Abstract
Throughout the COVID-19 pandemic, the new clinical entity of the post-COVID-19 condition, defined as a multisystemic condition of persistent symptoms following resolution of an acute severe acute respiratory syndrome coronavirus 2 infection, has emerged as an important area of clinical focus. While this syndrome spans multiple organ systems, cardiovascular complications are often the most prominent features. These include, but are not limited to, myocardial injury, heart failure, arrhythmias, vascular injury/thrombosis and dysautonomia. As the number of individuals with the post-COVID-19 condition continues to climb and overwhelm medical systems, summarizing existing information and knowledge gaps in the complex cardiovascular effects of the post-COVID-19 condition has become critical for patient care. In this Review, we explore the current state of knowledge of the post-COVID-19 condition and identify areas where additional research is warranted. This will provide a framework for better understanding the cardiovascular manifestations of the post-COVID-19 condition with a focus on pathophysiology, diagnosis and management.
Similar content being viewed by others
Main
The widespread study of COVID-19 has led to the discovery of various underlying pathological mechanisms and clinical sequelae1. Although primarily a respiratory pathogen, SARS-CoV-2 is now known to cause multiorgan dysfunction with systemic inflammation ranging from asymptomatic to fatal. The increasingly common condition following acute infection has been described in variable terms including long COVID-19 syndrome, long COVID, post-COVID-19 condition and post-acute sequelae of COVID (PASC)2. One common definition of the post-COVID-19 condition is a multisystemic condition of persistent, relapsing or new symptoms following an acute SARS-CoV-2 infection3. To date, there are over 23,000 publications regarding the post-COVID-19 condition. Many of these focus on defining the syndrome and identifying the patient populations most affected4. Other publications have delved into the molecular mechanisms at play and hypotheses surrounding the pathophysiology underlying clinical manifestations5. There has been considerable progress in attempting to determine the natural history of this syndrome and potential treatment approaches; however, many patients remain symptomatic years later and are considered to have a chronic health condition. This Review will provide a detailed overview into the currently known cardiovascular effects of the post-COVID-19 condition along with proposed management and future directions for this syndrome4.
Acute infection with SARS-CoV-2 is most classically marked by a febrile upper respiratory illness that commonly causes pneumonia and can lead to widespread organ dysfunction in severe cases; however, the infection can present in a multitude of ways6,7. In general, characteristics of patients found to be at higher risk of severe infection and COVID-19-related mortality include increasing age, male sex, minority race/ethnicity, a variety of comorbid medical conditions, obesity, active smoking, unvaccinated individuals, those with respiratory failure requiring mechanical ventilation and/or multisystem involvement, as well as those requiring intensive care8,9,10,11,12. Similarly, studies have shown that older patients with comorbidities are at higher risk for delayed recovery13. The immediate clinical manifestations of COVID-19 can affect nearly all organ systems and may include pneumonia, hypoxic respiratory failure, myocarditis, thromboembolism, stroke, acute myocardial infarction, acute kidney or liver failure, vasculitis and several immune phenomena14,15,16,17,18,19. While the majority of these processes are recoverable, the post-COVID-19 condition is subsequently diagnosed when a patient has unresolved acute illness, relapsing symptoms or incident symptoms occurring after acute COVID-19. The time course of this condition is variable. The World Health Organization developed a clinical case definition in 2021 of the post-COVID-19 condition as that which occurs in individuals with presumed or confirmed SARS-CoV-2 infection, usually 3 months from the onset of symptoms, lasting at least 2 months, and unable to be explained by other diagnoses20. A more recent and widely accepted definition uses a timeline of 30 days after infection to classify the post-COVID-19 condition3.
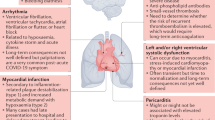
The components of the post-COVID-19 condition specifically relevant to the cardiovascular system that will be focused upon in this Review include myocardial injury, heart failure, thrombosis, dysautonomia and arrhythmias (Fig. 1)21. Special distinction has been given to two subpopulations of patients with a post-COVID-19 condition by the American College of Cardiology in their 2022 Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: those with cardiovascular risk factors and/or preexisting disease who develop a broad range of cardiovascular conditions versus patients with cardiovascular symptoms who lack objective evidence of cardiovascular disease22. The distinction is felt to be important regarding underlying pathophysiology and treatment and will be discussed further. Patients are being increasingly referred to cardiologists for management of the post-COVID-19 condition; therefore, a solid understanding of the mechanisms and treatment options is imperative for cardiovascular care providers.
Diagnosis
The post-COVID-19 condition can involve nearly every organ system and include dozens of symptoms. The most common non-cardiovascular effects reported include neurologic, psychologic, renal, respiratory and gastrointestinal dysfunction2. There is often a complex interplay between affected systems and resultant patient symptoms. Nonspecific complaints can include fatigue, dizziness, abdominal pain and memory loss. Certain underlying mechanisms such as systemic inflammation may explain the overlapping and vague symptoms. The post-COVID-19 condition has shown to be a highly variable disease process in terms of populations affected, time course, symptomatology and prognosis. Although the post-COVID-19 condition can be seen in nearly all ages and demographics, certain groups appear to be at higher risk including females, older patients, individuals who smoke and those with higher body mass index8. Additional risk factors include a variety of comorbid conditions, including individuals who required intensive care during their acute COVID-19 condition, and those with early pandemic variants, recurrent infections and incomplete vaccination status3,8 (Table 1). The recently published prospective observational cohort as part of the National Institutes of Health Researching COVID to Enhance Recovery (RECOVER) Initiative proposed a scoring system to help identify patients with the post-COVID-19 condition. They identified 12 important symptoms among patients 6 months out from index infection: post-exertional malaise, fatigue, brain fog, dizziness, gastrointestinal symptoms, palpitations, changes in sexual desire/capacity, loss/change in smell or taste, thirst, chronic cough, chest pain and abnormal movements3 (Table 1). Although over 200 symptoms have been reported in the post-COVID-19 condition, these appear consistent with the most frequent complaints in affected individuals. This study importantly notes that there is great heterogeneity in symptoms, and distinct phenotypes likely exist within all patients with the post-COVID-19 condition. Among the cardiovascular phenotypes proposed by the American College of Cardiology practice guideline document, the population with overt cardiovascular disease after COVID-19, deemed PASC-CVD, tend to be older with traditional cardiovascular risk factors and comorbidities. These patients are more commonly found to have demonstrable myocardial dysfunction, ischemia and/or inflammation. Those with cardiovascular symptoms (such as chest pain and palpitations) while lacking clear disease on diagnostic testing, termed PASC-CVS, are classically younger and healthier at baseline22. These represent two separate populations in terms of likely pathophysiology and thus proposed management.
