Abstract
Fractional flow reserve (FFR) is the current gold standard invasive assessment of coronary artery disease (CAD). FFR reports coronary blood flow (CBF) as a fraction of a hypothetical and unknown normal value. Although used routinely to diagnose CAD and guide treatment, how accurately FFR predicts actual CBF changes remains unknown. In this study, we compared fractional CBF with absolute CBF (aCBF, in ml min−1), measured with a computational method during standard angiography and pressure wire assessment, on 203 diseased arteries (143 patients). We found a substantial correlation between the two measurements (r = 0.89 and Cohen’s kappa = 0.71). Concordance between fractional and absolute CBF reduction was high when FFR was >0.80 (91%) but reduced when FFR was ≤0.80 (81%), 0.70–0.80 (68%) and, particularly, 0.75–0.80 (62%). Discordance was associated with coronary microvascular resistance, vessel diameter and mass of myocardium subtended, all factors to which FFR is agnostic. Assessment of aCBF complements FFR and may be valuable to assess CBF, particularly in cases within the FFR ‘gray zone’.
Similar content being viewed by others
Main
Ischemic heart disease is caused by insufficient CBF to the myocardium. Because CBF cannot be measured directly in the cardiac catheter laboratory (CCL), cardiologists have relied largely upon sensor-tipped wire technologies (pressure, thermistor and Doppler) to derive several semi-quantitative indices, each serving as an indirect proxy for estimating CBF restriction1. Of these, pressure-derived FFR has emerged as the most popular and evidence-based method for guiding revascularization in intermediate lesions2,3,4. The value of FFR is that CBF restriction can be quantified, which allows revascularization to be targeted at lesions that restrict CBF the most. FFR is, however, a semi-quantitative assessment of fractional CBF reduction, unable to account for variability in vessel size (diameter), the mass of myocardium subtended and microvascular resistance (MVR), all of which influence aCBF. A 15% CBF reduction (FFR 0.85) due to a left main coronary artery stenosis is likely to result in a greater absolute reduction in myocardial blood flow over a larger myocardial territory, with potentially more serious clinical sequelae, than a 25% CBF reduction (FFR 0.75) resulting from disease in a smaller, more distal vessel. However, the opposite may be inferred by the FFR, and intervention may, therefore, not be targeted at the most physiologically significant lesion. Thus, a method that fully quantifies CBF changes, accounting for these important factors, may be of potential value. Methods for estimating aCBF in the CCL have been described5,6,7,8. In addition, these methods simultaneously measure absolute microvascular resistance (aMVR), thus resolving another limitation of FFR. Despite the popularity and importance of FFR in guiding key treatment decisions, the relationship between fractional and absolute CBF changes remains unknown, as does the potential value of these additional measurements when assessing patients for intervention. In this study of aCBF, we investigated (1) the relationship between fractional (FFR) and absolute CBF changes and (2) concordance and discordance between fractional and absolute CBF changes.
Results
Case exclusions and software failures
In total, 256 arteries from 169 patients met the inclusion criteria and were recruited. Of these, 20 arteries had insufficient pressure gradient for determining aCBF, and 19 had inadequate angiographic views for modeling, leaving 217 suitable cases. Of these, seven failed to mesh, and seven failed to converge during numerical simulation. The final analysis included 203 arteries from 143 patients. Therefore, of those cases meeting the clinical inclusion criteria, the computational fluid dynamics (CFD) method computed physiological results successfully in 93.5% of cases. A software problem resulted in vessel diameter data being unavailable in 28 cases.
Baseline patient and vessel characteristics
The baseline demographic and clinical characteristics of all 143 patients are shown in Table 1. Artery-specific and lesion-specific characteristics are summarized in Table 2. Overall, 51% of arteries underwent percutaneous coronary intervention (PCI), and 49% were treated medically. Ninety-three percent of arteries with a positive FFR (≤0.80) underwent PCI, and 7% were treated with medical therapy, whereas 2% of cases with a negative FFR (>0.80) underwent PCI, and 98% were treated medically. Median hyperemic aCBF, coronary flow reserve (CFR) and MVR were 85.2 (63.5–116.3 ml min−1), 1.62 (1.32–2.04) and 0.71 (0.52–0.98 mmHg.min/ml), respectively. The aCBF was higher in the left main stem (LMS), left anterior descending artery (LAD) and right coronary artery (RCA) cases (95.6 ml min−1, 81.6 ml min−1 and 98.8 ml min−1, respectively) than the left circumflex artery (LCX), diagonal and obtuse marginal (OM) cases (78.1 ml min−1, 79.9 ml min−1 and 56.7 ml min−1, respectively), but these differences did not reach statistical significance.
