Abstract
Synthetic textiles are a significant source of microplastic fibre pollution. While the microplastic fibre release mechanism during the washing of textiles is well studied, little is known about the release of nanoplastics. The first investigations on the nanoplastic fraction released during the washing and abrasion of polyester textiles have been published; however, questions were raised regarding the chemical composition of the observed submicrometre particles. Using a combination of analytical methods, we show here that 12 different polyester textiles released 4.6 × 1010 to 8.9 × 1011 particles per gram of textile during washing, with a mean size of 122–191 nm. The number of released submicrometre particles was not significantly influenced by the cutting method nor by the textile structure, but positively correlated (P < 0.01) with the number of submicrometre particles present on the fibre surface before washing. We found that 34–89% of the extracted submicrometre particles were soluble in ethanol. These particles are most likely water-insoluble poly(ethylene terephthalate) oligomers. Our results clearly show the urgent need to better understand the contribution of water-insoluble oligomer particles to the pollution of the environment by anthropogenic nanoplastics.
Similar content being viewed by others
Main
Plastic pollution is hard to tackle because it is accumulative and persistent, penetrating all aspects of our daily lives. This form of pollution has attracted public attention as microplastics are now detected everywhere, especially in surface water and soils1. Fibres are the major type of microplastics found in environmental samples, with textiles being an important source of environmental microplastics2, especially those comprising fibres3. The domestic washing of synthetic textiles releases microplastic fibres (MPFs) at a scale ranging from a few to more than 10,000 MPFs per gram of textile washed4,5,6,7 and accounts for a notable proportion of the MPFs released worldwide. It has been estimated that between 200,000 and 500,000 tonnes of microplastics from textiles enter the global marine environment each year, representing a 9% share of the total environmental microplastics8,9. Recent studies on MPFs have revealed that they are produced before delivery to customers, from yarn production to textile cutting and finishing, remaining in polyester textiles until extracted during washing5,10,11.
The scientific community is now also paying increasing attention to nanoplastics, that is, plastic particles smaller than 1,000 nm, as potentially they pose greater risks than microplastics12,13. As evidence grows, scientists are calling for increased scientific effort to characterize the environmental and human health risks of nanoplastics14. Studies reporting the release of nanoplastics during the daily use of plastic products have attracted extensive public attention, for example, the release of nanoplastics from tea bags into hot water15. Compared with MPF release from synthetic textiles, nanoplastic release is less understood. The nanoparticles released during the washing of polyester textiles have similar near-edge X-ray absorption fine structure (NEXAFS) spectra to poly(ethylene terephthalate) (PET) reference nanoplastics16. Large quantities of PET nanoplastics released from plastic products could pose a threat to the environment and human health. An increasing number of studies exploring the potential adverse effects of PET nanoplastics have identified a number of effects, ranging from limited acute toxicity at the cellular level (inflation and the production of reactive oxygen species)17 to chronic lethal and sublethal toxicity (mortality in Nitocra spinipes and Danio rerio)18,19. The potential effects on human health have also been investigated using cells of the respiratory system, such as A549, HePG2 and Caco-2, with PET nanoplastics at concentrations of 50–80 µg ml−1 found to cause severe damage to mitochondrial activity20,21.
Polymers manufactured via polycondensation, such as polyesters or polyamides, contain oligomers that coexist with the polymer. These oligomers, defined by either the degree of polymerization (<40) or the molecular weight (<10,000 Da)22,23, are known to migrate into food simulants from food packaging or containers during simulations of cooking and are usually classified as non-intentionally added substances24,25. These plastic oligomers can be formed during incomplete polymerization and are able to migrate out of plastics during heating or plastic (bio)degradation23,26,27. Spectroscopic methods fail to distinguish oligomer molecules from nanoplastics as the oligomers and polymer share the same chemical bonds. Information on the size cut-off (degree of polymerization) between oligomer and nanoplastics is limited; however, oligomers with a few repeating units can be clearly classified as molecules rather than nanoplastics.
In this study, we developed a reliable test protocol to study the release of submicrometre particles from synthetic textiles during washing. By characterizing the number and size of submicrometre particles released from a representative set of 12 different polyester fabrics (detailed in Methods) using a combination of analytical methods, we aimed to understand the source and release mechanism of submicrometre particles during the domestic washing of synthetic textiles. In addition, the nanoplastic fraction of the released submicrometre particles was estimated after ethanol treatment to discriminate nanoplastics from oligomer submicrometre particles.
Optimization of the test protocol
The quantification of nanoparticles by nanoparticle tracking analysis (NTA) is usually accompanied by contamination arising from the analytical process16,28. We conducted a systematic series of experiments to understand the contribution of nanoparticle contamination from solutions, containers, filters and filtration units during the investigation. The results of the NTA of the blank samples are presented in Supplementary Table 1. The addition of a surfactant facilitates the release of nanoparticles from the textiles, but the NTA of pure linear alkylbenzene sulfonate (LAS) solutions (0.0075, 0.075 and 0.75 g l−1) yielded nanoparticle concentrations ranging from 1.5 × 108 to 1.9 × 109 particles ml−1, which is 75% of the total contamination (Supplementary Table 1 and Supplementary Fig. 1). The NTA signals are probably confounded by micelles formed in particle-free surfactant solutions29,30. Therefore, surfactants should not be applied in the quantification of nanoplastics by NTA. Other identified sources of contamination in the blanks were the Gyrowash steel beakers and the single-use polyethylene (PE) lids. Fewer particles (4.7 × 106 particles ml−1) were introduced into the filtrate during filtration after rinsing the syringes and filters with 20 ml deionized (DI) water before use. Although less than that released from the PE lids, the particles introduced by the steel beakers could not be further reduced, even with careful cleaning, while the nanoparticles from the PE lids were reduced rapidly after prewashing.
