Abstract
Study design:
Experimental design.
Objectives:
This descriptive study presents muscular responses from both the upper and the lower extremities during T11–12 segmental stimulation.
Setting:
Neuro Lab of the Texas Woman’s University (School of Physical Therapy, TX, USA).
Methods:
A total of 13 healthy subjects were electrically stimulated using surface electrodes. In trial 1, signals were recorded from the flexor hallucis brevis, soleus, vastus medialis and gluteus medius in the lower right extremity. In trial 2, responses were recorded from the abductor digiti minimi, abductor pollicis brevis (APB), flexor carpi radialis and biceps brachii in the right upper extremity. In trial 3, stimulation was carried out and signals were recorded for both the upper and the lower extremities simultaneously, using different muscle combinations. Five traces per muscle were averaged for each step of the testing. Amplitude and deflection latency were the measured parameters and were compared using descriptive statistics.
Results:
Results showed signal amplitudes ranging from 85 to 821 μV in the upper extremity and from 582 to 3927 μV in the lower extremity, with the largest signal recorded in the soleus muscle and the APB. Response latency varies between 5.5 and 14 ms in the upper limbs and between 7.7 and 27 ms in the lower limbs and was comparable in bilateral recording.
Conclusion:
These muscular responses seem to be elicited from electrical stimulation of motor nuclei in lower limb muscles or from pathways to those nuclei in upper limb muscles, and could be useful in testing patients with spinal disorders.
Similar content being viewed by others
Introduction
Testing spinal cord function depends largely on clinical assessment, imaging techniques or both. Location, extent and degree of disruption may be assessed by neurological examination of upper or lower limb neuromusculoskeletal dysfunction. Although these tests are convenient, findings are largely subjective. The test might miss certain pathways or fibers that are still in an active state, but in such a condition that if neglected may result in the loss of significant and necessary functions. Therefore, there is a need for detailed methods or procedures that accurately test such pathways. Electrophysiological testing procedures could meet this need, especially with its potential to assess circuitry conduction and function.
Using spinal cord stimulation to test patients with diseases or injuries has been of continuous interest to clinicians and physiologists. High voltage, percutaneous electrical stimulation of the lumbosacral spinal segment has been carried out to assess conduction in the cauda equina in healthy subjects.1 Recently, T11–12 stimulation has been reported to induce multisegmental monosynaptic responses in lower limb muscles in healthy subjects.2, 3, 4 These responses have been recorded in both lower limbs. However, no studies have been published on the response to T11–12 electrical stimulation of the upper limbs in healthy subjects. These spinal cord stimulation studies have created some interest for their utilization in patients with spinal cord injury.5 The nonspecificity of clinical and imaging testing could be complemented by electrophysiological tests. Using electrophysiological tests could add information about the location and extent of the pathology, which might suggest a better treatment approach. Most spinal cord stimulation studies reported using epidural techniques.5, 6, 7 However, noninvasive, percutaneous spinal cord stimulation, besides being safer, would provide electrophysiological information that could be used by rehabilitation professionals and clinicians.
As few articles have been published on the methods of eliciting and recording these multisegmental responses, there is a need for detailed reports of the procedures.
Previous reports have discussed lower limb muscular responses to T11–12 spinal stimulation. These responses are expected as lower limb muscles are innervated by lumbosacral cord segments. However, upper limb muscular responses to T11–12 stimulation are not expected. No direct motor pathways from lumbar spinal neurons are known to directly innervate upper limb muscles. However, propriospinal pathways between the lumbosacral and cervical segments might transmit signals that result in the activation of limb muscles. These pathways have not been well tested using electrophysiological techniques in humans. These ascending potentials would be useful in assessing the cervicolumbar spinal cord pathways for the upper extremities. They could also be useful in testing distally or proximally innervated muscle groups.
To our knowledge, no report has been published that showed simultaneous recording of such multisegmental motor responses from all four limbs using single focal stimuli. Such an approach would be important for limb muscular control by the spinal cord and could save time in testing patients with spinal cord pathologies.
The fact that these multisegmental responses could be tested bilaterally and in a large number of muscles makes it useful to identify those spinal segments that if disrupted would result in objective signal dysfunction. Even the degree of such a dysfunction could be carefully assessed, and thereby a better health-care decision made. Such needs have been identified in previous reports.8
These multisegmental responses could also be useful in testing physiological function and circuitries between the upper and lower limbs, as related to the spinal cord.2, 3
The purpose of this electromyographic study is to compare the multisegmental muscular responses of upper and lower limb muscles with electrical stimulation of T11–12 spinal segment in healthy subjects.
