract
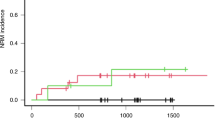
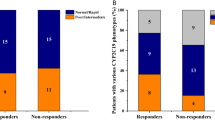
Cytochrome P450 enzymes (CYPs) and flavin-containing monooxygenases (FMOs) likely have a role in the oxidation of intermediate metabolites of busulfan (Bu). In vitro studies to investigate the involvement of these enzymes are cumbersome because of the volatile nature of the intermediate metabolite tetrahydrothiophene (THT) and the lack of sensitive quantitation methods. This study explored the association between the CYP2C9, CYP2C19, CYP2B6 and FMO3 genotypes and sulfolane (Su, a water soluble metabolite of Bu) plasma levels in children undergoing hematopoietic stem cell transplantation (HSCT). The relationship between these genotypes and the effectiveness of myeloablative conditioning was also analyzed. Sixty-six children receiving an intravenous Bu-based myeloablative conditioning regimen were genotyped for common functional variant alleles in CYP2C9 (*2 and *3), CYP2C19 (*2 and *17), FMO3 (rs2266780, rs2266782 and rs1736557) and CYP2B6 (*5 and *9). The plasma levels of Bu and its metabolite Su were measured after the ninth Bu dose in a subset of 44 patients for whom plasma samples were available. The ratio of Bu to Su was considered the metabolic ratio (MR) and was compared across the genotype groups. Higher MRs were observed in CYP2C9*2 and *3 allele carriers (mean±s.d.: 7.8±3.6 in carriers vs 4.4±2.2 in non-carriers; P=0.003). An increased incidence of graft failure was observed among patients with an MR>5 compared with those with MR values <5 (20% vs 0%; P=0.02). In contrast, a significantly higher incidence of relapse and graft failure (evaluated as event-free survival) was observed in patients with malignant disease who carried CYP2B6 alleles with reduced function on both chromosomes compared with carriers of at least one normal allele (100% vs 40%; P=0.0001). These results suggest that CYP2C9 has a role in the oxidation reactions of THT and indicate that it may be possible to predict the efficacy of Bu-based myeloablative conditioning before HSCT on the basis of CYP genotypes and Bu MRs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schattenberg AV, Levenga TH . Differences between the different conditioning regimens for allogeneic stem cell transplantation. Curr Opin Oncol 2006; 18: 667–670.
Czerwinski M, Gibbs JP, Slattery JT . Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos 1996; 24: 1015–1019.
Cooper AJ, Younis IR, Niatsetskaya ZV, Krasnikov BF, Pinto JT, Petros WP et al. Metabolism of the cysteine S-conjugate of busulfan involves a beta-lyase reaction. Drug Metab Dispos 2008; 36: 1546–1552.
Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol 1989; 36: 525–530.
Malar R, Sjoo F, Rentsch K, Hassan M, Gungor T . Therapeutic drug monitoring is essential for intravenous busulfan therapy in pediatric hematopoietic stem cell recipients. Pediatr Transplant 2011; 15: 580–588.
Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Theoret Y, Champagne MA et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant 2010; 45: 261–267.
Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R . Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos 2001; 29: 264–267.
Zwaveling J, Press RR, Bredius RG, van Derstraaten TR, den Hartigh J, Bartelink IH et al. Glutathione S-transferase polymorphisms are not associated with population pharmacokinetic parameters of busulfan in pediatric patients. Ther Drug Monit 2008; 30: 504–510.
Ansari M, Krajinovic M . Can the pharmacogenetics of GST gene polymorphisms predict the dose of busulfan in pediatric hematopoietic stem cell transplantation? Pharmacogenomics 2009; 10: 1729–1732.
ten Brink MH, Wessels JA, den Hartigh J, van der Straaten T, von dem Borne PA, Guchelaar HJ et al. Effect of genetic polymorphisms in genes encoding GST isoenzymes on BU pharmacokinetics in adult patients undergoing hematopoietic SCT. Bone Marrow Transplant 2012; 47: 190–195.