We also measured the number of submicrometre particles released from samples of Fleece polyester with or without adding a steel ball to simulate the mechanical force exerted by other clothes during laundry. The results of t-test analysis showed that adding one steel ball significantly (P = 0.001, Supplementary Table 2) increased the number of nanoparticles released during washing. Therefore, to minimize contamination and optimize the gain for the investigation of nanoparticles, the sample textiles were washed in a glass vial (closed with a prewashed PE lid) inside the Gyrowash steel beaker with one steel ball but no surfactant. Using this optimized procedure, six blank samples yielded an average of 4.9 × 107 particles ml−1, which is about an order of magnitude lower than most of the concentrations measured from the washing samples.
Nanoparticle release from different fabrics and cutting methods
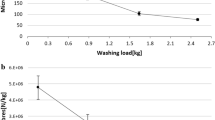
The average number of submicrometre particles released per wash varied widely, ranging from 4.6 × 1010 particles g−1 for scissor-cut Microfibre to 8.9 × 1011 particles g−1 for laser-cut Satin F (Fig. 1a and Supplementary Table 3). Most of the particles had an average hydrodynamic size between 100 and 200 nm (Fig. 1b and Supplementary Table 3). The average mass released from different fabrics was estimated on the basis of their size distributions (Supplementary Fig. 2), assuming that all of the particles were spherical, and the density of PET (1.38 g cm−3). One gram of laser-cut textile samples released 0.2 mg (Jersey S) to 1.2 mg (Satin F) of submicrometre particles and 1 g of scissor-cut samples released 0.1 mg (Plain B) to 1.6 mg (Satin F) of submicrometre particles. The mass release data are summarized in Supplementary Table 4.
The number of submicrometre particles released during the washing is not influenced by either the cutting method or the textile structure. a, Number of submicrometre particles (100–600 nm, few particles >600 nm were detected by NTA) released per gram of textile for different polyester fabrics cut by scissors or laser. The data are presented as the mean ± sd (n = 3 textile replicates). b, Box plots showing the hydrodynamic size distributions of the submicrometre particles measured by NTA. The black squares represent the mean, the black centre lines denote the median value (50th percentile), and the tops and bottoms of the boxes show the 75th (Q3) and 25th (Q1) percentiles of the dataset. The top and bottom whiskers denote Q3 + 1.5IQR and Q1 − 1.5IQR, respectively, where IQR is the interquartile range Q3 − Q1. The size box plot is converted from size distributions to relative counts at different sizes.
There was no significant difference (t-test = 0.92, P = 0.36) in the number of nanoparticles released by the two different cutting methods. However, one-way analysis of variance (ANOVA) suggested a significant difference in the number of submicrometre particles released from different polyester fabrics in both laser-cut (P < 0.05) and scissor-cut (P < 0.05) samples (Supplementary Table 5). The difference between the two cutting methods was not significant (P = 0.22). We grouped the 12 textiles into knit, woven and surface-treated categories. The group comparison test showed no difference in the number of particles released except for the comparison of knit with woven and surface-treated with woven in scissor-cut samples (P = 0.005 and 0.006, respectively; Supplementary Table 6). In addition, yarn type (filament or spun yarn) was found not to significantly affect the number of particles released (P = 0.08 and 0.15, respectively) for laser- and scissor-cut samples. However, we found different fabric types, cutting methods, yarn types and fabric groups to affect the size of submicrometre particles released during washing (Supplementary Table 6).
Repeated washes
Four textiles were selected and subjected to four wash cycles to investigate the influence of repeated washing on the release of submicrometre particles. The concentration of submicrometre particles decreased below the detection limit after the third wash for all four fabrics (Fig. 2 and Supplementary Table 7). Five to eight times more submicrometre particles were released in the first wash cycle compared with the second wash. Based on the total number of submicrometre particles released during the four washes, the first wash extracted 73–87% of all the submicrometre particles. The size distribution of the submicrometre particles (100–400 nm) during the repeated washing is shown in Fig. 2b–e. Although the total number of particles decreased, the size of the extracted particles remained about the same, although a slight trend towards larger sizes could be observed. However, the variability was greater due to the lower concentration.
The number of released submicrometre particles decreases during the repeated washing of the selected fabrics, while the size distribution remains largely unchanged. a, Concentration of submicrometre particles (100–600 nm) released during four repeated washes of four polyester textiles. The data are presented as the mean ± sd (n = 3 textile replicates). The contamination level of blanks (Lob, dashed line) was calculated29 as the mean blank + 1.63sd (n = 12 blanks). b–e, Hydrodynamic size distributions of the submicrometre particles (100–400 nm) released during four repeated washes (R1 to R4) of four different fabrics: Fleece (b), Interlock S (c), Plain F (d) and Satin F (e).
Submicrometre particles on the surface of polyester fibres
Polyester fibres from all 12 unwashed textiles were imaged by scanning electron microscopy (SEM), revealing that the fibres were not smooth and carried particles on their surfaces; the number of submicrometre particles on the surfaces was estimated per gram of textile from the SEM images (Supplementary Note 1). The number of released submicrometre particles determined by NTA during washing with nanopure water or LAS solution cannot be directly compared due to the false-positive signals introduced by the surfactant. However, the rough estimates of the number of nanoparticles on the fibre surfaces indicate that washing with LAS solution removed surface particles more efficiently than washing with nanopure water after four repeated washes (Supplementary Fig. 3).
In general, we observed a substantial number of submicrometre particles on the surfaces of the polyester fibres. As shown in Fig. 3a, most of these submicrometre particles are spherical and have the same morphology as those obtained from the washing liquids (Fig. 3b). The fibre surface was smoother with fewer submicrometre particles visible after repeated washes, especially after washing with LAS solution (Fig. 3c). Satin F was found to release the highest number of submicrometre particles during washing, as measured by NTA, while Microfibre released the lowest number of submicrometre particles of all of the textiles. The SEM image of an unwashed Satin F fibre surface (Fig. 3d) shows many more submicrometre particles than on the surface of an unwashed Microfibre fibre (Fig. 3e). There is a linear correlation (P = 0.008, slope = 1.91, R2 = 0.44) between the number of submicrometre particles quantified by NTA and that estimated by SEM, as shown in Fig. 3f. The numbers of submicrometre particles determined by NTA and SEM for all 12 fabrics are presented in Supplementary Table 8. The number of submicrometre particles released during washing with nanopure water, as measured by NTA, was, on average, three times (range of 1.3–4.5) higher than that estimated to be on the surface of the fibre surface by SEM image analysis.