Reduction in aCBF
The median reduction in aCBF (from hypothetical normal, caused by epicardial disease) was 20.6 (12.4–38.3) ml min−1. FFR-positive cases were associated with a greater reduction in aCBF than FFR-negative cases (34.6 versus 11.6 ml min−1, P < 0.0001). The threshold for significance for reduction in aCBF was determined as a reduction in aCBF of ≥23 ml min−1. Reduction in aCBF was numerically but statistically non-significantly higher in LMS, LAD and RCA cases (median values: 45.7 ml min−1, 21.1 ml min−1 and 23.7 ml min−1, respectively) compared to LCX, diagonal and OM branch cases (median values: 20.8 ml min−1, 17.8 ml min−1 and 12.8 ml min−1, respectively).
Relationship between fractional and absolute flow reduction
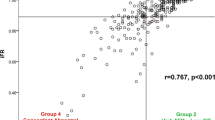
Fractional CBF reduction (measured by FFR) was plotted against aCBF reduction (measured by the CFD method). Despite a strong correlation between fractional and absolute CBF reduction (r = 0.89 and 0.86–0.92, P < 0.001), there was variability among individual cases (Fig. 1). When the cases were divided into those with significant or non-significant CBF reduction according to FFR (fractional reduction) and according to aCBF (absolute reduction), the Cohen’s kappa statistic was 0.71, indicating a substantial agreement between the two parameters.
Concordance between fractional and absolute CBF reduction is indicated by the labels according to the FFR range. Concordance means significant FFR (≤0.80) and significant reduction in aCBF (≥23 ml min−1) or insignificant FFR (>0.80) and insignificant reduction in aCBF (<23 ml min−1). The horizontal and leftward vertical dashed lines reflect the thresholds for physiological significance for FFR and aCBF. The asterisks indicate statistical significance for differences in concordance (z-scores) compared to the FFR >0.80 group at the *P < 0.05 and **P < 0.01** levels (exact P values (two-tailed) were 0.021 for the ≤0.80 group, <0.001 for the 0.70–0.80 and 0.75–0.80 groups and 0.02 for the ≤0.70 group, unadjusted for multiple comparisons). There were no replicates.
Concordance between fractional and absolute flow reduction
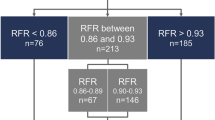
All 203 cases were categorized as physiologically concordant (significant FFR and significant aCBF reduction or non-significant FFR and non-significant aCBF reduction) and discordant. Discordance between fractional and absolute CBF reduction was low in FFR-negative (FFR >0.80) cases (9%). Discordance was significantly higher in FFR-positive cases (19%, P < 0.05), higher still when FFR was between 0.70 and 0.80 (32%, P < 0.01) and highest when FFR was in the 0.75–0.80 ‘gray zone’ (38%, P < 0.01). Discordance was 0% in cases with FFR <0.70 (Fig. 1).
Factors affecting discordance
When comparing the fractional and absolute reduction in CBF, differences in coronary MVR, vessel diameter and myocardial jeopardy were identified that were associated with discordance between these two measures (Fig. 2). In cases with FFR ≤0.80, aMVR was significantly higher (causing reduced aCBF) in discordant cases than concordant cases (1.02 versus 0.51 mmHg.min/ml, P < 0.01). The same was true of the FFR 0.70–0.80 and 0.75–0.80 groups (1.02 versus 0.57 mmHg.min/ml and 1.00 versus 0.55 mmHg.min/ml, respectively, P < 0.01 for both comparisons). In cases with FFR >0.80, aMVR was significantly lower (causing higher CBF) in discordant cases than concordant cases (0.57 versus 0.84 mmHg.min/ml, P < 0.01) (causing increased CBF). Myocardial jeopardy was consistently and significantly lower in discordant cases than concordant cases in all cases with FFR ≤0.80 (7.28 versus 5.28, P < 0.05), in those with FFR 0.70–0.80 (7.69 versus 5.28, P < 0.05) and in those with FFR 0.75–0.80 (8.27 versus 5.21, P < 0.05). In cases with FFR >0.80, myocardial jeopardy was numerically higher in discordant cases, but this difference did not reach statistical significance (7.71 versus 5.69, P = not significant (NS)). Vessel diameter was significantly greater in concordant than discordant in cases with FFR 0.70–0.80 and in those with FFR 0.75–0.80 (3.33 versus 2.85 mm, P < 0.05, and 3.51 versus 2.92 mm, P < 0.05). In cases with FFR >0.80 and in those with FFR ≤0.80, no significant difference was observed in vessel reference diameter between concordant and discordant cases (2.92 versus 2.81 mm, P = NS, and 3.14 versus 2.85 mm, P = NS). Thus, heterogeneity in aMVR, myocardial jeopardy and vessel size appeared to contribute to discordance between fractional and absolute CBF reduction.