A multidisciplinary approach involving various specialists such as cardiologists, pulmonologists, neurologists, rheumatologists, psychiatrists and infectious disease experts is often utilized to diagnose the post-COVID-19 condition. Initial diagnostic evaluation typically includes laboratory testing (such as complete blood count, basic metabolic panel, troponin, pro-brain natriuretic peptide and C-reactive protein), electrocardiogram and echocardiogram (Table 1). Based on the type and severity of patients’ symptoms and results of the initial testing, additional studies such as cardiac magnetic resonance imaging (MRI), coronary angiography, ambulatory rhythm monitor, chest imaging and pulmonary function tests may be pursued22.
Although the cardiovascular complications of the post-COVID-19 condition are highly publicized, the sequelae from this virus are not particularly unique. Cardiovascular effects including myocarditis have been long described following other viral illnesses such as influenza and Epstein–Barr virus23,24. Similarly, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) has several parallels to the post-COVID-19 condition and is hypothesized to arise after a percentage of Epstein–Barr virus and other viral infections25. However, the mortality rate and incidence of vascular complications is far greater in COVID-19.
Pathophysiology
The currently known pathophysiology underlying many of the cardiovascular complications of the post-COVID-19 condition can be grouped into those involving immune dysregulation and inflammation, endothelial dysfunction, microvascular injury and neurological signaling dysfunction26,27,28. Patients with the PASC-CVD phenotype are more likely to have endothelial dysfunction, microvascular injury and inflammation underlying their post-COVID-19 condition, while those with PASC-CVS tend to have more from immune dysregulation and neurological signaling dysfunction in addition to inflammation22. Immediate cardiovascular effects of COVID-19 are often thought to be caused by direct cytotoxic injury with viral cell entry via the angiotensin-converting enzyme 2 receptor or via downstream inflammation and cytokine release26,29. Hyperactivation of the immune system can induce endothelial dysfunction and immunothrombosis, further leading to microthrombotic complications, which can affect numerous organ systems by direct and indirect mechanisms2,28. Studies of patients with the post-COVID-19 condition have found plasma microthrombi in addition to decreased capillary density many months after acute infection, which support a hypothesis of long-term vascular dysfunction30,31. Furthermore, vascular activation biomarkers have been identified in individuals with the post-COVID-19 condition with angiogenesis proteins ANG-1 and P-SEL being highly sensitive and specific for predicting long COVID status32.
Ongoing inflammatory marker elevations have been detected in individuals with the post-COVID-19 condition suggesting a role of immune dysregulation pathways2. This is particularly relevant in the subpopulation of patients with PASC-CVS who typically lack cardiovascular disease at baseline. In the case of autonomic dysregulation, abnormal signaling in neurological pathways has been suggested as an underlying mechanism for this common post-COVID-19 condition manifestation33. There are also downstream effects of COVID-19 that secondarily impact cardiovascular disease. Patients recovered from COVID-19 have been found in multiple studies to have incident diabetes mellitus and hypertension as well as exacerbation of the disease for those with a preexisting diagnosis34,35,36. This undoubtedly contributes to increased risks of heart failure, atherosclerosis, atrial arrhythmias and endothelial dysfunction. Ultimately, further studies are needed to fully elucidate the major mechanisms involved in specific symptom clusters of the post-COVID-19 condition to help guide treatment strategies.
POTS
Since the start of the COVID-19 pandemic, postural orthostatic tachycardia syndrome (POTS) has been increasingly recognized among individuals who develop chronic symptoms after recovering from acute COVID-19 infection, particularly those with the PASC-CVS phenotype described above. Initially, a few case reports emerged in which POTS was observed immediately after the resolution of acute COVID infection37,38,39,40,41,42,43,44. As the pandemic continues, several studies have investigated autonomic function using various tests, including the head-up tilt-table test (HUTT), providing further evidence of autonomic dysfunction in the post-COVID-19 condition45,46,47,48,49,50,51,52,53,54,55,56,57,58 (Table 2). However, the prevalence of POTS among individuals with the post-COVID-19 condition varies widely across studies, as not all patients meet the criteria for POTS on a tilt-table test. Nonetheless, their clinical symptoms are essentially indistinguishable, with most patients experiencing orthostatic intolerance during the HUTT. It is important to note that the reproducibility of the HUTT is moderate to poor in patients without structural heart disease59,60,61. Therefore, the actual prevalence of POTS is likely much higher than reported thus far. A recent meta-analysis of published studies on cardiovascular autonomic dysfunction following COVID-19 infection revealed that the most common diagnosis in the post-COVID-19 condition (defined as symptom persistence for >28 days) was POTS62.
Typical symptoms of POTS include chronic fatigue, dizziness, palpitations, pre-syncope and brain fog, among many others, and these symptoms are often debilitating. POTS is defined by the presence of the above symptoms that last longer than 3 months and a sustained heart rate increase of at least 30 beats per minute (40 beats per minute for pediatric patients) or a heart rate of 120 beats per minute or more within 10 min of active standing or during the HUTT in the absence of orthostatic hypotension while reproducing the typical symptoms. POTS is a clinical syndrome, not a disease, with potentially heterogeneous etiologies. The COVID-19 pandemic and increasing incidence of POTS in patients with the post-COVID-19 condition may offer insights to the underlying mechanisms at play. When diagnosing POTS, it is important to recognize the patterns of symptoms, and the HUTT is not always necessary to establish the diagnosis if other conditions that mimic POTS, such as pulmonary embolism, interstitial lung disease or cardiac arrhythmia, have been ruled out. The HUTT should be considered as supporting evidence for POTS, rather than the sole diagnostic factor.