Materials and methods
A total of 13 subjects, 5 men and 8 women aged 20–45 years, signed informed consent approved by the review board of Texas Electrophysiology Services, to participate in the study. Recordings from upper limb muscles were taken with subjects standing and from lower limb muscles with subjects sitting. All subjects were healthy with no neck, arm or lower back pain during the previous 12 months and could tolerate strong electric pulses to the lower thoracic (T11–12) area. Subjects were excluded if they had metabolic or neurological diseases, arthritis or radiculopathy of the cervical or lumbar spine or cancer. The average age, weight and height (±s.d.) of the study subjects were 27.61±5.22 years, 65.31±16.29 kg and 168.08±14.20 cm, respectively.
Electrical stimulation and recording
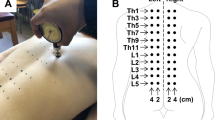
The T11–12 spinal segment was electrically stimulated using 1-ms square wave pulses at 0.2 pps (pulse per second). The T11–12 spinal segment was located by palpation during flexion/extension of the thoracolumbar spine and a cup electrode (1 cm diameter). An AgCl cathode with gel was affixed to the intervertebral space between T11 and T12 using a 3-m hypoallergic tape. For effective stimulation, pressure was applied on the electrode (cathode) during data collection. The anode electrode was a 5-cm square flexible pad (similar to that used with transcutaneous electrical nerve stimulation (TENS)), and the pad was applied on the anterior-superior iliac spine of the limb that was not being used for recording. Stimulation was the most critical step of the current experiment. Stimulus intensity was increased until maximum action potentials were recorded from the tested muscles at a comfortable level.
Muscular responses/action potentials were recorded using a four-channel Cadwell electromyographic unit (Cadwell Lab., Kennewick, WA, USA). Surface silver–silver chloride cup electrodes (1 cm diameter each for the active and reference embedded in a 3-cm plexiglass bar) with gel were applied to the muscles (motor points) using a 3-m hypoallergic tape. A metal ground electrode (3 cm diameter) with gel was applied to the subject’s fibular head. In trial 1, action potentials were recorded from the motor points of the following muscles in the right lower extremity: flexor hallucis brevis (FHB), soleus, vastus medialis obliquus (VMO) and gluteus medius (GM). Trial 2 included T11–12 electrical stimulation while recording action potentials from the following muscles of the right upper limbs: abductor digiti minimi (ADM), abductor pollicis brevis (APB), flexor carpi radialis (FCR) and biceps brachii (BB). In trial 3, T11–12 stimulation was carried out during bilateral recording from the four limbs, one muscle for each limb. The following muscles were tested during these combinations: ADM, APB, FCR, BB, FHB, soleus and GM. In bilateral recording, reference electrodes were applied on the acromion and the anterior-superior iliac spine of the right side of the body. Recording parameters were 100–1000 μV per div with a sweep speed of 5 ms per div, using 10 Hz–10 KHz Butterworth filter.
Flexor carpi radialis and soleus H-reflexes were also elicited and recorded from the right upper and lower extremities using the method by Sabbahi and Khalil.9 In this method, the median or the tibial nerves were electrically stimulated (1 ms, 0.2 pps at H-max) at the cubital or popliteal fossas using bar surface electrodes with the cathode proximal to the anode. FCR or soleus H-reflexes were recorded from the motor point of the muscle at the forearm (FCR) with the reference electrode placed lateral to the cathode. Soleus H-reflexes were recorded with surface bar electrodes applied on the soleus muscle 3 cm distal to the bifurcation of the medial and lateral gastrocnemius muscles and in line with the Achilles tendon. A metal ground electrode (2-cm diameter) was applied on the lateral epicondyle of the humerus (FCR) and on the body of the lateral gastrocnemius for the soleus muscle. Recording parameters were 200–2000 μv per div using a filter setting of 10 Hz–10 KHz.
