Abstract
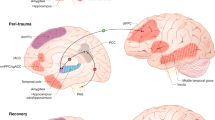
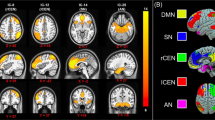
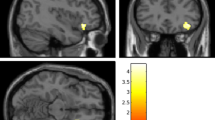
Although resilience is a dynamic process of recovery after trauma, in most studies it is conceptualized as the absence of specific psychopathology following trauma. Here, using the emergency department AURORA study (n = 1,835 with 63% women), we took a longitudinal, dynamic and transdiagnostic approach to define a static resilience (r) factor, which could explain greater than 50% of variance in mental well-being 6 months following trauma and a dynamic resilience factor, which represented recovery from initial symptoms. We then assessed its neurobiological profile across threat, inhibition and reward processes using functional magnetic resonance imaging collected 2 weeks post-trauma (n = 260). Our whole-brain and study-wide Bonferroni-corrected results suggest that resilience is promoted by activation of regions involved in higher-level cognitive functioning, reward valuation and salience detection in response to reward, whereas resilience is hampered by posterior default mode network activation to threat and reward. These findings serve to generate new hypotheses for brain mechanisms that could promote dynamic and multifaceted components of resilience following trauma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
We agree to make materials, data and associated protocols promptly available without undue qualifications. The data and/or research tools used in the preparation of this manuscript were obtained from the NDA. The NDA is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in mental health. The dataset identifier(s) include the NIMH Data Archive digital object identifier 10.15154/zwyn-rb26. The masks used for the ROI analyses are freely available. The Hammers atlas is available via https://brain-development.org/brain-atlases/ (ref. 72), the CITI168 subcortical atlas via https://osf.io/r2hvk/wiki/home/ (ref. 73), the WFUPickAtlas via https://www.nitrc.org/projects/wfu_pickatlas (ref. 74) and the Harvard/Oxford atlas via https://neurovault.org/collections/262/ (ref. 75). REX software is available via https://www.nitrc.org/projects/rex/ (ref. 19).
Code availability
We agree to make code promptly available without undue qualifications. More information is available at https://github.com/sjhvanrooij/rfactor (ref. 76).
Change history
26 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44220-024-00258-6
References
Resilience definition and meaning. Merriam-Webster https://www.merriam-webster.com/dictionary/resilience
Kalisch, R. et al. Deconstructing and reconstructing resilience: a dynamic network approach. Perspect. Psychol. Sci. J. Assoc. Psychol. Sci. 14, 765–777 (2019).
Roeckner, A. R., Oliver, K. I., Lebois, L. A. M., van Rooij, S. J. H. & Stevens, J. S. Neural contributors to trauma resilience: a review of longitudinal neuroimaging studies. Transl. Psychiatry 11, 508 (2021).
Kaldewaij, R. et al. Anterior prefrontal brain activity during emotion control predicts resilience to post-traumatic stress symptoms. Nat. Hum. Behav. 5, 1055–1064 (2021).
Richter, A., Krämer, B., Diekhof, E. K. & Gruber, O. Resilience to adversity is associated with increased activity and connectivity in the VTA and hippocampus. NeuroImage Clin. 23, 101920 (2019).
van Rooij, S. J. H. et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front. Psychiatry 7, 156 (2016).
van Rooij, S. J. H. et al. Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behav. Brain Res. 408, 113282 (2021).
Luthar, S. S. & Brown, P. J. Maximizing resilience through diverse levels of inquiry: prevailing paradigms, possibilities, and priorities for the future. Dev. Psychopathol. 19, 931–955 (2007).
Luthar, S. S., Cicchetti, D. & Becker, B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 71, 543–562 (2000).
Rutter, M. Resilience: some conceptual considerations. J. Adolesc. Health 14, 626–631 (1993).
Wallbrown, F. H., Blaha, J., Wallbrown, J. D. & Engin, A. W. The hierarchical factor structure of the wechsler intelligence scale for children-revised. J. Psychol. 89, 223–235 (1975).
Caspi, A. et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. J. Assoc. Psychol. Sci. 2, 119–137 (2014).
Caspi, A. & Moffitt, T. E. All for one and one for all: mental disorders in one dimension. Am. J. Psychiatry 175, 831–844 (2018).
van Harmelen, A.-L. et al. Adolescent friendships predict later resilient functioning across psychosocial domains in a healthy community cohort. Psychol. Med. 47, 2312–2322 (2017).