a, SEM image of a polyester fibre from an unwashed Fleece polyester textile sample. b, SEM image of selected particles recovered from the washing liquid of a Fleece sample by drop casting the liquid onto a cleaned silica wafer. c, SEM image of the surface of a polyester fibre from the same sample of Fleece after a fourth wash. d, SEM image of a fibre from an unwashed Satin F sample, which released the highest number of particles per gram textile, as quantified by NTA. e, SEM image of a fibre from an unwashed Microfibre fabric sample, which released the lowest number of particles per gram textile, as quantified by NTA. f, A good correlation (P < 0.01) is observed between the number of submicrometre particles present on the surface of 1 g of unwashed fibre estimated by SEM image analysis and the number of submicrometre particles released per gram of fabric during the first wash, as determined by NTA. The data are presented as the mean ± sd (n = 3 textile replicates). The calculations and complete dataset are presented in Supplementary Note 3 and Supplementary Table 9. Scale bars in a–e, 5 μm.
We next investigated the morphology of the submicrometre particles by transmission electron microscopy (TEM). TEM images of submicrometre particles from a sample of Fleece centrifuged onto TEM grids are shown in Fig. 4a,b. We selected a submicrometre particle to study the element distribution by scanning TEM energy-dispersive X-ray (STEM-EDX) analysis (Fig. 4c). The carbon (Fig. 4e) and oxygen (Fig. 4f) signals were observed to correlate very well with the high-angle annular dark-field (HAADF) image of this submicrometre particle (Fig. 4d), while the silica signals (Fig. 4g) were similar to the background noise. This suggests that this submicrometre particle contains only carbon and oxygen and is unlikely to be a dust particle or a silica-containing additive used during the manufacture of polyester textiles. The EDX spectrum of this particle recorded up to 12 keV is presented in Supplementary Fig. 4. The elemental mapping images of another group of submicrometre particles are presented in Supplementary Fig. 5.
The particle morphology revealed by STEM and elemental analysis of selected submicrometre particles excludes the possibility of the particles being N- or Si-containing additives. a,b, STEM images showing different morphologies of submicrometre particles released from Fleece samples during washing with nanopure water and centrifuged onto a TEM grid. c, The morphology of the particle highlighted in blue in b with a size of about 1,000 nm under higher magnification. d, HAADF image of the particle shown in c. e–g, Elemental distribution maps of carbon (e), oxygen (f) and silica (g) for the particle shown in c. Additional HAADF images and elemental distribution maps as well as the complete EDX spectrum are shown in Supplementary Figs. 4 and 5. The particles contain mainly hydrogen, carbon and oxygen, in accord with the molecular formula of PET.
Distinction between oligomer nanoparticles and nanoplastics
The recovered submicrometre particles could be either PET nanoplastics or PET oligomers agglomerated into nanoparticles that should be dissolvable in ethanol. First, the NTA instrument was calibrated using standard polystyrene (PS) nanoplastics diluted in ethanol. The results showed a linear working range from 107 to 1010 particles ml−1 (Supplementary Fig. 6). After vortexing for 60 s in 50% ethanol, the PET reference nanoplastics did not dissolve, but smaller particles were observed, indicating the likely separation of agglomerates of PET by ethanol (Fig. 5b). The kinetics of dissolution of the oligomer particles was assessed by increasing the mixing time after the addition of ethanol to an extract from Fleece samples. Vortexing for 60 s before NTA analysis was adequate to see a significant drop (P < 0.001) in the number of particles extracted from Fleece samples. Extending the vortexing time to 180 s did not result in further dissolution (Supplementary Fig. 7a,b). In the presence of ethanol, 34–89% of the submicrometre particles extracted from six selected fabrics dissolved, with an average percentage of dissolved particles of 71% for the six fabrics (Fig. 5a and Supplementary Table 9). After ethanol dissolution, only larger particles remained for all six selected fabrics, suggesting that the smaller particles are oligomers present in the form of submicrometre particles. The change in size (Fig. 5b and Supplementary Table 9) was not due to aggregation in ethanol, as demonstrated by the calibration with 100 nm PS nanoplastics and PET nanoplastics in both ethanol and DI water, where the ethanol separated agglomerated PET nanoplastics.
a, Submicrometre particle concentrations (n = 3 replicates) in water (green bars) and after the addition of ethanol (blue bars) for PET nanoplastic reference particles (NanoPET) and particles extracted from six selected fabric samples. The contamination level of blanks (Lob, dashed line) was calculated as the mean blank + 1.63sd (n = 3 blanks). The original solutions were mixed with ethanol (1:1 v/v) and vortexed for 60 s at room temperature. b, Size distributions of particles in the original (solid lines) and ethanol-treated (dotted lines) solutions are presented up to a size of 600 nm for NanoPET and six selected fabrics. The signal intensity of the ethanol-treated samples is increased tenfold (except nanoPET) to facilitate comparison of the size distributions.
Pyrolysis–gas chromatography–mass spectrometry
Figure 6 (selected samples) and Supplementary Fig. 8 (all analysed samples) show the pyrolysis–gas chromatography–mass spectrometry (pyrolysis–GC–MS) chromatograms for a range of relevant samples. The pyrolysis products of a PET standard were identified as benzene homologues and derivates, which were also observed in the pyrolysis products of a textile sample (Supplementary Fig. 8), but not in the wash solution. Some low-molecular-weight benzenes, as well as saturated alkanes and unsaturated olefin chains, were detected in methanol extracts of the PET standard and textile. In particular, benzoic acid at 7.64−7.97 min and vinyl benzoate at 7.24 min were specific pyrolysis products of the highly polymeric PET standard and textile sample, consistent with previous studies31. In addition, some benzenes incorporating the N heteroatom were detected in the pyrolysis products of the textile and methanol and ethanol extracts (Supplementary Fig. 8), possibly originating from additives used during manufacturing. The textile wash solution was separated into supernatant and sediment, and both textile wash samples contained almost the same pyrolysis products (Supplementary Fig. 9). Moreover, the pyrolysis products of the textile wash samples were more similar to the pyrolysis products of the methanol and ethanol extracts of the PET standard and textile than to the pyrolysis products of the PET standard and textile themselves, indicating that a major fraction of the submicrometre particles released during washing were methanol- and ethanol-soluble oligomers rather than PET nanoplastics.
