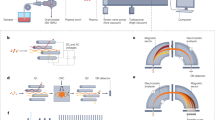
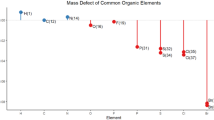
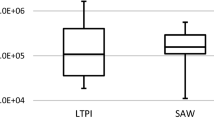
Abstract
We demonstrate the application of proton transfer time-of-flight mass spectrometry (PTR-TOF-MS) in monitoring the kinetics of disinfectant decay in water with a sensitivity one to three orders of magnitude greater than other analytical methods. Chemical disinfection inactivates pathogens during water treatment and prevents regrowth as water is conveyed in distribution system pipes, but it also causes formation of toxic disinfection by-products. Analytical limits have hindered kinetic models, which aid in ensuring water quality and protecting public health by predicting disinfection by-products formation. PTR-TOF-MS, designed for measuring gas phase concentrations of organic compounds, was able to simultaneously monitor aqueous concentrations of five inorganic haloamines relevant to chloramine disinfection under drinking water relevant concentrations. This novel application to aqueous analytes opens a new range of applications for PTR-TOF-MS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the finding of this study are available within this article and its Supplementary Information. The data that support the findings of this study are available via figshare at https://doi.org/10.6084/m9.figshare.25302220 (ref. 49).
References
Rosario-ortiz, B. F. et al. How do you like your tap water? Science 351, 912–914 (2016).
Sedlak, D. L. & von Gunten, U. The chlorine dilemma. Science 331, 42 LP–43 (2011).
Allaire, M., Wu, H. & Lall, U. National trends in drinking water quality violations. Proc. Natl Acad. Sci. USA 115, 2078–2083 (2018).
2017 Water Utility Disinfection Survey Report (American Water Works Association, 2018).
Monochloramine in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality (World Health Organization, 2004).
Valentine, R. L. & Jafvert, C. T. Reaction scheme for the chlorination of ammoniacal water. Environ. Sci. Technol. 26, 577–586 (1992).
Duirk, S. E. & Valentine, R. L. Modeling dichloroacetic acid formation from the reaction of monochloramine with natural organic matter. Water Res. 40, 2667–2674 (2006).
Amy, G., Siddigui, M., Zhai, W., DeBroux, J. & Odem, W. Survey of Bromide in Drinking Water and Impacts on DBP Formation (Water Research Foundation, 1994).
Allen, J. M. et al. Drivers of disinfection byproduct cytotoxicity in U.S. drinking water: should other DBPs be considered for regulation? Environ. Sci. Technol. 56, 392–402 (2022).
Dietrich, A. M. & Burlingame, G. A. Critical review and rethinking of USEPA secondary standards for maintaining organoleptic quality of drinking water. Environ. Sci. Technol. 49, 708–720 (2015).
Vidic, R. D., Brantley, S. L., Vandenbossche, J. M., Yoxtheimer, D. & Abad, J. D. Impact of shale gas development on regional water quality impact of shale gas development. Science 340, 6134 (2013).
Kirmeyer, G., Martel, K., Thompson, G. & Radder, L. Optimizing Chloramine Treatment (Water Research Foundation, 2004).
Wahman, D. G., Speitel, G. E. & Katz, L. E. Bromamine decomposition revisited: a holistic approach for analyzing acid and base catalysis kinetics. Environ. Sci. Technol. 51, 13205–13215 (2017).
Trofe, T. W., Inman, G. W. & Donald Johnson, J. Kinetics of monochloramine decomposition in the presence of bromide. Environ. Sci. Technol. 14, 544–549 (1980).
Pope, P. G. & Speitel, G. E. Reactivity of bromine-substituted haloamines in forming haloacetic acids. ACS Symp. Ser. 995, 182–197 (2008).
Kinani, S., Roumiguières, A. & Bouchonnet, S. A critical review on chemical speciation of chlorine-produced oxidants (CPOs) in seawater. Part 1: chlorine chemistry in seawater and its consequences in terms of biocidal effectiveness and environmental impact. Crit. Rev. Anal. Chem. https://doi.org/10.1080/10408347.2022.2139590 (2022).