Galatzer-Levy, I. R. et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the jerusalem trauma outreach and prevention study (J-TOPS). PLoS ONE 8, e70084 (2013).
McLean, S. A. et al. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry 25, 283–296 (2020).
Stevens, J. S. et al. Brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. Am. J. Psychiatry 178, 1037–1049 (2021).
Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019).
REX. NITRIC https://www.nitrc.org/projects/rex/ (2007).
O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J. & Andrews, C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4, 95–102 (2001).
Wallis, J. D. & Kennerley, S. W. Contrasting reward signals in the orbitofrontal cortex and anterior cingulate cortex. Ann. N. Y. Acad. Sci. 1239, 33–42 (2011).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Hu, S., Ide, J. S., Zhang, S. & Li, C.-S. R. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J. Neurosci. Off. J. Soc. Neurosci. 36, 12688–12696 (2016).
du Boisgueheneuc, F. et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain J. Neurol. 129, 3315–3328 (2006).
Brockett, A. T., Tennyson, S. S., deBettencourt, C. A., Gaye, F. & Roesch, M. R. Anterior cingulate cortex is necessary for adaptation of action plans. Proc. Natl Acad. Sci. USA 117, 6196–6204 (2020).
Doya, K. Modulators of decision making. Nat. Neurosci. 11, 410–416 (2008).
Behrens, T. E. J., Hunt, L. T., Woolrich, M. W. & Rushworth, M. F. S. Associative learning of social value. Nature 456, 245–249 (2008).
Dosenbach, N. U. F., Raichle, M. & Gordon, E. M. The brain’s cingulo-opercular action-mode network. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/2vt79 (2024).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
McTeague, L. M. et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 177, 411–421 (2020).
Mahayana, I. T., Tcheang, L., Chen, C.-Y., Juan, C.-H. & Muggleton, N. G. The precuneus and visuospatial attention in near and far space: a transcranial magnetic stimulation study. Brain Stimul. 7, 673–679 (2014).
Lee, J. et al. rTMS over bilateral inferior parietal cortex induces decrement of spatial sustained attention. Front. Hum. Neurosci. 7, 26 (2013).
Hommet, C. et al. Unilateral spatial neglect following right inferior parietal cortectomy. Epilepsy Behav. 5, 416–419 (2004).
Smallwood, J. et al. The default mode network in cognition: a topographical perspective. Nat. Rev. Neurosci. 22, 503–513 (2021).
Zhang, W., Xie, Y. & Yang, T. Reward salience but not spatial attention dominates the value representation in the orbitofrontal cortex. Nat. Commun. 13, 6306 (2022).
Anderson, B. A., Laurent, P. A. & Yantis, S. Value-driven attentional capture. Proc. Natl Acad. Sci. USA 108, 10367–10371 (2011).
David, O. A. & Magurean, S. Positive attention bias trained during the rethink therapeutic online game and related improvements in children and adolescents’ mental health. Children 9, 1600 (2022).
van Rooij, S. J. H. et al. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol. Psychiatry 84, 106–115 (2018).
Kim, Y. J. et al. Association between posttraumatic stress disorder severity and amygdala habituation to fearful stimuli. Depress. Anxiety 36, 647–658 (2019).
Wicking, M. et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol. Learn. Mem. 136, 116–126 (2016).
Chaposhloo, M. et al. Altered resting-state functional connectivity in the anterior and posterior hippocampus in post-traumatic stress disorder: the central role of the anterior hippocampus. NeuroImage Clin. 38, 103417 (2023).
Stevens, J. S. et al. Episodic memory after trauma exposure: medial temporal lobe function is positively related to re-experiencing and inversely related to negative affect symptoms. NeuroImage Clin. 17, 650–658 (2018).
Tanriverdi, B. et al. Hippocampal threat reactivity interacts with physiological arousal to predict PTSD symptoms. J. Neurosci. 42, 6593–6604 (2022).
Garavan, H., Ross, T. J. & Stein, E. A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl Acad. Sci. USA 96, 8301–8306 (1999).
van Rooij, S. J. H., Geuze, E., Kennis, M., Rademaker, A. R. & Vink, M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology 40, 667–675 (2015).
Luijten, M., Meerkerk, G.-J., Franken, I. H. A., van de Wetering, B. J. M. & Schoenmakers, T. M. An fMRI study of cognitive control in problem gamers. Psychiatry Res. 231, 262–268 (2015).