The submicrometre particles extracted from polyester textiles during washing are more likely to be aggregates of PET oligomers, as supported by the pyrolysis–GC–MS chromatograms of a textile (Satin F) wash solution (sedimented fraction after centrifugation), methanol and ethanol extracts of a PET standard, a PET standard and method blank control.
The origin and release mechanism of the submicrometre particles from textiles
This study contributes to a greater understanding of the plastic pollution from polyester textiles with an improved washing protocol for extracting submicrometre particles. No significant difference in the number of released submicrometre particles was observed for various fabric structures, yarn types and cutting methods. This result is counterintuitive because we expected submicrometre particles to share a similar release mechanism to MPFs5. As shown in a previous study, more MPFs are released from scissor-cut fabrics than from laser-cut fabrics, suggesting that the loosening of the fabric structure facilitates the release of MPFs5,10,32. This indicates that the release of submicrometre particles is governed by a process different from that of the release of MPFs. We have shown that the number of released particles measured by NTA correlates with the number of submicrometre particles visible on the surface of pristine fibres for all the investigated fabrics. After washing, these particles were removed from the fibre surfaces and the submicrometre particles recovered from the washing solutions shared similar morphologies to those observed on the fibre surfaces before washing. In addition, the decreasing number of submicrometre particles released over repeated washes indicates that they were not formed during washing, but instead were extracted. The above evidence suggests that the submicrometre particles obtained from washing polyester textiles emanate from a release mechanism rather than a formation mechanism. The fibre or fabric production process is therefore most likely responsible for the production of submicrometre particles that are later released during washing. It is also possible that these particles are formed after production during storage through the migration of oligomers to the surface and their precipitation as submicrometre particles. The differences between fabrics are therefore not related to the type of fabric, but rather to the grade of PET used to produce the fabric and the storage conditions after production (for example, temperature and time).
Chemical composition of the submicrometre particles
Various chemicals are added to fibres to achieve specific functionalities, such as fire resistance, high strength and chemical stability33. In a previous study, more micrometre- and submicrometre-sized particles were observed on the surfaces of polyester fibres containing silicon softeners than on untreated polyester fibres34. The EDX spectra of our samples showed no signals from silica, excluding the possibility that the submicrometre particles are silica-containing particles. In addition to additives, the raw materials and different fibre production (extrusion) techniques are also likely to influence the quality of the polyester fibre surface33. Atakan et al.35 revealed via SEM analysis that polyester textiles made of recycled PET pellets had more submicrometre particles on the fibre surfaces than those made with virgin PET, and fibres produced by different companies had different numbers of particles on their surfaces.
A previous study confirmed the presence of PET nanoplastics in the abrasion and washing samples of polyester textiles by single-nanoparticle NEXAFS spectral analysis16. Another study used the same analytical technique to identify PET nanoplastics in environmental samples36. However, a discussion recently emerged regarding the chemical composition of the nanoparticles released from polyester textiles, pointing out the possibility that they might consist of PET oligomers and not PET polymers37,38,39,40. Our study has demonstrated that PET nanoplastic standards are not dissolvable in ethanol, and that a major fraction of the submicrometre particles released from the surfaces of fibres are likely to be PET oligomers because they can be dissolved in ethanol. On average, 71% of the particles extracted from fabrics could be dissolved, which is in line with the conclusion in a previous study that ethanol treatment is needed to reduce the misidentification of ethanol-soluble oligomer particles as nanoplastic41. Pyrolysis–GC–MS analysis also revealed that the released submicrometre particles shared similar chemical components with the methanol and ethanol extracts of PET, indicating that they are more likely to be the precipitates of oligomers rather than PET nanoplastics. We cannot offer specific insights into the formation process of the ethanol-insoluble nanoplastics based on our current findings. However, it is worth noting that high-energy processes such as fibre spinning during the production of fibres, which have been shown to be the main source of fibre fragments in polyester textiles42, may also be linked to the generation of nanoplastics.
Synthetic textiles are a crucial source of plastic pollution at the submicrometre scale
The results of this study suggest that synthetic textiles are a crucial source of particulate pollution at the submicrometre scale. Twelve different textiles released an average of 1.7 × 1011 (4.6 × 109 to 8.9 × 1011) submicrometre particles (100–600 nm) per gram of textile (Supplementary Table 3) with an estimated average mass of 0.5 mg (0.1–1.6 mg) per gram of textile. Considering only the ethanol-insoluble PET nanoplastics, this number decreased to 4.5 × 1010 (4.3 × 108 to 3.7 × 1011) particles per gram of textile with an estimated mass ranging between 10−3 mg (Twill F) and 0.9 mg (Satin F) per gram of textile (size taken as 150 nm diameter; details of the calculations are presented in Supplementary Note 2). In contrast, the total oligomer content in PET fibres was measured to be 1–2% (refs. 43,44). The production of polyester textiles was predicted to reach 63 million metric tonnes by 202345, and more than half of this will be used in clothes that will be washed46. From the average release results obtained in this study, we can estimate that the amount of PET nanoplastics released during laundry worldwide could range from 36 to 36,000 metric tonnes per year. In addition, up to 0.1–0.4 million metric tonnes of oligomer particles are expected to be released. Although wastewater treatment plants can remove up to 99.4% of nanoplastics47,48, a substantial amount can still end up in the environment. This results in PET nanoplastics being the major type of nanoplastic in environmental samples, as about 2.7 µg l−1 of PET nanoplastics has been found in Greenland ice core samples49. In addition, more than 2 × 1011 nanoplastic particles have been found to be deposited per square metre of surface snow each week in the Alps, with PET identified as the major polymer type50.