Ansari M, Rezgui M, Théoret Y, Uppugunduri CRS, Mezziani S, Vachon MF et al. Glutathione S transferase gene variations influence busulfan pharmacokinetics and outcome of hematopoietic stem cell transplantation in pediatric patients. Bone Marrow Transplant 2013; 48: 939–946.
Gulbis AM, Culotta KS, Jones RB, Andersson BS . Busulfan and metronidazole: an often forgotten but significant drug interaction. Ann Pharmacother 2011; 45: e39.
Blanco JG, Harrison PL, Evans WE, Relling MV . Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos 2000; 28: 379–382.
Hakkola J, Pasanen M, Purkunen R, Saarikoski S, Pelkonen O, Maenpaa J et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human adult and fetal liver. Biochem Pharmacol 1994; 48: 59–64.
Treluyer JM, Gueret G, Cheron G, Sonnier M, Cresteil T . Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: in-vivo/in-vitro correlation and inducibility. Pharmacogenetics 1997; 7: 441–452.
Orr ST, Ripp SL, Ballard TE, Henderson JL, Scott DO, Obach RS et al. Mechanism-based inactivation (MBI) of cytochrome P450 enzymes: structure-activity relationships and discovery strategies to mitigate drug-drug interaction risks. J Med Chem 2012; 55: 4896–4933.
Appendix: Drug Metabolizing Enzymes and Biotransformation Reactions. In: Zhang D, Surapaneni S (eds) ADME-Enabling Technologies in Drug Design and Development. John Wiley & Sons, Inc.: Hoboken, New Jersey, 2012, pp 545–565.
Krueger SK, Williams DE . Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther 2005; 106: 357–387.
Damani LA, Houdi AA . Cytochrome P-450 and FAD-monooxygenase mediated S- and N-oxygenations. Drug Metabol Drug Interact 1988; 6: 235–244.
The International HapMap Project. Nature 2003; 426: 789–796.
Versace F, Uppugunduri CR, Krajinovic M, Theoret Y, Gumy-Pause F, Mangin P et al. A novel method for quantification of sulfolane (a metabolite of busulfan) in plasma by gas chromatography-tandem mass spectrometry. Anal Bioanal Chem 2012; 404: 831–838.
Ansari M, Théoret Y, Rezgui M, Peters C, Mezziani S, Desjean C et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematopoietic stem cell transplantation, on behalf of the pediatric disease working parties of the european blood and marrow transplant group. Ther Drug Monit 2013, (in press).
Rifai N, Sakamoto M, Lafi M, Guinan E . Measurement of plasma busulfan concentration by high-performance liquid chromatography with ultraviolet detection. Ther Drug Monit 1997; 19: 169–174.
Ansari M, Uppugunduri CR, Deglon J, Theoret Y, Versace F, Gumy-Pause F et al. A simplified method for busulfan monitoring using dried blood spot in combination with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2012; 26: 1437–1446.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone marrow transplant 1995; 15: 825–828.
Pedersen RS, Brasch-Andersen C, Sim SC, Bergmann TK, Halling J, Petersen MS et al. Linkage disequilibrium between the CYP2C19*17 allele and wildtype CYP2C8 and CYP2C9 alleles: identification of CYP2C haplotypes in healthy Nordic populations. Eur J Clin Pharmacol 2010; 66: 1199–1205.
McCune JS, Gibbs JP, Slattery JT . Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet 2000; 39: 155–165.
Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood 2004; 104: 1574–1577.
Balasubramanian P, Desire S, Panetta JC, Lakshmi KM, Mathews V, George B et al. Population pharmacokinetics of cyclophosphamide in patients with thalassemia major undergoing HSCT. Bone Marrow Transplant 2012; 47: 1178–1185.
de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH . Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 2005; 44: 1135–1164.
Timm R, Kaiser R, Lotsch J, Heider U, Sezer O, Weisz K et al. Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome P450 2C19. Pharmacogenomics J 2005; 5: 365–373.