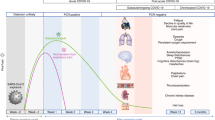
The underlying mechanism of POTS is not well understood. Interestingly, evidence of both sympathetic dysfunction and sympathetic hyperfunction can be found in various literature sources63,64,65,66,67,68,69,70,71. In the peripheral nervous system (PNS), multiple studies have demonstrated evidence of vasomotor and sudomotor dysfunction at the post-ganglionic level of the sympathetic nervous system. This dysfunction leads to orthostatic intolerance, brain fog, blood pooling and inadequate temperature regulation. Furthermore, impaired peripheral vasomotor function reduces cardiac preload, resulting in symptoms such as fatigue, exercise intolerance and exertional dyspnea72. In the central nervous system (CNS), various studies have shown intact or exaggerated baroreflex function in patients with POTS. Consequently, the reduced cardiac preload triggers a hyperadrenergic reaction, characterized by tachycardia, to maintain cerebral blood flow through the baroreflex. While this hyperadrenergic reaction compensates for the reduced cardiac preload, the heightened sympathetic nervous system activity itself gives rise to uncomfortable and debilitating symptoms, including disturbed sleep, anxiety, decreased appetite, indigestion, chronic nausea and constipation. In summary, patients with POTS experience both sympathetic dysfunction in the PNS and compensatory sympathetic hyperactivity in the CNS (Fig. 2). Individuals with the post-COVID-19 condition have been specifically studied and found to have reduced autonomic function, as evidenced by lower heart rate variability and other ambulatory electrocardiogram (EKG) metrics73,74.
The pathophysiology underlying POTS can involve both sympathetic dysfunction in the PNS and compensatory sympathetic hyperactivity in the CNS. In the post-COVID-19 condition, this is hypothesized to occur secondary to direct SARS-CoV-2 viral invasion versus autoimmune and/or inflammatory injury of the nervous system. GI, gastrointestinal.
The presence of both sympathetic dysfunction and hyperfunction in POTS poses unique challenges for clinicians. For instance, aggressive management of hyperadrenergic symptoms can exacerbate vasomotor symptoms, and vice versa. Therefore, when considering pharmacological management of POTS symptoms, it is essential to carefully consider the paradoxical nature of the underlying physiology and to avoid polypharmacy if possible. Currently, there are no treatments that have been shown to restore autonomic nerve dysfunction in POTS. The primary treatment approach aims to increase blood volume to support vasomotor function. Although volume expansion does not provide disease-modifying effects in POTS, it can alleviate many debilitating symptoms. In the early stages of treatment, it is crucial to establish realistic and functional goals, as volume expansion therapy requires active patient participation, including lifestyle modifications, frequent medication adjustments, dietary changes and long-term rehabilitation while offering only limited benefits.
There is an immediate and pressing need for research across various aspects of POTS. One research gap of high priority is the identification of the autoimmune subset of POTS, as it could pave the way for the development of disease-modifying treatments utilizing commercially available immune-modulating drugs. The COVID-19 pandemic has presented a unique opportunity to potentially uncover autoimmune markers, thanks to numerous research cohorts that have been tracking phenotypes following COVID-19 infection longitudinally. Currently, there is an ongoing phase 2 clinical trial for post-COVID POTS that involves the use of a neonatal fragment crystallizable (Fc) receptor antagonist75. Additionally, multiple research projects are underway to investigate viral persistence, microclots and neuroinflammation as underlying factors in the post-COVID-19 condition or post-COVID POTS.
Myocardial injury
Biomarker evidence of myocardial injury is common in acute COVID-19, typically manifested by elevated circulating levels of troponin. This is important because although acute myocardial injury may resolve fully, injury may persist in some patients or yield scarring, which may manifest as long-term sequelae of COVID-19 infection. The incidence of myocardial injury among patients with acute COVID-19 infection depends on the specific assay used, study population and phase of the pandemic; rates of up to 36% have been reported76. However, only up to 7% of individuals with acute COVID had chronic myocardial injury in one study77.
Myocardial injury may result from general critical illness facets of acute COVID-19 including hypoxemia and shock, for example78. Cardiac structural pathology may also contribute including ventricular systolic and diastolic dysfunction, pericardial effusion and valve disease, which may be preexisting and exacerbated by COVID or may be de novo79,80. Other mechanisms of myocardial injury include hypercoagulability leading to microthrombi or macrothromboses including venous thromboembolism and arterial thromboembolism and a hyperinflammatory state11,81,82.
Multimodality imaging techniques including cardiac magnetic resonance (CMR) imaging and echocardiography shed light on the longer-term implications of myocardial injury associated with COVID-19 (refs. 83,84). Individuals with persistent fatigue or dyspnea after COVID infection had CMR and were compared to controls. Approximately 7% of individuals with chronic COVID symptoms had late gadolinium enhancement on CMR compared to zero controls85. Such abnormalities are also observed on CMR in individuals with persistent COVID symptoms who did not have initial biochemical myocardial injury86. This suggests that our current understanding of the causal pathway between COVID infection, myocardial injury, resolution or persistence of myocardial injury, subsequent imaging findings suggesting myocardial pathology and the presence or absence of persistent COVID symptoms is incomplete. For example, a case–control comparison of patients who were hospitalized with COVID who did versus did not have myocardial injury was performed87. All patients underwent CMR, and the rate of structural cardiac abnormality was 61% in cases compared to 31–36% in controls. These abnormalities included 17% of cases with ventricular dysfunction versus 3–7% of controls and 13% of cases with infarction versus 2–7% of controls. Adverse event rates to 12 months occurred in 10% of cases and 6% of controls (P = 0.7). Scar formation on MRI was a predictor of adverse outcomes, while troponin elevation itself was not87. This study and others illustrate the potential utility of CMR to quantify myocardial scarring in individuals with cardiac dysfunction after COVID-19 infection; however, larger and longer-term terms are needed for accurate risk stratification of patients for adverse cardiovascular events.
Overall, the cardiovascular outcomes after COVID-associated myocardial injury are variable and depend on the study population, mechanism of myocardial injury and the endpoint selected. Acute myocardial injury related to severe COVID requiring hospitalization or intensive care unit stay is associated with high short-term risk for mortality88. Chronic myocardial injury was associated with a nearly fourold increased risk for medium-term (6-month) mortality77. Mortality is not the only endpoint of importance to patients, and studies with patient-reported outcomes and functional outcomes are needed.