Implications and recommendations
In light of our research findings, it is possible that the reported release of nanoplastics during the use of plastic products such as tea bags15, cups51 and milk bottles52 may be overestimated. This overestimation could result from the release of water-insoluble oligomer nanoparticles, as shown in our study. However, in plastic product release studies, it is important to distinguish the first release of nanoplastics generated in production from the continuous release of nanoplastics by formation during use. In the context of environmental monitoring of nanoplastics in environmental samples, it is noteworthy that the majority of these studies rely on MS-based techniques31,49,50. This study successfully illustrates the capability of these methods to distinguish nanoplastics from oligomers. However, to determine the size distribution and number concentration of nanoplastic particles, supplementary techniques such as light scattering-based and microscopic techniques are needed.
As the significance of oligomers becomes clearer, it is imperative to define the boundary between oligomers and nanoplastics. Given that PET oligomers are soluble in ethanol25,26,53, one approach to distinguish oligomers from nanoplastics is to examine their characteristics, such as their solubility in ethanol. However, it is worth noting that this method may not be applicable to other polymer types.
Further research is essential to comprehend the involvement of oligomers and/or small molecules in the potential adverse consequences of nanoplastics. The currently available information on PS and polylactic acid oligomers and monomers suggests that at least some oligomer particles might pose even greater concerns than nanoplastics27,54. More controlled studies with a special focus on the differences in the physicochemical properties of nanoplastics and oligomer particles are needed. In addition, the stability of oligomer particles in the environment remains poorly investigated and there is a need for more studies to elucidate their persistence and environmental relevance.
Tackling nanoplastic pollution requires effort from different stakeholders. There is increasing awareness on the part of consumers to take action against MPF pollution from synthetic textiles, including the use of point-of-use filters or devices to catch MPFs55. However, a method to remove large fibres is not effective for submicrometre particles. It is challenging for customers to identify clothing items with a low potential for releasing nanoplastics solely through visible textile characteristics. For instance, avoiding processed fabrics such as Fleece may not always guarantee a reduction in nanoplastic release, although this is feasible for microplastic fibre release5. We have identified the critical processes for the generation of submicrometre particles in the synthetic textile production chain: they are either introduced during the production of polyester fibres or formed after the migration of oligomers to the fibre surface during storage. This underscores the importance of manufacturers taking responsibility for minimizing nanoplastic pollution at the pre-consumer stage, where the issue may be less apparent to consumers.
Methods
Materials
We tested 12 different polyester fabrics (purchased directly from textile manufacturers) that can be grouped into woven, knit and surface-treated subgroups according to their fabric structure and properties. The 12 fabrics have various applications in household, transportation and clothing and have already been extensively studied for their potential to release MPFs during washing and abrasion5,16,56,57. They were obtained from two retailers and their physical properties (fabric structure and fibre surface) were characterized by SEM (Hitachi S6200, 2.0 kV, ×40). The densities of the polyester fabrics were determined by weighing three pieces of 10 cm × 4 cm samples. The physical properties (the fabric structures and fibre diameters are summarized in Supplementary Table 10) of the fabrics were characterized by SEM in previous MPF release studies5,56. Fabrics labelled with the suffix F denote fabrics made from filament yarns (endless fibre bundles), while those labelled S were made from spun yarns (staple length fibres). Plain B and Fleece are fabrics that have been subjected to special surface treatments by mechanical forces that intentionally damage their surfaces to create fuzzy and soft textures. The Microfibre textile (a surface-treated fabric with a woven structure) is made from much thinner fibres than the other textiles. The 12 fabrics were cut with either a laser cutter (tt-1300, Times Technology) or scissors into 2 cm × 2 cm samples. The scissors were carefully cleaned with ethanol and nanopure water to reduce cross-contamination. The average sample weight ranged from 0.03 to 0.09 g per piece of fabric. PET reference nanoplastics were synthesized according to an established protocol by dissolution and reprecipitation58.
Washing
The washing experiments were conducted in a Gyrowash machine (James Heal, Gyrowash Model 1615) with eight steel containers that simulate the domestic washing process under controlled conditions based on International Organization for Standardization (ISO) standard 105-C06 (ISO 1994)59. Modifications were made to reduce the nanoparticles present in blank samples while optimizing those in the treatment of fabrics. In the measurement of nanoparticles by NTA, high values in blank samples are a problem28. We conducted a series of experiments to analyse possible sources of contamination during the washing process. The sources that we considered included the washing containers, wash solutions and filtration units. In addition, we designed experiments to determine the influence of different washing settings on the release of submicrometre particles from Fleece fabrics to improve the washing protocol used for this study.
Based on the results of the contamination analysis, we decided to wash the sample fabrics in 25-ml glass vials closed with a (prewashed) PE snap cap. Ten millilitre nanopure water (Chorus 1 Analytic, ELGA LabWater) was used as the washing solution without the addition of detergent, which produced no signal in the method blank analysis by NTA. The volume of wash solution used is less than that specified by the standard ISO washing programme, representing a compromise aimed at maintaining a less contaminated environment. One steel ball (reduced from ten in proportion to the reduction in the volume of the washing liquid) was added to simulate the mechanical force generated during washing59. As a closed environment for the test sample fabric, the glass vial was placed in the Gyrowash steel container buffered with distilled water. The water bath was heated to 40 ± 2 °C before washing, and each round of standard washing took 45 min with a rotation speed of 40 r.p.m.
Up to four repeated washes were conducted on four selected fabrics to investigate the extractability of submicrometre particles. Although the washes reported in this Article were performed without detergent due to the high number of particles measured in blank samples by NTA, we also conducted the repeated washing experiments with 0.75 g l−1 LAS solution (Supplementary Table 1 and Supplementary Fig. 1).
All experiments were performed in triplicate. After washing, the fabrics were squeezed and removed from the Gyrowash machine with tweezers. The washing solution was then filtered and the filtrate was transferred for characterization by NTA. Nanopure water was used for the method blank samples, which were subjected to the washing and filtration processes without fabrics.