Kim IW, Yun HY, Choi B, Han N, Kim MG, Park S et al. Population pharmacokinetics analysis of cyclophosphamide with genetic effects in patients undergoing hematopoietic stem cell transplantation. Eur J Clin Pharmacol 2013; 69: 1543–1551.
Begg EJ, Helsby NA, Jensen BP . Pharmacogenetics of drug-metabolizing enzymes: the prodrug hypothesis. Pharmacogenomics 2012; 13: 83–89.
Helsby NA, Hui CY, Goldthorpe MA, Coller JK, Soh MC, Gow PJ et al. The combined impact of CYP2C19 and CYP2B6 pharmacogenetics on cyclophosphamide bioactivation. Br J Clin Pharmacol 2010; 70: 844–853.
Hassan M, Andersson BS . Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics 2013; 14: 75–87.
Rezvani AR, McCune JS, Storer BE, Batchelder A, Kida A, Deeg HJ et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant 2013; 19: 1033–1039.
Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone marrow transplant 2011; 46: 344–349.
Zhu ZH, Sun ML, Li ZS, Yang ZC, Zhang TB, Heng ZC et al. An investigation of the maximum allowable concentration of sulfolane in surface water. Hua Xi Yi Ke Da Xue Xue Bao 1987; 18: 376–380.
Cooper AJ, Pinto JT, Callery PS . Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expert Opin Drug Metab Toxicol 2011; 7: 891–910.
Melanson SE, Stevenson K, Kim H, Antin JH, Court MH, Ho VT et al. Allelic variations in CYP2B6 and CYP2C19 and survival of patients receiving cyclophosphamide prior to myeloablative hematopoietic stem cell transplantation. Am J Hematol 2010; 85: 967–971.
Black JL, Litzow MR, Hogan WJ, O'Kane DJ, Walker DL, Lesnick TG et al. Correlation of CYP2B6, CYP2C19, ABCC4 and SOD2 genotype with outcomes in allogeneic blood and marrow transplant patients. Leuk Res 2012; 36: 59–66.
Rocha V, Porcher R, Fernandes JF, Filion A, Bittencourt H, Silva W Jr. et al. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia 2009; 23: 545–556.
Acknowledgements
We are grateful to all patients and their parents for participating in this study. This investigation was supported by grants provided by CANSEARCH, the Geneva Cancer League and the Hans Wilsdorf and Charles Bruneau Foundations. We thank MF Vachon, C Desjean and M Labuda for the collection, maintenance and organization of biological material and clinical data. We thank Professor Yves Théoret for the first dose pharmacokinetic-guided dosing in the cohort. We thank Ms Pauline Mathot for her assistance in genotyping the samples for some of the SNPs.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Uppugunduri, C., Rezgui, M., Diaz, P. et al. The association of cytochrome P450 genetic polymorphisms with sulfolane formation and the efficacy of a busulfan-based conditioning regimen in pediatric patients undergoing hematopoietic stem cell transplantation. Pharmacogenomics J 14, 263–271 (2014). https://doi.org/10.1038/tpj.2013.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2013.38
Keywords
This article is cited by
-
Vitamin D levels and busulphan kinetics in patients undergoing hematopoietic stem cell transplantation, a multicenter study
Bone Marrow Transplantation (2021)
-
Predicting the presence and mechanism of busulfan drug-drug interactions in hematopoietic stem cell transplantation using pharmacokinetic interaction network–based molecular structure similarity and network pharmacology
European Journal of Clinical Pharmacology (2021)
-
Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients
Clinical Pharmacokinetics (2021)
-
Population pharmacokinetic analysis of intravenous busulfan: GSTA1 genotype is not a predictive factor of initial dose in Chinese adult patients undergoing hematopoietic stem cell transplantation
Cancer Chemotherapy and Pharmacology (2020)
-
Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation
Bone Marrow Transplantation (2018)