Heart failure andmyocarditis
Myocardial injury, as outlined above, may be associated with direct end-organ cardiac impact that can have important implications in the long-term cardiovascular sequelae of COVID-19. One cause of acute myocardial injury in the context of viral infection includes myocarditis89,90. Myocarditis is an inflammatory condition involving the myocardium, and can arise from a variety of etiologies including infections, toxins and immune diseases. Acute symptoms include chest pain syndrome, heart failure symptoms and palpitations or syncope in the setting of arrhythmias. Diagnosis is typically based on clinical presentation and a combination of diagnostic testing including cardiac biomarkers, CMR (using the modified Lake Louise Criteria) to identify signs of edema, fibrosis or hyperemia, and, in some cases, endomyocardial biopsy (using the Dallas Criteria) to identify histopathological evidence of myocardial inflammation83,91,92,93. However, caution has been given to using the modified Lake Louise Criteria with CMR to diagnose post-COVID-19 myocarditis due to existing definitions of myocarditis relying on evidence of focal areas of necrosis, while the pathology after COVID-19 is that of more heterogenous and diffuse inflammation. CMR in these patients thus will often show evidence of inflammatory phenomena without classical myocarditis22. Patients hospitalized for COVID-19 infection showed approximately 16 times the risk of myocarditis compared to those hospitalized for other reasons, according to a study by the Centers for Disease Control and Prevention94. Despite these findings, acute myocarditis in the setting of COVID-19 infection is an infrequently reported complication, with autopsy studies suggesting a much lower rate of true myocarditis. In a systematic review of studies examining autopsied hearts after COVID-19 infection, among 277 cases the rate of histologically reported myocarditis was 7.2%; however, the investigators proposed a true prevalence of less than 2% given the diagnostic inconsistencies across included studies95.
Although COVID-19 often initially manifests as a respiratory illness period, it may later manifest with multiorgan system involvement several weeks later. Although relatively rare, multisystem inflammatory syndrome may develop, which is a clinical syndrome occurring approximately 4 weeks after acute COVID-19 infection and characterized by fevers, laboratory evidence of active inflammation and end-organ involvement96. From a cardiac standpoint, it is characterized by an acute, fulminant myocarditis-like syndrome including heart failure and, in some cases, cardiogenic shock97. Patients are managed with intravenous corticosteroids and immunoglobulins as well as hemodynamic support, which may include mechanical circulatory support in severe cases98.
The incidence of post-acute myocarditis or recurrent myocarditis in COVID-19 is not yet well defined. CMR studies of patients who have recovered from COVID-19 have reported upwards of 15% rates of myocardial abnormalities on delayed gadolinium enhancement imaging consistent with myocarditis, suggesting the possibility of longer-term subclinical myocardial sequelae98,99. Acute and subacute myocardial inflammation is known to increase the risk of future ventricular arrhythmias and cardiomyopathy due to development of cardiac fibrosis in other forms of myocarditis100,101. Future studies are needed to better understand these implications in COVID-19. However, it is reasonable to extrapolate current standards of myocarditis care to COVID-19, including exercise restrictions and cardioprotective medications93,102. These include prescribing beta blockers and angiotensin receptor blockers/neprilysin inhibitors or angiotensin-converting enzyme inhibitors as tolerated. Pericarditis or myopericarditis, which can initially be responsive to anti-inflammatory therapies, carries the longer-term complication of risk of recurrence and, therefore, affected individuals require continued monitoring, particularly when weaning off treatment.
Whether clinically overt or subclinical, acute myocardial injury can lead to myocardial dysfunction and resultant cardiomyopathy from COVID-19 infection80. Causes of cardiomyopathy in the context of COVID-19 include stress cardiomyopathy, myocarditis, consequences of multisystem inflammatory syndrome and an acute coronary syndrome mechanism103. Future longer-term studies of COVID-19-related myocardial injury will be needed to determine the risk of developing cardiomyopathy with ventricular dysfunction in individuals with acute COVID-19 myocardial injury. When there is concern for ventricular dysfunction, an accurate assessment of ejection fraction including more sensitive markers of ventricular dysfunction such as global longitudinal strain should be obtained. Patient functional status using thorough heart failure symptom assessment should be addressed accordingly. For example, cardiopulmonary symptoms such as dyspnea (shortness of breath) may need to be further evaluated to distinguish between cardiac and pulmonary sequelae of COVID-19 infection, whereas heart failure-specific symptoms such as orthopnea (shortness of breath while lying flat) and bendopnea (shortness of breath when bending over) would prompt directed treatment with loop diuretics and guideline-directed medical therapies104. Right ventricular cardiomyopathy may occur after COVID-19 for a myriad of reasons, including advanced lung disease or pulmonary embolism leading to right ventricular strain105. In these cases, individuals with right ventricular cardiomyopathy may not physiologically tolerate afterload reduction and beta blockade but may require mild diuretics. Individuals with reduced cardiac function require close monitoring and serial assessment to monitor for recovery. In those with persistent left ventricular dysfunction, an implantable cardioverter defibrillator may be indicated to prevent sudden cardiac death, based on current guidelines104.
Arrhythmias
Acute infection with COVID-19 has been associated with notable increases in cardiac ectopy and arrhythmias, which can persist or present later in patients diagnosed with the post-COVID-19 condition106,107,108. Sinus tachycardia is commonly seen in the post-COVID-19 condition and may be a secondary effect from or component of autonomic dysfunction, deconditioning, hypoxia, heart failure or ongoing inflammation as described above. In addition, atrial and ventricular tachyarrhythmias, bradyarrhythmias and heart block have been described in the COVID-19 literature106. Aside from sinus tachycardia, the most common incident arrhythmia reported has been atrial fibrillation107,109. This often presents in the acute infectious period and more often in critically ill patients; however, analysis of the Veterans Health Administration data showed a higher incidence of cardiac dysrhythmias including atrial fibrillation in patients both 6 and 12 months after COVID-19 infection compared to control patients without COVID-19 (refs. 21,110,111,112). The relation of incident atrial fibrillation specifically with the post-COVID-19 condition remains unclear. Individuals diagnosed with the post-COVID-19 condition very frequently report subjective palpitations and tachycardia, but diagnosis of true arrhythmia has been limited by a paucity of studies including ambulatory EKG monitoring in this population113. Reports of ventricular arrhythmias are largely limited to those hospitalized with acute COVID-19, with few studies reporting ambulatory EKG monitoring data in individuals with the post-COVID-19 condition. However, one study of patients 3 months after COVID-19 hospitalization with 24-h ambulatory EKG monitoring showed 5% of patients had nonsustained ventricular tachycardia and 18% had frequent premature ventricular contractions114. Mechanisms underlying cardiac arrhythmias in the setting of the post-COVID-19 condition are primarily thought to involve inflammatory cytokine release, persistent immune dysfunction, myocardial scarring and fibrosis, and potential gap junction dysfunction101,113. In general, the treatment approach for cardiac arrhythmias in the post-COVID-19 condition is not different from the treatment of arrhythmias unrelated to COVID-19 and should be guideline based.