Particle separation
The wash solutions were filtered through 25 mm polycarbonate cyclopore membranes (Whatman) with a pore size of 2 μm in a polycarbonate filtration unit using 10 ml polypropylene syringes. To reduce contamination by the membrane and filtration system, 20 ml nanopure water was filtered and discarded before filtering sample or blank solutions. The filtrates were kept in clean, dried glass vials and transferred for NTA measurement as soon as possible (on the same day) to reduce the agglomeration of nanoparticles in the absence of surfactants. Nanoparticles recovered after washing Fleece samples were centrifuged onto a TEM grid (EM Resolution C200Cu25) at 754.6 g over 40 min. Details of the centrifugation and deposition methods have been described previously16.
Nanoparticle tracking analysis
Seventy-two polyester textile samples from 12 different fabrics and cut by two different methods were analysed to quantify the number of released particles. Submicrometre particles were analysed by NTA, a well-established method for characterizing the particle number and size of nanoparticles and nanoplastics60,61. NTA determines the Brownian motion of submicrometre particles and converts it to the hydrodynamic diameter, but the instrument cannot distinguish particles by their chemical properties. The NTA analyses were performed using a NanoSight LM20 device. Particles were counted and analysed using the NTA image analysis software, giving the attenuated particle size distribution and number concentration curves. PS nanoplastics with a size of 100 nm (Thermo Scientific, Nanosphere 3100A) were used to calibrate the number concentration and size distribution results reported by the instrument (Supplementary Note 3). The linear working range of this instrument was found to be 106–1010 particles ml−1 (Pearson’s correlation coefficient = 0.999, Supplementary Table 11 and Supplementary Fig. 9). The particle concentration fell into this range for all samples and blanks. The standardized measurement consisted of three steps. First, the instrument was calibrated with a 108 particles ml−1 PS nanoplastics solution. After calibrating the position of the camera vision and camera level, the mean particle size was determined to be 100 ± 10 nm with a number concentration of (1.0 ± 0.1) × 108 particles ml−1. To analyse another sample, the viewing cell was first rinsed with two injections of 500 µl DI water, followed by two injections of the next sample solution. Before drawing samples into the syringes, the glass vials were stirred with a vortex mixer for 15 s to diffuse the particles in the suspension. The NTA data were truncated to a size range of 100–600 nm before normalizing the number of released particles to submicrometre particles per gram of textile to compare different fabrics. Based on the findings of our previous study, we are confident that most of the released particles larger than 100 nm exhibit peaks characteristic of PET in their NEXAFS spectra16. Due to the weak light scattering of organic particles, the detection of particles smaller than 100 nm is limited even if they are present. Furthermore, it is worth noting that previous studies have demonstrated that NTA measurements of polydisperse particles can lead to inaccuracies in estimating smaller nanoparticles due to light scattered by larger particles61. These facts justify our decision to exclude nanoparticles smaller than 100 nm from our analysis.
Characterization of nanoparticles and surfaces by SEM and STEM-EDX
The surfaces of fibres and surface structures of different polyester textiles were characterized by SEM (Hitachi SU5000, 1–5 kV) without coating. Submicrometre particles released from sample textiles during washing were drop cast onto a silica wafer and sputter-coated with a 7 nm layer of Au/Pd using a high-vacuum sputter coater (LEICA EM ACE600) before observation by SEM (Quanta FEI 650, 5 kV, magnification up to ×20,000, resolution 1,536 × 1,103 pixels, dwell time 5 μs). STEM investigations were performed using a Talos F200X (FEI) microscope operating at 200 kV in STEM mode. The collecting angle of the HAADF detector was 60–200 mrad and the camera length was 98 mm. EDX mapping was performed up to 12 keV for elemental analysis using a Super-X EDX device in STEM mode with Esprit software (Bruker). The particle deposition method has been described previously16.
Ethanol treatment of released submicrometre particles
PET oligomers in PET food packaging can be extracted with ethanol26,53,62 (20–95 vol%). Therefore, we used ethanol to distinguish the dissolvable fraction of the extracted submicrometre particles. The linear working range of NTA was calibrated with solutions of PS standard diluted with ethanol (95 vol%) to compensate for the different viscosity of ethanol. To find the optimal vortex time, 1 ml of solution extracted from Fleece fabrics was vortexed for 0, 30, 60 and 180 s after the addition of 1 ml ethanol; the optimal setting was 60 s. Then, PET reference nanoparticles16 (diluted to ~108 particles ml−1) and the submicrometre particles extracted from six selected fabrics were treated with ethanol with a volume ratio of 1:1 (sample/95% ethanol) and vortexed at 1,500 r.p.m. for 60 s. All samples were analysed in triplicate at room temperature.
Pyrolysis–GC–MS
To distinguish oligomers and polymeric PET, pyrolysis–GC–MS was used to identify their specific pyrolysis products. Pyrolysis–GC–MS measurements were conducted using a Multi-Shot EGA/PY-3030D pyrolyser (Frontier Laboratories) connected to an Agilent 7890A gas chromatograph equipped with an HP-5MS column and an Agilent 5975C mass spectrometer detector. The pyrolysis was performed using the parameters reported previously31,63 with a single-shot mode pyrolysis temperature of 650 °C for 0.2 min and an interface temperature of 320 °C. The split ratio used to inject the pyrolysis products was 50:1. Details of the single-shot pyrolysis–GC–MS conditions are listed in Supplementary Table 12. Standard PET microplastics, a sample of the used textile (Satin F), the supernatant and sediment of the textile washing solution, and methanol and ethanol extracts of the PET standard and textile were analysed by pyrolysis–GC–MS to compare the pyrolysis products of these samples. Details of the pretreatment procedures of some samples are provided in Supplementary Note 4. The species responsible for the MS peaks were identified by comparing their full-scan mass spectra with the analytical pyrolysis library64.
Statistics
The size distribution of the particles for each sample was derived from the average concentration of particles of triplicate measurements by NTA performed at 1 nm intervals. The data are provided up to 600 nm because only a few signals were detected above 600 nm. The difference in the two cutting methods for the 12 textiles was tested using paired t-test analysis. The effect of fabric type and cutting method on the number of released nanoparticles (per gram of textile) was tested using one-way ANOVA performed in Rstudio (R version 4.0.5). Fabrics were classified into woven, knit and surface-treated groups; group comparisons were performed using the Benjamini–Hochberg group comparison method, also performed in Rstudio. The effect of yarn type (filament or spun) was also tested using the same method. The difference in the hydrodynamic size distribution of the recovered nanoparticles was tested using the same methods. P values of less than 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Details about experimental methods, numerical data on the number, size and morphology of nanoparticles, additional photos and SEM images are compiled in the Supplementary Information .Source data are provided with this paper.