Solanto, M. V., Schulz, K. P., Fan, J., Tang, C. Y. & Newcorn, J. H. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J. Neuroimaging 19, 205–212 (2009).
Powers, A. et al. Right inferior frontal gyrus and ventromedial prefrontal activation during response inhibition is implicated in the development of PTSD symptoms. Eur. J. Psychotraumatology 13, 2059993 (2022).
van Rooij, S. J. H. et al. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. J. Psychiatry Neurosci. 39, 330–338 (2014).
Grieder, M. et al. Right inferior frontal activation during alcohol-specific inhibition increases with craving and predicts drinking outcome in alcohol use disorder. Front. Psychiatry 13, 909992 (2022).
Kleerekooper, I. et al. The effect of aging on fronto-striatal reactive and proactive inhibitory control. NeuroImage 132, 51–58 (2016).
Admon, R. et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb. Cortex 23, 28–35 (2013).
Vythilingam, M. et al. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry Res. 172, 75–77 (2009).
Ben-Zion, Z. et al. Neural responsivity to reward versus punishment shortly after trauma predicts long-term development of posttraumatic stress symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 150–161 (2022).
Elliott, M. L. et al. What is the test–retest reliability of common task-functional mri measures? new empirical evidence and a meta-analysis. Psychol. Sci. 31, 792–806 (2020).
Ben-Zion, Z. et al. Evaluating the evidence for brain-based biotypes of psychiatric vulnerability in the acute aftermath of trauma. Am. J. Psychiatry 180, 146–154 (2023).
Bernstein, D. P., Ahluvalia, T., Pogge, D. & Handelsman, L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348 (1997).
Connor, K. M. & Davidson, J. R. T. Development of a new resilience scale: the Connor–Davidson Resilience Scale (CD-RISC). Depress. Anxiety 18, 76–82 (2003).
Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale (c) 2005 Update 2008 (eds Gennarelli, T. & Woodzin, E.) (AAAM, 2016).
Bortsov, A. V. et al. Pain distribution and predictors of widespread pain in the immediate aftermath of motor vehicle collision. Eur. J. Pain 17, 1243–1251 (2013).
Pilkonis, P. A. et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS): depression, anxiety, and anger. Assessment 18, 263–283 (2011).
PROMIS. HealthMeasures https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis
Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K. & Domino, J. L. The posttraumatic stress disorder checklist for dsm-5 (PCL-5): development and initial psychometric evaluation. J. Trauma. Stress 28, 489–498 (2015).
Hamilton, C. M. et al. The PhenX Toolkit: get the most from your measures. Am. J. Epidemiol. 174, 253–260 (2011).
Cyders, M. A., Littlefield, A. K., Coffey, S. & Karyadi, K. A. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addict. Behav. 39, 1372–1376 (2014).
Stevens, J. S. et al. Disrupted amygdala–prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 47, 1469–1478 (2013).
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C. & Fiez, J. A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 84, 3072–3077 (2000).
Pruim, R. H. R., Mennes, M., Buitelaar, J. K. & Beckmann, C. F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage 112, 278–287 (2015).
Hammers, A. et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 19, 224–247 (2003).
Jovanovic, T. et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex J. Devoted Study Nerv. Syst. Behav. 49, 1884–1891 (2013).
Tyszka, J. M. & Pauli, W. M. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum. Brain Mapp. 37, 3979–3998 (2016).
Hammers atlas database. Brain Development https://brain-development.org/brain-atlases/.
An in vivo high resolution atlas of the subcortical human brain. OSF https://osf.io/r2hvk/wiki/home/.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19, 1233–1239 (2003).
Gorgolewski, K. J. et al. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the brain. Front. Neuroinform. 9, 8 (2015).
van Rooij, S. J. rfactor. GitHub https://github.com/sjhvanrooij/rfactor (2023).