Experimental procedures
Stimulation and recording electrode locations were cleansed using alcohol and dried, followed by placement of the electrodes with conducting gel. Subjects, having signed informed consent, underwent trials 1 and 3 (standing) and 2 (sitting). In trial 2, subjects were comfortably seated in a chair with their arms placed on their laps. This was followed by testing the FCR and soleus H-reflexes. Subjects tolerated the electrical stimuli but requested rest periods between trials. Resting times between trials were 3–5 min. Subjects were told to relax during the testing periods. At the end of testing, electrodes were removed, the skin was cleansed and the subject dismissed. Figure 1 shows the electrodes’ location.
Signal and statistical analyses
Five traces were recorded and averaged for each muscle in the three trials. In addition, five traces of FCR and soleus H-reflexes were recorded and averaged. Peak-to-peak amplitude and deflection latency were the dependent parameters in all trials. Signals were pooled for all subjects using descriptive statistics, with the mean and s.d. analyzed using the SPSS-11.0 statistical package (SPSS Inc, Chicago, IL, USA). Multisegmental responses as well as FCR and soleus H-reflex latency were compared. Signals were correlated with subject’s height and age using Spearman’s rho correlation.
Results
T11–12 spinal stimulation showed robust and consistent responses in all upper and lower limb muscles tested. We recorded these potentials simultaneously in the upper and lower limbs. Electrical stimulation at the T11–12 level was more comfortable for the subjects than cervical spine stimulation found in this issue. The data presented in this study have been obtained from all tested participants confirming the validity of results. For testing reliability, these signals were recorded in a single subject (one of the authors) during several sessions (days) confirming repeatability.
T11–12 stimulation and lower limb responses
Large amplitude muscular responses at the FHB, soleus, VMO and GM muscles were recorded with single focal stimuli after T11–12 stimulation. In general, the signal amplitude was larger in the soleus and VMO and smaller in the GM and FHB (Figure 2). This figure showed data from a sample subject during repetitive stimulation (five stimuli) while keeping the stimulation intensity constant. A similar approach was used in all other figures. Average amplitudes of 3.9 mV (soleus), 1.8 mV (VMO), 1.9 mV (GM) and 0.58 mV (FHB) (Table 1) were recorded. The peak-to-peak amplitude of the signal varied greatly between subjects (Table 1 and Figure 3). Intrasubject variability was small and is shown in Figure 2.
T11–12 stimulation and upper limb responses
T11–12 spinal stimulation resulted in simultaneous large amplitude muscular responses at the APB, ADM, FCR and BB muscles with single focal stimuli (Figure 4). Signal amplitude was larger in distal limb muscles, such as the APB and ADM, and smaller in the more proximal limb muscles, namely the FCR and BB. It showed average amplitudes of 0.82 mV in the APB, 0.56 mV in the ADM, 0.3 mV in the FCR and 0.085 mV in the BB (Table 2). Again, the peak-to-peak amplitude of the signal varied greatly between subjects (Table 2 and Figure 3). Intrasubject variability was small (Figure 4).
T11–12 stimulation and bilateral recording from the upper and lower limbs
T11–12 stimulation resulted in simultaneous large amplitude muscular responses in all muscles tested (namely the ADM, APB, FCR, BB, FHB, soleus and GM) in the four limbs. Figure 5 shows simultaneous recording of muscular responses in both the upper and the lower limbs with focal T11–12 stimulation. Signal amplitude was larger in the soleus, APB and GM than in the FHB and BB muscles (Figure 5).
Signal latency
Latency for action potentials was compatible with the distance between the stimulation electrode at T11–12 and the muscle. The average response latencies for the lower limb were 27 ms for the FHB, 19 ms for the soleus, 11.2 ms for the VMO and 7.7 ms for the GM (Table 1). The average response latencies for the upper limb were 14 ms for the ADM, 14.1 ms for the APB, 7.8 ms for the FCR and 5.42 ms for the BB (Table 2). Muscle response latency varied based on subject’s height. However, response latency variability was substantially less than response amplitude variability (Figure 3). The response latency for the FCR multisegmental motor responses (MMR) (7.79 ms) was almost half that of the FCR H-reflex (16.67 ms), whereas the MMR for the soleus (18.9 ms) was a little more than half of the (16.5±0.71) soleus H-reflexes (25.5 ms).