Change history
13 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44221-024-00214-9
References
Hurley, R., Woodward, J. & Rothwell, J. J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 11, 251–257 (2018).
Kawecki, D. & Nowack, B. Polymer-specific modeling of the environmental emissions of seven commodity plastics as macro- and microplastics. Environ. Sci. Technol. 53, 9664–9676 (2019).
Koelmans, A. A. et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 155, 410–422 (2019).
Hernandez, E., Nowack, B. & Mitrano, D. M. Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 51, 7036–7046 (2017).
Cai, Y. et al. Systematic study of microplastic fiber release from 12 different polyester textiles during washing. Environ. Sci. Technol. 54, 4847–4855 (2020).
De Falco, F. et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 236, 916–925 (2018).
Carney Almroth, B. M. et al. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 25, 1191–1199 (2018).
Mapping of Global Plastics Value Chain and Plastics Losses to the Environment: With a Particular Focus on Marine Environment (United Nations Environment Programme, 2018).
Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe (European Environment Agency, 2022).
Cai, Y., Mitrano, D. M., Heuberger, M., Hufenus, R. & Nowack, B. The origin of microplastic fiber in polyester textiles: the textile production process matters. J. Clean. Prod. 267, 121970 (2020).
Pinlova, B., Hufenus, R. & Nowack, B. Systematic study of the presence of microplastic fibers during polyester yarn production. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2022.132247 (2022).
Gigault, J. et al. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 16, 501–507 (2021).
Mitrano, D. M., Wick, P. & Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 16, 491–500 (2021).
Wagner, S. & Reemtsma, T. Things we know and don’t know about nanoplastic in the environment. Nat. Nanotechnol. 14, 300–301 (2019).
Hernandez, L. M. et al. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 53, 12300–12310 (2019).
Yang, T., Luo, J. & Nowack, B. Characterization of nanoplastics, fibrils, and microplastics released during washing and abrasion of polyester textiles. Environ. Sci. Technol. 55, 15873–15881 (2021).
Magrì, D. et al. PET nanoplastics interactions with water contaminants and their impact on human cells. Environ. Pollut. 271, 116262 (2021).
Ji, Y. et al. Realistic polyethylene terephthalate nanoplastics and the size- and surface coating-dependent toxicological impacts on zebrafish embryos. Environ. Sci. Nano 7, 2313–2324 (2020).
Bejgarn, S., MacLeod, M., Bogdal, C. & Breitholtz, M. Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere 132, 114–119 (2015).
Tolardo, V. et al. In vitro high-throughput toxicological assessment of nanoplastics. Nanomaterials 12, 1947 (2022).
Zhang, H., Zhang, S., Duan, Z. & Wang, L. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ. Int. 162, 107177 (2022).
Silano, V. et al. Review and priority setting for substances that are listed without a specific migration limit in Table 1 of Annex 1 of Regulation 10/2011 on plastic materials and articles intended to come into contact with food. EFSA J. 18, e06124 (2020).
Schreier, V. N. et al. Evaluating the food safety and risk assessment evidence-base of polyethylene terephthalate oligomers: protocol for a systematic evidence map. Environ. Int. 167, 107387 (2022).
Hoppe, M., de Voogt, P. & Franz, R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates—a review. Trends Food Sci. Technol. 50, 118–130 (2016).
Castle, L., Mayo, A., Crews, C. & Gilbert, J. Migration of poly(ethylene terephthalate) (PET) oligomers from PET plastics into foods during microwave and conventional cooking and into bottled beverages. J. Food Prot. 52, 337–342 (1989).
Brenz, F., Linke, S. & Simat, T. Linear and cyclic oligomers in polybutylene terephthalate for food contact materials. Food Addit. Contam. 35, 583–598 (2018).
Wang, M. et al. Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01329-y (2023).
Lambert, S. & Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145, 265–268 (2016).
Mehtala, J. G. & Wei, A. Nanometric resolution in the hydrodynamic size analysis of ligand-stabilized gold nanorods. Langmuir 30, 13737–13743 (2014).
Bai, K., Barnett, G. V., Kar, S. R. & Das, T. K. Interference from proteins and surfactants on particle size distributions measured by nanoparticle tracking analysis (NTA). Pharm. Res. 34, 800–808 (2017).
Xu, Y. et al. Assessing the mass concentration of microplastics and nanoplastics in wastewater treatment plants by pyrolysis gas chromatography–mass spectrometry. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.2c07810 (2023).
Kärkkäinen, N. & Sillanpää, M. Quantification of different microplastic fibres discharged from textiles in machine wash and tumble drying. Environ. Sci. Pollut. Res. 28, 16253–16263 (2021).
Mahltig, B. & Grethe, T. High-performance and functional fiber materials-a review of properties, scanning electron microscopy SEM and electron dispersive spectroscopy EDS. Textiles 2, 209–251 (2022).
Parvinzadeh, M. & Hajiraissi, R. Macro- and microemulsion silicone softeners on polyester fibers: evaluation of different physical properties. J. Surfactants Deterg. 11, 269–273 (2008).
Atakan, R., Sezer, S. & Karakas, H. Development of nonwoven automotive carpets made of recycled PET fibers with improved abrasion resistance. J. Ind. Text. 49, 835–857 (2018).
Foetisch, A., Filella, M., Watts, B., Vinot, L.-H. & Bigalke, M. Identification and characterisation of individual nanoplastics by scanning transmission X-ray microscopy (STXM). J. Hazard. Mater. 426, 127804 (2022).
Busse, K. et al. Comment on “Plastic teabags release billions of microparticles and nanoparticles into tea”. Environ. Sci. Technol. 54, 14134–14135 (2020).
Hernandez, L. M. et al. Response to comment on “Plastic teabags release billions of microparticles and nanoparticles into tea”. Environ. Sci. Technol. 54, 14136–14137 (2020).