Potential treatments
Unfortunately, there are no curative therapies for the post-COVID-19 condition; however, there are some effective therapies for subsets of patients especially targeted toward those with ME/CFS, which have been reviewed recently and are beyond the scope of the current review2. An important concept in this population is that exercise is not recommended, with conflicting results in early studies and one study suggesting harm with exercise therapy to treat fatigue and related symptoms in the post-COVID-19 condition115,116,117. For the most prominent cardiovascular-related post-COVID-19 condition diagnosis, POTS, several treatment options exist, typically initiated in a POTS subspeciality clinic. The approach to managing POTS after establishing a diagnosis involves a general approach of patient education, supportive care to address reversible causes (such as fluid status) and management of chronic disease if pertinent. Other standard treatments include compression stockings to prevent venous blood pooling, graded exercise programs and if palpitations are present, beta blockers may be helpful118. If symptoms persist despite conservative measures, other pharmacological interventions may be considered including fludrocortisone (causes fluid and salt retention thus increasing blood pressure), clonidine (decreases sympathetic nervous system outflow) or ivabradine (reduces heart rate). In terms of POTS-targeted therapy in the setting of the post-COVID-19 condition, several ongoing trials are underway (Table 4). One ongoing randomized control trial is studying the efficacy of efgartigimod, used to treat myasthenia gravis, which has been shown to reduce IgG levels, including IgG autoantibodies, which may underlie some of the autonomic disease manifestations in individuals with the post-COVID-19 condition who have POTS. Another trial in POTS/post-COVID-19 condition is focused on ivabradine for ameliorating symptoms suggestive of dysautonomia. Many trials are ongoing that are focused on the most prominent and debilitating post-COVID-19 condition syndromes including POTS and ME/CFS.
In terms of the management of post-COVID-19 heart failure, treatment centers around standard guideline-directed medical therapy as described above119. Similarly, the approach to patients who develop acute or chronic coronary syndromes after COVID-19 are guideline based120,121. Given the probable pathophysiology of a cardiovascular post-COVID-19 condition, several trials are currently underway to assess the efficacy of an anti-inflammatory approach for cardiovascular involvement with the study of statins and colchicine. Other studies focus on thromboprophylaxis including with apixaban and rivaroxaban (ClinicalTrials.gov IDs NCT04801940 and NCT04904536; https://www.georgeinstitute.org.in/projects/Colchicine-for-Long-COVID). Recently, one randomized control trial was presented at the annual European Associated of Cardiovascular Imaging conference (May 2023), and showed that hyperbaric oxygen compared to placebo, significantly improved cardiac dysfunction over time as measured by echocardiographic strain indices122. A large study in the UK will evaluate multiple therapies in randomized control trials nested within the larger study initially focusing on rivaroxaban, colchicine and famotidine/loratadine for the post-COVID-19 condition with a focus on both cardiovascular and non-cardiovascular outcomes (STIMULATE-ICP)123. Other promising ongoing trials for the post-COVID-19 condition but not specific to cardiovascular complications include baricitinib, an immunomodulatory medication, and drugs used for acute COVID-19 are being repurposed and studied for the post-COVID-19 condition including remdesivir and nirmatrelvir/ritonavir (Paxlovid), thought to target viral persistence. Other trials are focused on cardiac rehabilitation, vitamin supplementation (such as vitamins K and D) and melatonin (Tables 3 and 4).
Knowledge gaps and future directions
With the increasing number of COVID-19 survivors, important progress has been made in characterizing the various clinical phenotypes and potential mechanisms underlying the post-COVID-19 condition. However, there are several important knowledge gaps that require further investigation. First, the natural history and long-term prognosis of the post-COVID-19 condition are not well understood. While some studies suggest spontaneous recovery over time, others indicate persistent symptoms lasting beyond a year after the initial infection124,125. Therefore, large-scale longitudinal studies are needed to better understand the medium-term and long-term implications of the post-COVID-19 condition.
Despite the multitude of diagnostic testing options for individuals with a suspected post-COVID-19 condition, findings are often nonspecific or normal in this population. Other more specific diagnostic tools for the post-COVID-19 condition are in development, and many overlap with biomarker research in ME/CFS, including proteins in extracellular vesicles, specific immune markers and tests of saliva, erythrocyte deformation, electrical impedance and isocapnic buffering (the mean percentage of oxygen use in the aerobic-to-anaerobic transition phase)2,126,127,128. Further research on epidemiologic, genetic and clinical risk factors and potential biomarkers and immune mediators will help improve the diagnostic evaluation of patients with the post-COVID-19 condition.
Lastly, there are limited data on effective treatment for the post-COVID-19 condition. Given the heterogeneity of clinical presentations and lack of specific pharmacological therapies approved for the post-COVID-19 condition, current treatment approaches are largely focused on symptom management. However, studies of antivirals to prevent the post-COVID-19 condition have shown promising results. Both molnupiravir and Paxlovid (combination of nirmatrelvir and ritonavir) were found to reduce the risk of developing the post-COVID-19 condition when received during the acute infection129,130. There are ongoing clinical trials evaluating both pharmacological therapies including, but not limited to, cardiac drugs, antivirals and anti-inflammatory agents as well as non-pharmacological therapies such as vagus nerve stimulation (Tables 3 and 4). The results of these trials will be instrumental in developing a standardized approach to the treatment of the post-COVID-19 condition. In addition to treating current patients with the post-COVID-19 condition, further research in this area can help more optimally prepare for future pandemics. Given the associations of cardiovascular risk factors and adverse outcomes following COVID-19 infection, including development of the post-COVID-19 condition, the importance of risk reduction at the population level is another salient lesson learned from the current literature.