Acknowledgements
The investigators thank the trauma survivors participating in the AURORA study. Their time and effort during a challenging period of their lives make our efforts to improve recovery for future trauma survivors possible. The AURORA project was supported by the National Institute of Mental Health (NIMH) under U01MH110925, the US Army MRMC, One Mind, and the Mayday Fund. The authors that were supported financially by funding for the AURORA project are as follows: R.C.K., K.J.R., K.C.K., S.A.M., S.L.H., F.L.B., X.A., J.S.S., T.C.N., G.D.C., T.J., S.D.L., L.T.G., K.A.B., S.L.R., J.P.H., A.B.S., C.L., P.I.M., P.L.H., S.S., C.W.J., B.E.P., R.A.S., V.P.M., J.L.P., M.J.S., E.H., C.P., D.A.P., R.C.M., R.M.D., N.K.R., B.J.O., L.D.S., S.E.B., J.J., D.A.P., J.F.S. and S.E.H. This paper was also supported by the NIMH under K01MH121653 (S.J.H.R.). The content is solely responsibility of the authors and does not necessarily represent the official views of any of the funders. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Verily Life Sciences and Mindstrong Health provided some of the hardware and software used to perform study assessments. The Many Brains Project provided software for neurocognitive assessments. This paper reflects the views of the authors and may not reflect the opinions or views of the NIH or of the submitters submitting original data to the NIMH Data Archive (NDA).
Author information
Authors and Affiliations
Contributions
S.A.M., R.C.K., K.J.R., K.C.K., S.J.H.v.R., J.S.S., J.L.S., C.A.H., T.D.E., N.H., V.M., L.L., T.J., S.L.H. and S.B. contributed to the conceptualization, including formulation or evolution of overarching research goals and aims, of the study. F.L.B., X.A., T.C.N., G.D.C., S.D.L., L.T.G., K.A.B., S.L.R., J.J., D.A.P., J.F.S., S.E.H., S.A.M., R.C.K., K.J.R., K.C.K., S.J.H.v.R., J.S.S, J.L.S., C.A.H., T.D.E., N.H., V.M., L.L., T.J., S.L.H. and S.B. contributed to the methodology of the study, including development or design of methodology and creation of models. S.J.H.v.R., J.S.S, J.L.S., C.A.H., T.D.E., N.H., V.M., L.L., T.J., S.L.H., S.B. and K.J.R. contributed to the neuroimagingdata collection, formal analyses and validation of the study. S.L.H., F.L.B., X.A., J.S.S., T.C.N., G.D.C., T.J., S.D.L., L.T.G., K.A.B., S.L.R., J.P.H., A.B.S., C.L., P.I.M.Jr, P.L.H., S.S., C.W.J., B.E.P., R.A.S., J.L.P., M.J.S., E.H., C.P., D.A.P., R.C.M., R.M.D., N.K.R., B.J.O., L.D.S., S.A.M., R.C.K., K.J.R. and K.C.K. conducted the research and investigation process, specifically, performing the experiments or data and evidence collection. S.L.H., F.L.B., X.A., J.S.S., T.C.N., G.D.C., T.J., S.D.L., L.T.G., K.A.B., S.L.R., J.P.H., A.B.S., C.L., P.I.M.Jr, P.L.H., S.S., C.W.J., B.E.P., R.A.S., J.L.P., M.J.S., E.H., C.P., D.A.P., R.C.M., R.M.D., N.K.R., B.J.O., L.D.S., S.A.M., R.C.K., K.J.R. and K.C.K. provided the resources for the study, including provision of study materials, patients, laboratory samples, instrumentation, computing resources or other analysis tools. S.L.H., F.L.B., X.A., J.S.S., T.C.N., G.D.C., T.J., S.D.L., L.T.G., K.A.B., S.L.R., S.A.M., R.C.K., K.J.R. and K.C.K. were responsible for data curation, including management activities to annotate, scrub data and maintain research data for initial use and later reuse. S.J.H.v.R., J.S.S., J.L.S., C.A.H., N.H., T.J. and K.J.R. were responsible for writing the original draft, including prepration, creation and presentation of the published work, and writing initial draft. All authors contributed to the paper by reviewing and editing the original draft. S.J.H.v.R., J.S.S., J.L.S. and C.A.H. were responsible for data visualization, including preparing, creating and presenting the published work, specifically, visualization and data presentation. S.A.M., R.C.K., K.J.R., K.C.K., S.J.H.v.R. and J.S.S. were responsible for supervision, including oversight and leadership for the research activity planning and execution, including mentorship external to the core team. S.L.H., F.L.B., J.S.S., T.J., J.P.H., A.B.S., C.L. P.I.M.Jr, P.L.H., S.S., C.W.J., B.E.P., R.A.S., J.L.P., M.J.S., E.H., C.P., D.A.P., R.C.M., R.M.D., N.K.R., N.J.O., L.D.S. and S.B. were responsible for project administration, including management and coordination, responsibility for the research activity planning and execution. S.A.M., R.C.K., K.J.R. and K.C.K. were responsible for acquisition of the financial support for the project leading to this publication. S.J.H.v.R. was responsible for funding supporting her effort on this publication.