Stark, M. Comment on “Characterization of nanoplastics, fibrils, and microplastics released during washing and abrasion of polyester textiles”. Environ. Sci. Technol. 56, 10543–10544 (2022).
Yang, T. & Nowack, B. Reply to comment on “Characterization of nanoplastics, fibrils, and microplastics released during washing and abrasion of polyester textiles”. Environ. Sci. Technol. 56, 10545–10546 (2022).
Li, D. et al. Alcohol pretreatment to eliminate the interference of micro additive particles in the identification of microplastics using Raman spectroscopy. Environ. Sci. Technol. 56, 12158–12168 (2022).
Pinlova, B., Hufenus, R. & Nowack, B. Systematic study of the presence of microplastic fibers during polyester yarn production. J. Clean. Prod. 363, 132247 (2022).
Kawahara, Y. et al. Oligomer deposition on the surface of PET fiber in supercritical carbon dioxide fluid. Macromol. Mater. Eng. 291, 11–15 (2006).
Li, H. et al. Extraction of cyclic oligomer and their influence on polyester dyeing in a silicone waterless dyeing system. Polymers 13, 3687 (2021).
Tiseo, I. Global demand for polyester fibers 2017–2023. Statista https://www.statista.com/statistics/831459/demand-polyester-fibers-worldwide/#statisticContainer (2021).
Palacios-Mateo, C., van der Meer, Y. & Seide, G. J. E. S. E. Analysis of the polyester clothing value chain to identify key intervention points for sustainability. Environ. Sci. Eur. 33, 2 (2021).
Pulido-Reyes, G. et al. Nanoplastics removal during drinking water treatment: laboratory- and pilot-scale experiments and modeling. J. Hazard. Mater. 436, 129011 (2022).
Ramirez Arenas, L., Ramseier Gentile, S., Zimmermann, S. & Stoll, S. Fate and removal efficiency of polystyrene nanoplastics in a pilot drinking water treatment plant. Sci. Total Environ. 813, 152623 (2022).
Materić, D. et al. Nanoplastics measurements in northern and southern polar ice. Environ. Res. 208, 112741 (2022).
Materić, D., Ludewig, E., Brunner, D., Röckmann, T. & Holzinger, R. Nanoplastics transport to the remote, high-altitude Alps. Environ. Pollut. 288, 117697 (2021).
Zangmeister, C. D., Radney, J. G., Benkstein, K. D. & Kalanyan, B. Common single-use consumer plastic products release trillions of sub-100 nm nanoparticles per liter into water during normal use. Environ. Sci. Technol. 56, 5448–5455 (2022).
Li, D. et al. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 1, 746–754 (2020).
Ubeda, S., Aznar, M. & Nerín, C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC–MS–QTOF. Anal. Bioanal. Chem. 410, 2377–2384 (2018).
Zhang, Y. et al. Role of residual monomers in the manifestation of (cyto)toxicity by polystyrene microplastic model particles. Environ. Sci. Technol. 57, 9925–9933 (2023).
McIlwraith, H. K. et al. Capturing microfibers—marketed technologies reduce microfiber emissions from washing machines. Mar. Pollut. Bull. 139, 40–45 (2019).
Cai, Y., Mitrano, D. M., Hufenus, R. & Nowack, B. Formation of fiber fragments during abrasion of polyester textiles. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.1c00650 (2021).
Yang, T., Gao, M. & Nowack, B. Formation of microplastic fibers and fibrils during abrasion of a representative set of 12 polyester textiles. Sci. Total Environ. 862, 160758 (2023).
Rodríguez-Hernández, A. G., Muñoz-Tabares, J. A., Aguilar-Guzmán, J. C. & Vazquez-Duhalt, R. A novel and simple method for polyethylene terephthalate (PET) nanoparticle production. Environ. Sci. Nano 6, 2031–2036 (2019).
ISO 105-C06:2010 Textiles—Tests for Colour Fastness. Part C06: Colour Fastness to Domestic and Commercial Laundering (ISO, 1994).
Lambert, S., Sinclair, C. J., Bradley, E. L. & Boxall, A. B. A. Effects of environmental conditions on latex degradation in aquatic systems. Sci. Total Environ. 447, 225–234 (2013).
Filipe, V., Hawe, A. & Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 27, 796–810 (2010).
Alberto Lopes, J., Tsochatzis, E. D., Karasek, L., Hoekstra, E. J. & Emons, H. Analysis of PBT and PET cyclic oligomers in extracts of coffee capsules and food simulants by a HPLC–UV/FLD method. Food Chem. 345, 128739 (2021).
Ribeiro, F. et al. Quantitative analysis of selected plastics in high-commercial-value Australian seafood by pyrolysis gas chromatography mass spectrometry. Environ. Sci. Technol. 54, 9408–9417 (2020).
Gomiero, A., Øysæd, K. B., Palmas, L. & Skogerbø, G. Application of GCMS–pyrolysis to estimate the levels of microplastics in a drinking water supply system. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2021.125708 (2021).
Acknowledgements
We thank X. Wang for contributing to the pyrolysis–GC–MS measurements.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Author information
Authors and Affiliations
Contributions
T.Y. carried out the experiments. Y.X. performed the pyrolysis–GC–MS measurements, analysed the data and wrote the corresponding text, supervised by G.L. T.Y. analysed the data and wrote the paper. B.N. edited and wrote the paper with input from all authors. B.N. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Nanna Hartmann and Markus Sillanpää for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figs. 1–9, Tables 1–12 and Notes 1–4.
Source data
Source Data Fig. 1
Unprocessed and processed data for the box plot in Fig. 1b.
Source Data Fig. 2
Unprocessed data for Fig. 2b–e.
Source Data Fig. 5
Unprocessed data for Fig. 5b.
Source Data Fig. 6
Unprocessed data for Fig. 6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, T., Xu, Y., Liu, G. et al. Oligomers are a major fraction of the submicrometre particles released during washing of polyester textiles. Nat Water 2, 151–160 (2024). https://doi.org/10.1038/s44221-023-00191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-023-00191-5
This article is cited by
-
Exploring the continuum between nanoplastics and oligomers
Nature Water (2024)