References
Yuki, K., Fujiogi, M. & Koutsogiannaki, S. COVID-19 pathophysiology: a review. Clin. Immunol. 215, 108427 (2020).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023). This comprehensive review of long COVID highlights important underlying pathophysiology and includes detailed information on both cardiac and noncardiac effects of this condition.
Thaweethai, T. et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA https://doi.org/10.1001/jama.2023.8823 (2023).
Bonilla, H. et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 14, 1129459 (2023).
Phetsouphanh, C. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 23, 210–216 (2022).
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 324, 782–793 (2020).
Long, B. et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am. J. Emerg. Med. 54, 46–57 (2022).
Tsampasian, V. et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern. Med. 183, 566–580 (2023).
Dessie, Z. G. & Zewotir, T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 21, 855 (2021).
Lundberg, D. J. et al. COVID-19 mortality by race and ethnicity in US metropolitan and nonmetropolitan areas, March 2020 to February 2022. JAMA Netw. Open 6, e2311098 (2023).
Minhas, A. S. et al. The role of sex and inflammation in cardiovascular outcomes and mortality in COVID-19. Int. J. Cardiol. 337, 127–131 (2021).
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022).
Tenforde, M. W. et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March–June 2020. MMWR Morb. Mortal Wkly Rep. 69, 993–998 (2020).
Hu, B., Guo, H., Zhou, P. & Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19, 141–154 (2021).
Zaim, S., Chong, J. H., Sankaranarayanan, V. & Harky, A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 45, 100618 (2020).
Sette, A. & Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880 (2021).
Wong, K. et al. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann. Med. Surg. 74, 103249 (2022).
Long, B., Brady, W. J., Koyfman, A. & Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 38, 1504–1507 (2020).
Luo, W., Liu, X., Bao, K. & Huang, C. Ischemic stroke associated with COVID-19: a systematic review and meta-analysis. J. Neurol. 269, 1731–1740 (2022).
World Health Organization. A clinical case definition of post-COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (2021).
Xie, Y., Xu, E., Bowe, B. & Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590 (2022). This unique article utilized a large national database to highlight the heightened risk of several cardiovascular complications following COVID-19 infection, which helps frame the importance of a solid knowledge base in this topic for healthcare providers.
Gluckman, T. J.et al. 2022 ACC Expert Consensus Decision Pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 79, 1717–1756 (2022). This important expert consensus document discusses different phenotypes of post-COVID-19 condition cardiovascular disease and provides recommendations on screening for myocardial involvement.
Khan, M. S. et al. Cardiovascular implications of COVID-19 versus influenza infection: a review. BMC Med. 18, 403 (2020).
Chimenti, C. et al. Intramyocyte detection of Epstein–Barr virus genome by laser capture microdissection in patients with inflammatory cardiomyopathy. Circulation 110, 3534–3539 (2004).
Deumer, U.-S. et al. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): an overview. J. Clin. Med. 10, 4786 (2021).
Zheng, Y. -Y., Ma, Y. -T., Zhang, J. -Y. & Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 17, 259–260 (2020).
Raman, B., Bluemke, D. A., Lüscher, T. F. & Neubauer, S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 43, 1157–1172 (2022).
Bonaventura, A. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329 (2021).
Beyerstedt, S., Casaro, E. B. & Rangel, É. B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 40, 905–919 (2021).
Pretorius, E. et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 20, 172 (2021).
Osiaevi, I. et al. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 26, 53–61 (2023).
Patel, M. A. et al. Elevated vascular transformation blood biomarkers in long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol. Med. 28, 122 (2022).
Proal, A. D. & VanElzakker, M. B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 12, 698169 (2021).
Naveed, Z. et al. Association of COVID-19 infection with incident diabetes. JAMA Netw Open 6, e238866 (2023).
Mohiuddin Chowdhury, A. T. M. et al. Clinical characteristics and the long-term post-recovery manifestations of the COVID-19 patients—a prospective multicenter cross-sectional study. Front. Med. 8, 663670 (2021).
Gyöngyösi, M. et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 119, 336–356 (2023). This is a thorough Scientific Statement describing long COVID epidemiology, diagnosis, pathophysiology, and management with specific emphasis on the cardiovascular system.
Miglis, M. G. et al. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 30, 449–451 (2020).
Kanjwal, K., Jamal, S., Kichloo, A. & Grubb, B. P. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J. Innov. Card. Rhythm Manag. 11, 4302–4304 (2020).
Ishibashi, Y., Yoneyama, K., Tsuchida, T. & Akashi, J. Y. Post-COVID-19 postural orthostatic tachycardia syndrome. Intern. Med. 60, 2345 (2021).
O’Sullivan, J. S., Lyne, A. & Vaughan, C. J. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 14, e243585 (2021).
Schofield, J. R. Persistent antiphospholipid antibodies, mast cell activation syndrome, postural orthostatic tachycardia syndrome and post-COVID syndrome: 1 year on. Eur. J. Case Rep. Intern. Med. 8, 002378 (2021).
Johansson, M. et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 3, 573–580 (2021).
Blitshteyn, S. & Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol. Res. 69, 205–211 (2021).
Bosco, J. & Titano, R. Severe post-COVID-19 dysautonomia: a case report. BMC Infect. Dis. 22, 214 (2022).
Wallukat, G. et al. Functional autoantibodies against G-protein-coupled receptors in patients with persistent long-COVID-19 symptoms. J. Transl. Autoimmun. 4, 100100 (2021).
Buoite Stella, A. et al. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J. Neurol. 269, 587–596 (2022).
Shouman, K. et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin. Auton. Res. 31, 385–394 (2021).
Campen, C. L. M. C., van, Rowe, P. C. & Visser, F. C. Orthostatic symptoms and reductions in cerebral blood flow in long-haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina 58, 28 (2021).
Novak, P. et al. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann. Neurol. 91, 367–379 (2022).
Jamal, S. M. et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J. Am. Coll. Cardiol. 79, 2325–2330 (2022).