Kinani, S., Roumiguières, A. & Bouchonnet, S. A Critical review on chemical speciation of chlorine-produced oxidants (CPOs) in seawater. Part 2: sampling, sample preparation and non-chromatographic and mass spectrometric-based methods. Crit. Rev. Anal. Chem. https://doi.org/10.1080/10408347.2022.2135984 (2022).
Roumiguières, A., Bouchonnet, S. & Kinani, S. A critical review on chemical speciation of chlorine-produced oxidants in seawater. Part 3: chromatographic- and mass spectrometric-based methodologies. Crit. Rev. Anal. Chem. https://doi.org/10.1080/10408347.2023.2220129 (2023).
Brodfuehrer, S. H., Wahman, D. G., Alsulaili, A., Speitel, G. E. & Katz, L. E. Role of carbonate species on general acid catalysis of bromide oxidation by hypochlorous acid (HOCl) and oxidation by molecular chlorine (Cl2). Environ. Sci. Technol. 54, 16186–16194 (2020).
Luh, J. & Mariñas, B. J. Kinetics of bromochloramine formation and decomposition. Environ. Sci. Technol. 48, 2843–2852 (2014).
Method 10020, Chloramine (Mono) and Nitrogen, Free Ammonia (Hach Company, 2019).
Method 10070, Chlorine, Free and Total, High Range (Hach Company, 2018).
Pope, P. G. Haloacetic Acid Formation During Chloramination: Role of Environmental Conditions, Kinetics, and Haloamine Chemistry (University of Texas at Austin, 2006).
Hu, W., Lauritsen, F. R. & Allard, S. Identification and quantification of chloramines, bromamines and bromochloramine by membrane introduction mass spectrometry (MIMS). Sci. Total Environ. 751, 142303 (2021).
Blake, R. S., Monks, P. S. & Ellis, A. M. Proton-transfer reaction mass spectrometry. Chem. Rev. 109, 861–896 (2009).
Thompson, J. M. Introduction. in Mass Spectrometry 1–56 (Pan Stanford Publishing Pte., 2018).
Roumiguières, A., Bouchonnet, S. & Kinani, S. Challenges and opportunities for on-line monitoring of chlorine-produced oxidants in seawater using portable membrane-introduction Fourier transform-ion cyclotron resonance mass spectrometry. Anal. Bioanal. Chem. 413, 885–900 (2021).
Wu, T. et al. Real-time measurements of gas-phase trichloramine (NCl3) in an indoor aquatic center. Environ. Sci. Technol. 55, 8097–8107 (2021).
Arata, C. et al. Volatile organic compound emissions during HOMEChem. Indoor Air 31, 2099–2117 (2021).
Bhattacharyya, N. et al. Bleach emissions interact substantially with surgical and KN95 mask surfaces. Environ. Sci. Technol. 57, 6589–6598 (2023).
Senthilmohan, S. T. et al. Detection of monobromamine, monochloramine and dichloramine using selected ion flow tube mass spectrometry and their relevance as breath markers. Rapid Commun. Mass Spectrom. 22, 677–681 (2008).
Shrivastava, A. & Gupta, V. B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2, 21–25 (2011).
Mensah, A. T., Berne, F., Allard, S., Soreau, S. & Gallard, H. Kinetic modelling of the bromine-ammonia system: formation and decomposition of bromamines. Water Res. 224, 119058 (2022).
Demeestere, K., Dewulf, J., De Witte, B. & Van Langenhove, H. Sample preparation for the analysis of volatile organic compounds in air and water matrices. J. Chromatogr. A 1153, 130–144 (2007).
Furman, C. S. & Margerum, D. W. Mechanism of chlorine dioxide and chlorate ion formation from the reaction of hypobromous acid and chlorite ion. Inorg. Chem. 37, 4321–4327 (1998).
Troy, R. C. & Margerum, D. W. Non-metal redox kinetics: hypobromite and hypobromous acid reactions with iodide and with sulfite and the hydrolysis of bromosulfate. Inorg. Chem. 30, 3538–3543 (1991).
Kumar, K., Day, R. A. & Margerum, D. W. Atom-transfer redox kinetics: general-acid-assisted oxidation of iodide by chloramines and hypochlorite. Inorg. Chem. 25, 4344–4350 (1986).
Lei, H., Mariñas, B. J. & Minear, R. A. Bromamine decomposition kinetics in aqueous solutions. Environ. Sci. Technol. 38, 2111–2119 (2004).
