Abstract
Study design:
Single case report.
Objectives:
We present an incomplete paraplegic patient with lower back and hip pain, diagnosed and treated as the piriformis syndrome (PS).
Setting:
University hospital, Turkey.
Case:
A 62-year-old woman with T3 paraplegia of American Spinal Injury Association Impairment Scale grade D presented with lower back and right hip pain accompanied by pain and numbness radiating to her right leg. After detailed anamnesis and physical examination, she was pre-diagnosed as having PS. The marked relief of pain following the ultrasound-guided piriformis muscle injection of 4 cc of lidocaine 2%+1 cc of betametazone confirmed the diagnosis.
Conclusion:
Although the compressive neuropathies and musculoskeletal injuries of the upper limb leading to neuropathic and musculoskeletal pain in persons with spinal cord injury (SCI) are well described in literature, there is limited information regarding those of lower limbs. To the best of our knowledge, this is the first reported case of PS in a patient with SCI. PS should be kept in mind as a pain generator, especially in active and ambulatory SCI patients.
Similar content being viewed by others
Introduction
Piriformis syndrome (PS), which is characterized by pain radiating to gluteal and posterior leg regions, is regarded as an underdiagnosed cause of sciatalgia. It is attributed to ~6–8% of lower back pain cases.1
The development of pain following spinal cord injury (SCI) is a debilitating complaint that affects 70–80% of the SCI patients.2 There are many different pain types, including compressive neuropathic and musculoskeletal pain, almost all of which described in literature belong to upper limbs.
After a detailed current literature survey, we could not find any report about compressive neuropathies or overuse injuries of the lower limbs. To the best of our knowledge, this is the first reported case of PS in a patient with SCI.
Case
A 62-year-old woman with an incomplete SCI at the level of T3, graded on the American Spinal Injury Association Impairment Scale as grade D, presented with lower back and right hip pain accompanied by pain and numbness radiating to her right leg. She had incurred a traumatic SCI after falling from a tree 2 years ago, and she was ambulatory in the community with bilateral axillary crutches since then. Her complaints had started 6 months ago after falling onto her right buttock. She reported that her pain, graded as 8 over 10 on the numeric rating scale (NRS), had gradually increased and had recently become unresponsive to medical treatment.
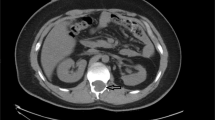
Her physical examination revealed mild tenderness in the lower lumbar vertebrae, whereas severe radicular pain in the right gluteal region with deep palpation of the piriformis muscle (PM). The straight leg raising and Laseque tests were negative, whereas the tests that are known to stretch or contract the PM over the sciatic nerve such as FAIR, Freiberg, Pace and Beatty on the right side were positive. With a pre-diagnosis of PS, we performed an ultrasound-guided PM injection of 4 cc of lidocaine 2%+1 cc of betametazone for diagnostic and therapeutic purposes (Figure 1). The injection was performed to the point of maximum tenderness on the PM with a 22-gauge 88-mm Spinocan (B.Braun Melsungen AG, Germany). A 5–10-MHz linear probe (Diasus Dynamic Imaging, Livingston, UK) was used during the procedure. Her pain relieved drastically after the injection, and hence the patient was diagnosed as having PS.
When the possible causative factors were asked after the diagnosis, it was found that she had a habit of eating on the floor table and prolonged sitting with one leg folded under the other. After she was warned about pain-exacerbating activities, meloxicam 10 mg (1x1) and paracetamol 500 mg (3×1) for 10 days and PM stretching exercise, which involved hip and knee flexion, hip abduction and external rotation in supine position, were given. There was a gradual decrease in her complaints and NRS scores at the first (NRS=2), third (NRS=1) and sixth month (NRS=0) evaluations after the injection. One year after the injection, she still did not have any complaints.
Discussion
The International Spinal Cord Injury Pain (ISCIP) Classification system that categorizes the types of pain following SCI has recently been accepted by many leading SCI and pain professional associations.3 This classification system is composed of three tiers. In the first tier, SCI-related pain is divided into nociceptive, neuropathic, other and unknown pain. In the second tier, nociceptive pain is further classified as musculoskeletal, visceral and other nociceptive pain; whereas neuropathic pain is further classified according to injury level as at-level, below-level and other neuropathic pain.
Both compressive neuropathies and musculoskeletal injuries of the upper limb are well defined in literature.4 However, there is a big gap in literature about compressive neuropathies and musculoskeletal injuries of the lower limbs in patients with SCI.
PS is as a neuromuscular disorder that either involves the compression of the sciatic nerve under the PM causing sciatic-type complaints such as numbness, pain and tingling along the sciatic nerve pathway, or it can simply present as a tight, hypertrophic and tender PM without sciatic nerve entrapment. Despite the ongoing debates about the diagnostic and treatment methods of PS, local anesthetic injections with or without corticosteroids are accepted as a diagnostic method of choice by many authors. The drastic and almost immediate relief of pain after the PM injection confirms the diagnosis.5
Most of the studies about neuropathic pain in SCI patients have focused on overall neuropathic pain. There is limited information regarding primary pain sources for the neuropathic pain subtypes. PS whether being under the category of ‘musculoskeletal pain’ or ‘other neuropathic pain’ according to the classification of ISCIP, it should be kept in mind as a pain generator of lower extremities, especially in active and ambulatory SCI patients.
References
Hallin RP . Sciatic pain and the piriformis muscle. Postgrad Med 1983; 74: 69–72.
Widerstrom-Noga EG, Felipe-Cuervo E, Broton JG, Duncan RC, Yezierski RP . Perceived difficulty in dealing with consequences of spinal cord injury. Arch Phys Med Rehabil 1999; 80: 580–586.
Saulino M . Spinal cord injury pain. Phys Med Rehabil Clin N Am 2014; 25: 397–410.
Apple DF, Cody R, Allen A . Overuse syndrome of the upper limb in people with spinal cord injury. In: Apple DF (ed.) Physical fitness: a guide for individuals with spinal cord injury. J Rehabil Res Dev 1996; 26: 97–108.
Niu C-C, Lai P-L, Fu T-S, Chen L-H, Chen W-J . Ruling out piriformis syndrome before diagnosing lumbar radiculopathy. Chang Gung Med J 2009; 32: 182–187.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Misirlioglu, T., Palamar, D. & Akgun, K. Piriformis syndrome in an incomplete paraplegic patient: a case report. Spinal Cord Ser Cases 1, 15009 (2015). https://doi.org/10.1038/scsandc.2015.9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2015.9
This article is cited by
-
Four symptoms define the piriformis syndrome: an updated systematic review of its clinical features
European Journal of Orthopaedic Surgery & Traumatology (2018)