Eldokla, A. M. & Ali, S. T. Autonomic function testing in long-COVID syndrome patients with orthostatic intolerance. Auton. Neurosci. 241, 102997 (2022).
Campen, C. L. M. Cvan & Visser, F. C. Long-haul COVID patients: prevalence of POTS are reduced but cerebral blood flow abnormalities remain abnormal with longer disease duration. Healthcare 10, 2105 (2022).
Kwan, A. C. et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. Nat. Cardiovasc. Res. 1, 1187–1194 (2022).
Chung, T. H. & Azar, A. Autonomic nerve involvement in post-acute sequelae of SARS-CoV-2 syndrome (PASC). J. Clin. Med. 12, 73 (2022).
Eslami, M. et al. Postural orthostatic tachycardia syndrome and orthostatic hypotension post COVID-19. Infect. Disord. Drug Targets 23, e100622205846 (2023).
Hira, R. et al. Objective hemodynamic cardiovascular autonomic abnormalities in post-acute sequelae of COVID-19. Can. J. Cardiol. 39, 767–775 (2023).
Zanin, A. et al. Parasympathetic autonomic dysfunction is more often evidenced than sympathetic autonomic dysfunction in fluctuating and polymorphic symptoms of ‘long-COVID’ patients. Sci. Rep. 13, 8251 (2023).
González-Hermosillo G, J. A. et al. Exaggerated blood pressure elevation in response to orthostatic challenge, a post-acute sequelae of SARS-CoV-2 infection (PASC) after hospitalization. Auton. Neurosci. 247, 103094 (2023).
Chen, X. C. et al. Reproducibility of head-up tilt-table testing for eliciting susceptibility to neurally mediated syncope in patients without structural heart disease. Am. J. Cardiol. 69, 755–760 (1992).
Pavri, B. B., Ruskin, J. N. & Brooks, R. The yield of head-up tilt testing is not significantly increased by repeating the baseline test. Clin. Cardiol. 19, 494–496 (1996).
Lamarre-Cliche, M. & Cusson, J. The fainting patient: value of the head-upright tilt-table test in adult patients with orthostatic intolerance. CMAJ 164, 372–376 (2001).
Abbate, G. et al. Postural orthostatic tachycardia syndrome after COVID-19: a systematic review of therapeutic interventions. J. Cardiovasc. Pharmacol. 82, 23–31 (2023).
Low, P. A. et al. Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. J. Auton. Nerv. Syst. 50, 181–188 (1994).
Goldstein, D. S. et al. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation 106, 2358–2365 (2002).
Bonyhay, I. & Freeman, R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110, 3193–3198 (2004).
Peltier, A. C. et al. Distal sudomotor findings in postural tachycardia syndrome. Clin. Auton. Res. 20, 93–99 (2010).
Jacob, G. et al. Abnormal norepinephrine clearance and adrenergic receptor sensitivity in idiopathic orthostatic intolerance. Circulation 99, 1706–1712 (1999).
Haensch, C.-A., Tosch, M., Katona, I., Weis, J. & Isenmann, S. Small-fiber neuropathy with cardiac denervation in postural tachycardia syndrome. Muscle Nerve 50, 956–961 (2014).
Goldstein, D. S. et al. Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation 111, 839–845 (2005).
Muenter Swift, N., Charkoudian, N., Dotson, R. M., Suarez, G. A. & Low, P. A. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am. J. Physiol. Heart. Circ. Physiol. 289, H1226–H1233 (2005).
Garland, E. M., Raj, S. R., Black, B. K., Harris, P. A. & Robertson, D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 69, 790–798 (2007).
Joseph, P. et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest 160, 642–651 (2021).
Shah, B. et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol. J. 22, 70–76 (2022).
Marques, K. C., Quaresma, J. A. S. & Falcão, L. F. M. Cardiovascular autonomic dysfunction in ‘Long COVID’: pathophysiology, heart rate variability, and inflammatory markers. Front. Cardiovasc. Med. 10, 1256512 (2023).
NIH National Library of Medicine. ClinicalTrials.gov. Efficacy and safety study of efgartigimod in adults with post-COVID-19 POTS. https://clinicaltrials.gov/ct2/show/NCT05633407
Lala, A. et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 76, 533–546 (2020).
Kini, A. et al. Types of myocardial injury and mid-term outcomes in patients with COVID-19. Eur. Heart J. Qual. Care Clin. Outcomes 7, 438–446 (2021).
Metkus, T. S. et al. Myocardial Injury in severe COVID-19 compared with non-COVID-19 acute respiratory distress syndrome. Circulation 143, 553–565 (2021).
Giustino, G. et al. Characterization of myocardial injury in patients with COVID-19. J. Am. Coll. Cardiol. 76, 2043–2055 (2020).
Minhas, A. S. et al. Myocardial work efficiency, a novel measure of myocardial dysfunction, is reduced in COVID-19 patients and associated with in-hospital mortality. Front. Cardiovasc. Med. 8, 667721 (2021).
Deitelzweig, S. et al. Thrombotic and bleeding events, mortality, and anticoagulant use among 546,656 hospitalized patients with COVID-19 in the United States: a retrospective cohort study. J. Thromb. Thrombolysis 53, 766–776 (2022).
Wei, Z.-Y., Geng, Y.-J., Huang, J. & Qian, H.-Y. Pathogenesis and management of myocardial injury in coronavirus disease 2019. Eur. J. Heart Fail. 22, 1994–2006 (2020).
Petersen, S. E. et al. Cardiovascular magnetic resonance for patients with COVID-19. JACC Cardiovasc. Imaging 15, 685–699 (2022).
Goerlich, E. et al. Multimodality imaging for cardiac evaluation in patients with COVID-19. Curr. Cardiol. Rep. 23, 44 (2021).
Kravchenko, D. et al. Cardiac MRI in patients with prolonged cardiorespiratory symptoms after mild to moderate COVID-19. Radiology 301, E419–E425 (2021).
Puntmann, V. O. et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 28, 2117–2123 (2022).
Artico, J. et al. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter, observational study. Circulation 147, 364–374 (2023).
Giustino, G. et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC Focus Seminar. J. Am. Coll. Cardiol. 76, 2011–2023 (2020). This paper outlines in great detail the mechanisms of myocardial injury in COVID-19 and discusses the clinical impact of these complications.