Bilateral recording resulted in signal latency that was comparable between both the upper and the lower limbs for the same muscle tested. Latency was also compatible with the location of the muscle and distance from the stimulation site (T11–12). Response latency was correlated with the subject’s height (Spearman’s rho: 0.79 for the ADM, Spearman’s rho: 0.84 for the APB) (Table 3). The degree of correlation was higher for upper (ADM, APB) than for lower (soleus) limb muscles.
Discussion
Focal T11–12 spinal stimulation produced multisegmental muscular responses in several lower limb muscles. This is probably due to stimulation of dorsal roots that enter the lower segments of the spinal cord and activate α-motoneurons projecting to lower limb muscles. This is in agreement with recently published reports.2, 3 We recorded responses from muscles supplied by L4, L5, S1 and, possibly, S2 spinal segments. Stimulation of these spinal segments using a focal stimulus indicates a convergence of afferent inputs on the adjacent (possibly upper and lower) motor nuclei. Previous studies have reported branching of spindle afferents with monosynaptic inputs on several homonymous and possible contralateral motor nuclei.7, 10
Responses for the soleus, VMO and GM muscles were greater than for the FHB muscle. This is probably due to the higher input–output relationship required for those antigravity muscles to meet the demands of weight bearing. We found that H-reflexes could be recorded easier in the resting soleus and VMO muscles than in any other muscles of the lower limb.9
Response variability was substantially high in all upper and lower limb muscles tested. This is probably due to variation in stimulation intensity and changes in impedance (of the skin and electrodes) with the longer testing time.11 This resulted in the lack of constant recording of the compound action potential in certain muscles (Figure 5; R-ADM, L-ADM, R-GM). No progressive variability changes were recorded, but rather it was random. It is believed that such variability was intensity related, especially with a large-sized body. Subjects’ body sizes varied greatly. Slim subjects showed large amplitude muscular signal with smaller stimulation intensity and smaller degree of variability throughout the three trials. Larger subjects required higher stimulus intensity, which were sometimes noxious and resulted in smaller response amplitudes. Stimulation currents were limited in our study by a maximum pulse duration of 1 ms and an amplitude of 100 mA. The stimulus intensity in this study varied from 20 to 50 mA. Previous studies3 used 2-ms pulses that produced higher electric output with possibly more noxious sensations.
The latency for these muscular responses was correlated with subject height. This is probably due to longer transmission times in taller subjects.
Do these evoked potentials then represent genuine reflexes (monosynaptic or polysynaptic) or something else? Could it be spinal cord stimulation with direct responses of lower or upper limb muscles to dorsal root stimulation (or motor nuclei)? Gerasimenko et al.6 reported early, intermediate and late responses to epidural spinal cord stimulation in rats. They suggested that early responses may be due to direct responses, whereas intermediate and late responses could be synaptically mediated. Early responses after S1 stimulation were recorded at almost 3 ms (in rats) and can be correlated with our muscular response latency in humans. Similar responses were also reported in cats using intraspinal microstimulation.7 The question of possible signal sources is valid especially when comparing lower and upper limb responses. The latency for these responses was short and almost half that of the soleus and FCR H-reflexes. Tendon vibration inhibited these lower limb responses similar to soleus H-reflex behavior.2 However, tendon vibration might cause a presynaptic inhibition effect of the α-motoneurons that are activated directly by electrical stimulation.10
Focal T11–12 stimulation produced multisegmental responses in all tested muscles in both upper extremities. These upper limb responses may not be genuine reflex responses but rather represent integrative volitional functions. The pathways for such responses would be ascending motor pathways to motor nuclei at the cervical region and would cause upper limb muscular contractions. The only known such pathway is formed by propriospinal pathways (spinospinalis fibers), which ascend along the spinal cord between the lumbar and cervical segments.12 It has been shown that short and long propriospinal neurons run up and down the spinal cord between cervical and lumbar levels in monkeys.13 This has been mapped using the horseradish peroxidase method in cats and monkeys.14, 15, 16 Such pathways have been shown to mediate monosynaptic and disynaptic excitatory and inhibitory effects on motoneurons.17 These neuronal connections serve the intersegmental and interlimb connection between upper and lower limb functions during locomotion. It is interesting to note that such interspinal connections ascend almost the entire length of the spinal cord, but seldom descend for more than two segments.18
In our companion study using electrical stimulation of the cervical spinal segment, we recorded no signal in any lower limb muscles, indicating that the propriospinal pathway only ascended the spinal cord. It indicates that lower limb muscles/activity may drive the upper limb functions and not vice versa. This may be seen in upper limb alternation during volitional walking in healthy subjects. If this is true, such information might be useful in setting the strategy for interlimb rehabilitation and function. T11–12 stimulation also resulted in simultaneous activation of the four limb muscles. Does this spinal location represent a center of divergence to all limb muscles? We could not find such a site in the cervical region, which could be expected because it is closer to the brain than the thoracolumbar region. Determining the function of a center of divergence will be the focus of future studies.