Box and whisker plots demonstrating the difference between concordant (white boxes) and discordant (shaded boxes) cases in each FFR subgroup on the basis of aMVR (a), vessel diameter (b) and myocardial jeopardy (c). The direction of these differences is associated with discordance between absolute and fractional coronary blood flow changes. Concordance was 100% in the FFR <0.70 group, hence the absence of a plot for this subgroup. The box defines the IQR; the line and ‘x’ indicate the median and mean; and the bars represent variability outside the upper and lower quartiles, excluding outliers. Asterisks indicate statistical significance at the P ≤ 0.05 level (P values for a are all <0.001; P values for b are 0.03 and 0.02; and P values for c are 0.018, 0.019 and 0.021—calculated in Rstudio (two-sided Mann–Whitney U-test) with no adjustment for multiple comparisons). The numbers in gray indicate n for each group in each comparison (no replicates).
Discussion
The main findings of this study were that there was a substantial correlation between fractional and absolute flow reduction, but that individual case variation resulted in discordance in 9% of cases with a physiologically non-significant FFR and 19% of those with a physiologically significant FFR. Discordance was greatest in cases within the FFR ‘gray zone’ where it reached 38%. Discordance between fractional and absolute CBF reduction appeared to be associated with variability in three related factors: coronary MVR, myocardial jeopardy (a marker of mass of myocardium subtended) and vessel size.
The ability to fully quantify aCBF changes may extend the benefits currently provided by FFR. It would be reasonable to infer that a lesion that reduces CBF by 50 ml min−1 is more likely to cause symptoms and clinical sequelae than a lesion that reduces CBF by 10 ml min−1. FFR is effectively normalized for vessel size but is agnostic to differences in absolute flow. Whether symptoms and clinical sequelae are better predicted by the absolute or the fractional flow reduction remains unknown. This was not the subject of this study. Rather, this study demonstrated that there was variability between absolute and fractional CBF reduction. In the current study, disparity between fractional and absolute CBF reduction was associated with changes in three related factors: vessel size (diameter), myocardial resistance and the mass of myocardium subtended by that artery. FFR does not account for these parameters, all of which are important determinants of CBF. Instead, in real-world practice, the operator assesses these factors automatically by ‘eye-balling’ the angiogram. Similar relationships have been demonstrated previously with CFR and the index of microcirculatory resistance (IMR). Variability in IMR accounts for discordance between FFR and Doppler-derived CFR9,10.
The novel method also predicted aMVR, which may be advantageous for several reasons. First, naturally occurring and pathological variability in aMVR is known to explain discordance between pressure-based and flow-based intracoronary assessment10,11. Second, MVR assessment is helpful in the diagnosis of coronary microvascular disease and is now supported by a 2A recommendation in the European Society of Cardiology guidelines12. Microvascular disease responds to medical therapy but is rarely diagnosed in the CCL because standard tests, such as FFR, cannot identify or measure it13. Third, it has been suggested that concomitant microvascular dysfunction may be one reason why 20% of patients do not achieve full symptomatic relief with PCI, even when FFR-guided4. It is, therefore, interesting that the rate of discordance in FFR-‘positive’ patients in our study was similar at 19%.