Cooper, L. T. Myocarditis. N. Engl. J. Med. 360, 1526–1538 (2009).
Trachtenberg, B. H. & Hare, J. M. Inflammatory cardiomyopathic syndromes. Circ. Res. 121, 803–818 (2017).
Friedrich, M. G. et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J. Am. Coll. Cardiol. 53, 1475–1487 (2009).
Aretz, H. T. et al. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1, 3–14 (1987).
Caforio, A. L. P. et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 34, 2636–2648, 2648a–2648d (2013).
Boehmer, T. K. et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020–January 2021. MMWR Morb. Mortal. Wkly Rep. 70, 1228–1232 (2021).
Halushka, M. K. & Vander Heide, R. S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 50, 107300 (2021).
Patel, P. et al. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review. JAMA Netw. Open 4, e2126456 (2021).
Centers for Disease Control and Prevention (CDC). Multisystem Inflammatory Syndrome in Adults (MIS-A) Case Definition and Information for Healthcare Providers (2023).
Rajpal, S. et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 6, 116–118 (2021).
Clark, D. E. et al. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC). J. Cardiovasc. Magn. Reson. 23, 106 (2021).
Peretto, G. et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J. Am. Coll. Cardiol. 75, 1046–1057 (2020).
Isakadze, N. et al. C-reactive protein elevation is associated With QTc interval prolongation in patients hospitalized with COVID-19. Front. Cardiovasc. Med. 9, 866146 (2022).
Ruberg, F. L. et al. Utilization of cardiovascular magnetic resonance (CMR) imaging for resumption of athletic activities following COVID-19 infection: an expert consensus document on behalf of the American Heart Association Council on Cardiovascular Radiology and Intervention (CVRI) Leadership and endorsed by the Society for Cardiovascular Magnetic Resonance (SCMR). J. Cardiovasc. Magn. Reson. 24, 73 (2022).
Hendren, N. S., Grodin, J. L. & Drazner, M. H. Unique patterns of cardiovascular involvement in coronavirus disease-2019. J. Card. Fail. 26, 466–469 (2020).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 79, e263–e421 (2022).
Satoskar, M. A. et al. Improving risk prediction for pulmonary embolism in COVID-19 patients using echocardiography. Pulm. Circ. 12, e12036 (2022).
Coromilas, E. J. et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 14, e009458 (2021).
Desai, A. D., Boursiquot, B. C., Melki, L. & Wan, E. Y. Management of arrhythmias associated with COVID-19. Curr. Cardiol. Rep. 23, 2 (2020).
Driggin, E. et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 75, 2352–2371 (2020).
Goerlich, E. et al. Left atrial function in patients with coronavirus disease 2019 and its association with incident atrial fibrillation/flutter. J. Am. Soc. Echocardiogr. 34, 1106–1109 (2021).
Dixit, N. M., Churchill, A., Nsair, A. & Hsu, J. J. Post-acute COVID-19 syndrome and the cardiovascular system: what is known? Am. Heart J. Plus 5, 100025 (2021).
Musikantow, D. R. et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin. Electrophysiol. 7, 1120–1130 (2021).
Al-Aly, Z., Xie, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Huseynov, A., Akin, I., Duerschmied, D. & Scharf, R. E. Cardiac arrhythmias in post-COVID syndrome: prevalence, pathology, diagnosis, and treatment. Viruses 15, 389 (2023).
Ingul, C. B. et al. Cardiac dysfunction and arrhythmias 3 months after hospitalization for COVID-19. J. Am. Heart Assoc. 11, e023473 (2022).
National Institute for Health and Care Excellence. Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management (NICE, 2021).
Centers for Disease Control and Prevention (CDC). Treatment of ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (2021).
Wright, J., Astill, S. L. & Sivan, M. The relationship between physical activity and long COVID: a cross-sectional study. Int. J. Environ. Res. Public Health 19, 5093 (2022).
Olshansky, B. et al. Postural orthostatic tachycardia syndrome (POTS): a critical assessment. Prog. Cardiovasc. Dis. 63, 263–270 (2020).
Yancy, C. W. et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136, e137–e161 (2017).
Gulati, M. et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 144, e368–e454 (2021).
Collet, J.-P. et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367 (2021).
Leitman, M. et al. The effect of hyperbaric oxygen therapy on myocardial function in post-COVID-19 syndrome patients: a randomized controlled trial. Sci. Rep. 13, 9473 (2023).
Forshaw, D. et al. STIMULATE-ICP: a pragmatic, multi-centre, cluster randomised trial of an integrated care pathway with a nested, phase III, open label, adaptive platform randomised drug trial in individuals with Long COVID: a structured protocol. PLoS ONE 18, e0272472 (2023).
Du, Y., Zhang, J., Wu, L. J., Zhang, Q. & Wang, Y. X. The epidemiology, diagnosis and prognosis of long-COVID. Biomed. Environ. Sci. 35, 1133–1139 (2022).
Han, Q., Zheng, B., Daines, L. & Sheikh, A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 11, 269 (2022).
Peluso, M. J. et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann. Neurol. 91, 772–781 (2022).
Galán, M. et al. Persistent overactive cytotoxic immune response in a Spanish cohort of individuals with long-COVID: identification of diagnostic biomarkers. Front. Immunol. 13, 848886 (2022).
Esfandyarpour, R., Kashi, A., Nemat-Gorgani, M., Wilhelmy, J. & Davis, R. W. A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Proc. Natl Acad. Sci. USA 116, 10250–10257 (2019).
Xie, Y., Choi, T. & Al-Aly, Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern. Med. 183, 554–564 (2023).
Xie, Y., Choi, T. & Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of COVID-19: cohort study. BMJ 381, e074572 (2023).
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. A.G.H., W.S.P. and E.G. created the review structure and outline. E.G., T.H.C., G.H.H., T.S.M., N.A.G. and A.G.H. all drafted respective review sections. E.G. compiled and finalized the manuscript. All authors assisted with editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Valentina Puntmann, Ziyad Al-Aly and Sameer M. Jamal for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goerlich, E., Chung, T.H., Hong, G.H. et al. Cardiovascular effects of the post-COVID-19 condition. Nat Cardiovasc Res 3, 118–129 (2024). https://doi.org/10.1038/s44161-023-00414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00414-8