Moving the stimulation electrode one segment above or below T11–12 reduces the muscular responses of the lower and upper limbs. Does it mean that the T11–12 segment might simulate the C3–4 segment in controlling upper limb movement. The large responses of the soleus muscle (extensor) to T11–12 stimulation might parallel the wrist extensor in the upper limb.T11–12 stimulation resulted in simultaneous recording of the MMR signal in all four limbs. This is probably due to convergence of pathways from the T11–12 spinal segment to the upper and lower limbs. This time-saving procedure could be useful in testing patients with spinal cord injuries and diseases.
The response latency reported for lower limb responses is compatible with previous studies.3, 4 The short latency for upper extremity responses indicated fast transmission in the propriospinal pathways to upper limb motor nuclei, with subsequent activation of upper limb muscles supplied by those nuclei.
For this study, subjects were in standing or sitting posture (loading). Sensory afferents from the feet (cutaneous during sitting and cutaneous and proprioceptive during standing) may be the contributing factors in the large amplitude of the signal in both the upper and the lower limbs. Harkema et al.19 reported a strong correlation between the loading effect of subjects with spinal cord injuries and electromyographic responses of lower limb muscles. In addition, Obata et al.20 reported enhanced excitability of the ankle extensor corticospinal pathway in healthy subjects in sitting or standing postures. These studies could support our lower limb response results. However, our upper extremity response results may need a different interpretation and will be the focus of future studies. It is important to note that that we did not compare the effect of loading (standing) with that of unloading (lying position) in this study. This would also be the focus of future research. Scientists and clinicians continue to search for a stimulation site that activates upper and lower limb muscles simultaneously. It could result in a shorter testing protocol for spinal cord injuries and diseases. On the basis of our results, it seems that the T11–12 segment could be that site. Such a spinal segment is located toward the end of the spinal cord that usually terminates between the L1 and L2 vertebral segments.21 Why such a common stimulation site is located at the end and not the middle (for example, mid-thoracic or cervical) spinal segment is a question for future investigation. As mentioned, stimulation of C7 in a joint study showed no muscular response in lower limb muscles, indicating a lack of motor control or drive of the upper limbs to the lower limbs during locomotion, although the opposite is true.
Delayed or absent responses and smaller amplitudes in the upper limbs in patients with spinal cord injury or disease, with T11–12 stimulation, will indicate compromised propriospinal ascending pathways, cervical motor nuclei function or both. Similarly, the lack of lower limb responses will indicate compromised lumbosacral motor nuclei functions.
Patients with spinal cord injury or disease might need serial testing using these multisegmental responses to follow changes in disrupted circuitry. Results of such tests could be helpful in directing, or terminating, rehabilitation programs. A testing protocol could be customized for each patient based on the type and level of injury.
The results of this study are limited by the fact that testing was carried out in standing or sitting positions. Most patients with spinal cord injury or disease would be tested lying down at the time of injury. The postural supporting reaction and vestibular effect would have a significant effect of the results of spinal cord injury patients tested for these responses. These studies are necessary for future interpretation of the physiological and pathological findings in these pathologies. Caution must be exercised when comparing results of patients in lying positions with these results. Another limitation is the need to optimize electrical stimulation. As mentioned in the ‘Materials and methods’ section, the cathode cup-stimulating electrode was applied with manual pressure on the T11–12 segment. The fluctuation in manual pressure causes possible variation in the current applied to the spinal segment and resulted in the decrease in some action potentials that are seen in blank traces in Figure 5. When constant pressure was applied, the signal was constantly recorded (Figure 5, FHB, L-GM, L-FCR, SOL). Furthermore, it was not the intent of this study to evaluate the effect of posture (standing versus lying or sitting) on the recorded signal. This will be the subject of future studies.