FFR was least reliable at predicting aCBF changes in cases with FFR 0.70–0.80 (68% concordance) and poorest in the range 0.75–0.80 (62% concordance). This is interesting because this FFR range corresponds with what has become known as the FFR ‘gray zone’. The DEFER trial originally proposed FFR >0.75 as the threshold for deferring intervention in favor of medical therapy14, whereas the seminal FAME and FAME-2 trials supported a threshold of FFR >0.80 (refs. 15,16). The higher threshold increases the sensitivity for detecting flow-limiting lesions but comes at the expense of reducing specificity. It is, therefore, interesting that the current study identified those with an FFR in the same range as being most discordant with aCBF.
The values of aCBF reduction are lower than those reported for the continuous infusion thermodilution (CIT) method17. There are two main reasons for this. First, our population had hemodynamically significant epicardial coronary artery disease, whereas data supporting the CIT method are largely derived from those with unobstructed coronary arteries. Second, the novel CFD method determines the coronary outlet flow, whereas CIT predicts inlet flow, the former being much greater due to the loss of flow to side branches, particularly in the LAD, which supplies multiple branches to the lateral wall and septum. Outlet flow is a lesion-specific parameter that allows the reduction in CBF due to proximal disease to be derived, which may be useful when predicting the physiological value of PCI and making revascularization decisions. Finally, the two methods are distinct, and a systematic or methodological influence cannot be excluded.
The methods used in this study do not compete with or invalidate FFR. Quite the opposite is true. FFR predicts CBF as a fraction of an hypothetical and unknown value. The novel method translates this into a value, converting a semi-quantitative into a fully-quantitative measurement. Moreover, the novel method requires the data acquired during FFR and the mathematics used in its derivation. In this way, the two methods may be complementary. Given that agreement between FFR-predicted flow reduction and aCBF reduction was high in cases where FFR was >0.80 and perfect when FFR was ≤0.70, a hybrid approach to coronary assessment may have value, with assessment of aCBF being required only if the FFR value lay in the range of 0.70–0.80. If this approach were applied to the current dataset, FFR alone would be sufficient in 65% of cases, and additional assessment of aCBF would be required in 35%.
The study was observational, and so the results are hypothesis generating. The clinical benefits of assessing CBF reduction in absolute terms are yet to be proven. The application of the proposed hybrid assessment is hypothetical. Currently, the CFD method does not account for flow to side branches, which underestimates flow in the proximal vessel. The novel method awaits in vivo validation but has been tested in vitro, in unbranched phantom models. It requires a pressure gradient of at least 4 mmHg, which roughly equates to a trans-lesional pressure ratio of 0.95. The latter means that, at the current stage of development, the method cannot be used in clearly unobstructed epicardial coronary arteries, which is a limitation in patients under investigation for ischemia with no obstructive coronary disease. Due to an error in the software, vessel diameter data were unavailable in 28 cases (13.7%).
Conclusions
In patients with CAD, a substantial correlation was observed between (pressure-derived) fractional and (computed) aCBF reduction. Concordance between fractional and absolute flow reduction was high when FFR was >0.80 and <0.70 but was poorer when 0.70–0.80 and poorest in the 0.75–0.85 ‘gray zone’ range. Assessment of aCBF reduction may complement FFR and extend its benefits in selected cases.
Methods
Patient inclusion and exclusion criteria
This study was approved by the South Yorkshire Health Research Authority Regional Ethics Committee (16/NW/0897 and 08/H1308/193). Patients were eligible for inclusion if they were being investigated for chest pain by invasive coronary angiography and pressure wire assessment, were aged 18 years or older and provided informed consent where appropriate. Participants were not compensated for inclusion and were recruited from elective and inpatient cardiac catheter lists at Sheffield Teaching Hospitals NHS Foundation Trust. Only cases suitable for pressure wire assessment were included, and so results may not be representative in other cases. Patients with chronic and acute coronary syndromes were included, but, in acute cases, only non-culprit arteries were studied. Patients with ST-segment elevation myocardial infarction within 60 days, contraindication to adenosine or contrast media, previous coronary artery bypass surgery, chronic total occlusion, severe valvular disease, inability to consent or without angiographically significant CAD were excluded. The clinical data of all patients are reported in Supplementary Dataset 1.