Brodfuehrer, S. H., Goodman, J. B., Wahman, D. G., Speitel, G. E. & Katz, L. E. Apparent reactivity of bromine in bromochloramine depends on synthesis method: implication bromine chloride and molecular bromine as important bromine species. J. Environ. Eng. 148, 12 (2022).
de Gouw, J. & Warneke, C. Measurements of volatile organic compounds in the earth’s atmosphere using proton‐transfer‐reaction mass spectrometry. Mass Spectrom. Rev. 26, 223–257 (2007).
Warneke, C., van der Veen, C., Luxembourg, S., de Gouw, J. A. & Kok, A. Measurements of benzene and toluene in ambient air using proton-transfer-reaction mass spectrometry: calibration, humidity dependence, and field intercomparison. Int. J. Mass Spectrom. 207, 167–182 (2001).
Krechmer, J. et al. Evaluation of a new reagent-ion source and focusing ion–molecule reactor for use in proton-transfer-reaction mass spectrometry. Anal. Chem. 90, 12011–12018 (2018).
Blomdahl, D. C. Optimization of Volatile Organic Compound Sensitivity of Vocus PTR-ToF-MS for Quantifying Disinfection Byproducts (Univ. Texas at Austin, 2021).
Riva, M. et al. Evaluating the performance of five different chemical ionization techniques for detecting gaseous oxygenated organic species. Atmos. Meas. Tech. 12, 2403–2421 (2019).
Hall, E. C. et al. Varying humidity increases emission of volatile nitrogen-containing compounds from building materials. Build. Environ. 205, 108290 (2021).
Sreeram, A., Blomdahl, D., Misztal, P. & Bhasin, A. High resolution chemical fingerprinting and real-time oxidation dynamics of asphalt binders using Vocus Proton Transfer Reaction (PTR-TOF) mass spectrometry. Fuel 320, 123840 (2022).
Holzinger, R. PTRwid: a new widget tool for processing PTR-TOF-MS data. Atmos. Meas. Tech. 8, 3903–3922 (2015).
AWWA Disinfection Systems Committee. Committee report: disinfection survey, part—alternatives, experiences, and future plans. Am. Water Works Assoc. 100, 110–124 (2008).
Brodfuehrer, S. H. et al. Data for ‘Simultaneous time-resolved inorganic haloamine measurement empowers robust analysis of disinfectant degradation kinetics and by-product formation’. figshare https://doi.org/10.6084/m9.figshare.25302220 (2024).
Acknowledgements
This work was supported by the National Science Foundation under grant 1953206. The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the US Environmental Protection Agency. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the US Environmental Protection Agency. EPA and its employees do not endorse any commercial products, services or enterprises.
Author information
Authors and Affiliations
Contributions
P.K.M. and L.E.K. conceived the research. S.H.B., D.C.B., P.K.M. and L.E.K. designed the research. S.H.B. and D.C.B. performed the experimental work. S.H.B., D.C.B., D.G.W., G.E.S., P.K.M. and L.E.K. contributed to interpreting the data. S.H.B. and D.C.B. wrote the original draft. S.H.B., D.C.B., D.G.W., G.E.S., P.K.M. and L.E.K. contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Said Kinani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–6 and Tables 1–4.
Supplementary Data 1
Mass spectrum of ambient lab air.
Supplementary Data 2
Haloamine standard curves.
Supplementary Data 3
Haloamine specific kinetic experiments.
Supplementary Data 4
Example kinetic data from experiments performed by Luh and Marinas.
Supplementary Data 5
Kinetic experiment with NOM.
Source data
Source Data Fig. 1
Individual haloamine mass spectra.
Source Data Fig. 2
PTR-TOF-MS response following sample introduction.
Source Data Fig. 3
Dihaloamine interferences.
Source Data Fig. 4
Colorimetric methods used during kinetic experiments.
Source Data Fig. 5
Haloamine specific kinetic experiments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brodfuehrer, S.H., Blomdahl, D.C., Wahman, D.G. et al. Simultaneous time-resolved inorganic haloamine measurements enable analysis of disinfectant degradation kinetics and by-product formation. Nat Water (2024). https://doi.org/10.1038/s44221-024-00227-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44221-024-00227-4