Corresponding author
Ethics declarations
Competing interests
T.C.N. has received research support from NIH, VA and Rainwater Charitable Foundation and consulting income from Jazz Pharmaceuticals. In the past 3 years, G.D.C. has received research funding from the NSF, NIH and LifeBell AI and unrestricted donations from AliveCor Inc., Amazon Research, the Center for Discovery, the Gates Foundation, Google, the Gordon and Betty Moore Foundation, MathWorks, Microsoft Research, Nextsense Inc, One Mind Foundation, the Rett Research Foundation and Samsun Research. G.D.C. has financial interest in AliveCor Inc. and Nextsense Inc. He is also the CTO of MindChild Medical and CSO of LifeBell AI and has ownership in both companies. These relationships are unconnected to the current work. L.T.G. receives funding from the National Institute of Mental Health (R01 MH121617) and am on the board of the Many Brains Project. Her family also has equity in Intelerad Medical Systems, Inc. S.L.R. reports grants from NIH during the conduct of the study; personal fees from the Society of Biological Psychiatry paid roles as secretary, other from Oxford University Press royalties, other from American Psychiatric Publishing Inc. royalties, other from the Veterans Administration per diem for oversight committee and other from Community Psychiatry/Mindpath Health paid board service, including equity outside the submitted work; other from National Association of Behavioral Healthcare for paid Board service; other from Springer Publishing royalties; and Leadership roles on Board or Council for SOBP, the Anxiety and Depression Association of America and the National Network of Depression Centers. S.S. has received funding from the Florida Medical Malpractice Joint Underwriter’s Association Dr. Alvin E. Smith Safety of Healthcare Services Grant, Allergan Foundation, the NIH/NIA-funded Jacksonville Aging Studies Center (JAX-ASCENT; R33AG05654), the Substance Abuse and Mental health Services Administration (1H79TI083101-01) and the Florida Blue Foundation. C.W.J. has no competing interest related to this work, though he has been an investigator on studies funded by AstraZeneca, Vapotherm, Abbott and Ophirex. J.J. receives consulting payments from Janssen Pharmaceuticals. Over the past 3 years, D.A.P. has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Concert Pharmaceuticals, Engrail Therapeutics, Neumora Therapeutics (former BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka Pharmaceuticals and Takeda Pharmaceuticals; honoraria from the Psychonomic Society (for editorial work) and Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation and Millennium Pharmaceuticals. In addition, he has received stock option from Neumora Therapeutics (former BlackThorn Therapeutics), Compass Pathways, Engrail Therapeutics, and Neuroscience Software. S.E.H. has no competing interest related to this work, though in the past 3 years he has received research funding from Aptinyx and Arbor Medical Innovations and consulting payments from Aptinyx, heron Therapeutics and Eli Lilly. In the past 3 years, R.C.K. was a consultant for Cambridge Health Alliance, Canandaigua VA Medical Center, Holmusk, Partners Healthcare, Inc., RallyPoint Networks, Inc. and Sage Therapeutics. He has stock options in Cerebral Inc., Mirah, PYM and Roga Sciences. K.C.K’s research has been supported by the Robert Wood Johnson Foundation, the Kaiser Family Foundation, the Harvard Center on the Developing Child, Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard, the NIH, One Mind, The Anonymous Foundation and Cohen Veterans Bioscience. She has been a paid consultant for Baker Hostetler, Discovery Vitality and the Department of Justice. She has been a paid external reviewer for the Chan Zuckerberg Foundation, the University of Cape Town and Capita Ireland. She has had paid speaking engagements in the past 3 years with the American Psychological Association, European Central Bank. Sigmund Freud University—Milan, Cambridge Health Alliance and Coverys. She receives royalties from Guilford Press and Oxford University Press. S.A.M. served as a consultant for Walter Reed Army Institute for Research and for Arbor Medical Innovations. K.J.R. has performed scientific consultation for Bioxcel, Bionomics, Acer and Jazz Pharm; serves on Scientific Advisory Boards for Sage, Boehringer Ingelheim, Senseye and the Brain Research Foundation; and has received sponsored research support from Alto Neuroscience. The other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Saurabh Shaw, Liliane Vilete and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Rooij, S.J.H., Santos, J.L., Hinojosa, C.A. et al. Defining the r factor for post-trauma resilience and its neural predictors. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00242-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44220-024-00242-0