Angiographic and pressure wire data collection
Invasive coronary angiography was performed according to standard clinical protocols. Operators were encouraged to optimize target artery opacification, acquire clear views of the stenosis region and minimize vessel overlap and panning to facilitate arterial reconstruction18. A panel of three cardiologists, independent of physiological simulation, evaluated each angiogram to assess the reconstruction. Each panel member had to be satisfied for a case to be included. The panel also assessed global and vessel-specific myocardial ischemic jeopardy index and the percentage lesion stenosis for all cases. The myocardial jeopardy index is a lesion-specific measure of the number of myocardial segments jeopardized by a stenosis and is, therefore, a marker of the mass of myocardium subtended19. Pressure wire assessment also proceeded according to standard protocols with trans-lesional pressure measurements acquired under baseline and hyperemic conditions. Hyperemia was induced with an intravenous infusion of adenosine (140 mcg/kg/min)20. The method for deriving aCBF (detailed below) uses data from angiography and a 0.014-inch pressure wire. In this study, the PressureWire X (Abbott Laboratories) and PrimeWire Prestige (Philips Volcano) were used. Any lesion with FFR ≤0.80 was regarded as physiologically significant and treated accordingly. FFR reports CBF as the fraction of CBF that remains, in an epicardial coronary artery, relative to an hypothetical normal value for that artery, under maximal flow conditions. Fractional (percentage) reduction in flow was, therefore, calculated as 1 − FFR, multiplied by 100. The hemodynamic data are included in Supplementary Dataset 2.
Deriving absolute coronary flow and absolute coronary microvascular resistance
aCBF was computed by the CFD model. Three-dimensional (3D) coronary arterial anatomy was reconstructed from two angiographic projections ≥30° apart, acquired during ECG-gated end diastole, using an epipolar line transaction method21. The panel of cardiologists assessed all arterial reconstructions and models against the angiographic images to ensure that each reconstructed model was based on the artery in question. The reconstructed arterial volume was discretized; measured pressures were applied as boundary conditions; and a numerical simulation based upon solving the 3D, incompressible Navier–Stokes and continuity equations was performed. The principal model output was a calculation of aCBF, distal to the epicardial stenosis/es, at the location of the pressure wire transducer. A detailed description of the method was previously published5. Only cases with FFR ≤0.95 or ≥4 mmHg trans-lesional pressure gradient were included because an insufficient pressure gradient is associated with increased model error. By combining measurements of aCBF and pressure, aMVR and the reduction in aCBF due to epicardial disease were also calculated. aMVR (mmHg.min/ml) was calculated according to the hydraulic equivalent of Ohm’s law, as the ratio of distal pressure (Pd) and aCBF (\(\frac{{P_d}}{{{{{\mathrm{aCBF}}}}}}\)). The aCBF in the hypothetical non-stenosed artery was calculated as the ratio of aCBF and FFR (\(\frac{{{{{\mathrm{aCBF}}}}}}{{{{{\mathrm{FFR}}}}}}\)), and the reduction in aCBF due to epicardial coronary disease was calculated as the difference between this and aCBF. These physiological parameters were calculated on a vessel-specific basis under baseline and hyperemic conditions.
The primary outcome was to assess the relationship (correlation, concordance and discordance) between fractional (FFR) CBF reduction and absolute CBF reduction in a population of patients with CAD. A subgroup analysis was also performed according to clinically relevant ranges of FFR (≤0.80, >0.80, 0.70–0.80, 0.75–0.80 and ≤0.70). Simulations were performed on a Dell Precision T5600 computer (Intel Xeon E5 2560, 2-GHz processor, 32 GB RAM) or a Dell Precision 5540 mobile workstation (Intel Core i9-9980HK processor, 32 GB RAM).