Conclusions
Focal electrical stimulation to the T11–12 segment elicited strong muscular responses of short latency in both lower and upper limb muscles in all of our healthy subjects. These responses could be of reflex origin (in the case of lower limb responses) or propriospinal pathway activation of cervical motor neurons causing upper limb responses. These responses could be useful in testing patients with spinal cord injury or disease.
References
Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD . Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psych 1988; 51: 174–181.
Courtine G, Harkema S, Dy C, Gerasimenko Y, Dyhre-Poulsen P . Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol 2007; 582 (Part 3): 1125–1139.
Kitano K, Koceja D . Spinal reflex in human lower leg muscles evoked by transcutaneous spinal cord stimulation. J Neurosci Methods 2009; 180: 111–115.
Minassian K, Persy I, Ratty F, Dimitrijevic M, Hofer C, Kern H . Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007; 35: 327–336.
Minassain K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 2004; 42: 401–416.
Gerasimenko YP, Lavrov I, Courtine G, Ichiyama RM, Dy CJ, Zhong H et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 2006; 157: 253–263.
Gaunt R, Prochazka A, Mushahwar K, Guevremont L, Ellaway P . Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol 2006; 96: 2995–3006.
Edgerton VR, Tillakaratne NJK, Bigbee AJ, Leon RD, Roy R . Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 2004; 27: 145–167.
Sabbahi MA, Khalil M . Segmental H-reflex studies in upper and lower limbs of normal subjects. Arch Phys Med Rehab 1990; 71: 216–222.
Rudomin P . Presynaptic control of synaptic effectiveness of muscle spindle and tendon organ afferents in the mammalian spinal cord. In: Binder MD, Mendell LM (eds). The Segmental Motor System. Oxford University Press: Oxford, 1990, pp 349–380.
Dumitru D . Electrodignostic Medicine. Hanley & Belfus: Philadelphia, 1995, pp 72–73.
Malmgren K, Pierrot-Deseilligny E . Evidence for non-monosynaptic Ia excitation of human flexor motoneurones possibly via propriospinal neurons. J Physiol 1988; 405: 747–764.
Ghez C . The control of movement. In: Kandel ER, Schwartz JH, Jessell TM (eds). Principles of Neural Science, 3rd edn. Elsevier Science: New York, 1991, pp 540–541.
Burton H, Loewy AD . Descending projections from the marginal cell layer and other regions of the monkey spinal cord. Brain Res 1976; 116: 485–491.
Molenaar I, Kuypers HG . Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res 1978; 152: 429–450.
Matsushita M, Ikeda M, Hosoya Y . The location of spinal neurons with long descending axons (long descending propriospinal tract neurons) in the cat: a study with the horseradish peroxidase technique. J Comp Neurol 1979; 184: 63–80.
Jankowska E, Lundberg A, Roberts WJ, Stuart D . A long propriospinal system with direct effect on motoneurones and on interneurones in the cat lumbosacral cord. Exp Brain Res 1974; 21: 169–194.
Kiernan J . Barr's The Human Nervous System–An Anatomical Viewpoint, 8th edn. Lippincott Williams & Wilkins: New York, 2005, p 79.
Harkema S, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton R . Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 1997; 77: 797–811.
Obata H, Sekiguchi H, Nakazawa K, Ohtsuki T . Enhanced excitability of the corticospinal pathway of the ankle extensor and flexor muscles during standing in humans. Exp Brain Res 2009; 197: 207–213.
Moore K, Dalley A . Clinically Oriented Anatomy, Moore KL and Dalley AF (eds), 5th edn. Lippincott Williams & Wilkins: New York, 2006, p 522.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sabbahi, M., Sengul, Y. Thoracolumbar multisegmental motor responses in the upper and lower limbs in healthy subjects. Spinal Cord 49, 741–748 (2011). https://doi.org/10.1038/sc.2010.165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.165
Keywords
This article is cited by
-
The effect of combined transcranial pulsed current stimulation and transcutaneous electrical nerve stimulation on lower limb spasticity in children with spastic cerebral palsy: a randomized and controlled clinical study
BMC Pediatrics (2021)
-
Cervical multisegmental motor responses in healthy subjects
Spinal Cord (2012)
-
Editorial Note on: Neurophysiological assessment of spine disorders: old fashion techniques for modern clinical problems
Spinal Cord (2012)
-
Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots
Experimental Brain Research (2012)