Statistical analysis
Statistical analysis was performed in RStudio 1.2.1335 (R, version 3.6.1) and in Microsoft Excel (16.16.27). Normality of distribution was assessed with histograms, Q–Q plots and the Shapiro–Wilk test. Parametric and non-parametric continuous data are presented as mean (±s.d.) and median (interquartile range (IQR)), respectively. Wilcoxon signed-rank and Mann–Whitney U-tests were used to compare paired and unpaired grouped data. Levene’s test for homoscedasticity was used, and we found evidence of heteroscedasticity. Therefore, the Kruskal–Wallis test was used to compare groups of data (rather than a standard ANOVA). Proportions were compared by calculating the z-score. To determine the threshold for significance for aCBF reduction, a power regression model (\(y = {{{\mathrm{a}}}} \cdot x^b\)) was applied to absolute CBF and fractional CBF reduction (1 − FFR), which were plotted against each other, at a point equivalent to FFR = 0.80. Correlation between aCBF and percentage flow reduction was assessed with Pearson’s correlation coefficient (r) after log-transformation for continuous data and with Cohen’s kappa correlation coefficient (κ) for categorical data (concordance and discordance), which adjusts for agreement expected by chance and is a number between −1.0 and 1.0, with values of 0, 0.10–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, 0.81–0.90 and 1.0, indicating none (equivalent to chance), slight, fair, moderate, substantial, near-perfect and perfect agreement, respectively22. Negative values indicate agreement worse than that expected by chance. To detect a 15% reduction in concordance (α = 0.05) with 80% power, we required around 50 cases in each subgroup. We aimed to include 200 cases in total, to ensure ≥50 in the major subgroup comparisons (FFR-positive versus FFR-negative and versus 0.70–0.80). P values were not corrected for type 1 error (alpha) for multiple comparisons.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All the data supporting the findings in this research letter are provided as source data and supplementary information to this manuscript.
References
Demir, O. M. et al. Invasive and non-invasive assessment of ischaemia in chronic coronary syndromes: translating pathophysiology to clinical practice. Eur. Heart J. 43, 105–117 (2021).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020).
Fihn, S. D. et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 126, 3097–3137 (2012).
Stergiopoulos, K. et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern. Med. 174, 232–240 (2014).
Morris, P. D. et al. A novel method for measuring absolute coronary blood flow and microvascular resistance in patients with ischaemic heart disease. Cardiovasc. Res. 117, 1567–1577 (2021).
Everaars, H. et al. Continuous thermodilution to assess absolute flow and microvascular resistance: validation in humans using [15O]H2O positron emission tomography. Eur. Heart J. 40, 2350–2359 (2019).
van ‘t Veer, M. et al. Novel monorail infusion catheter for volumetric coronary blood flow measurement in humans: in vitro validation. EuroIntervention 12, 701–707 (2016).
Aarnoudse, W. et al. Direct volumetric blood flow measurement in coronary arteries by thermodilution. J. Am. Coll. Cardiol. 50, 2294–2304 (2007).
Meuwissen, M. et al. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation 103, 184–187 (2001).
Johnson, N. P., Kirkeeide, R. L. & Gould, K. L. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc. Imaging 5, 193–202 (2012).
van de Hoef, T. P. et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ. Cardiovasc. Interv. 7, 301–311 (2014).
Kunadian, V. et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology working group on coronary pathophysiology & microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 16, 1049–1069 (2021).
Ford, T. J. et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J. Am. Coll. Cardiol. 72, 2841–2855 (2018).
Pijls, N. H. et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 49, 2105–2111 (2007).
Xaplanteris, P. et al. Five-year outcomes with PCI guided by fractional flow reserve. N. Engl. J. Med. 379, 250–259 (2018).
De Bruyne, B. et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 367, 991–1001 (2012).
Fournier, S. et al. Normal values of thermodilution-derived absolute coronary blood flow and microvascular resistance in humans. EuroIntervention 17, e309–e316 (2021).
Ghobrial, M. et al. The new role of diagnostic angiography in coronary physiological assessment. Heart 107, 783–789 (2021).
Whitlow, P. L. et al. Relationship of extent of revascularization with angina at one year in the Bypass Angioplasty Revascularization Investigation (BARI). J. Am. Coll. Cardiol. 34, 1750–1759 (1999).
Tonino, P. A. et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 55, 2816–2821 (2010).
Solanki, R. et al. The importance of three dimensional coronary artery reconstruction accuracy when computing virtual fractional flow reserve from invasive angiography. Sci. Rep. 11, 19694 (2021).
Cohen, J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 20, 37–46 (1960).
Acknowledgements
P.D.M. was funded by the Wellcome Trust (214567/Z/18/Z). For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. R.G. was supported by a National Institute for Health and Care Research UK Clinical Lectureship. The authors are grateful to J. Gaudoin (School of Mathematics and Statistics at the Open University) for statistical advice when revising the manuscript.
Author information
Authors and Affiliations
Contributions
P.D.M. and J.P.G. conceived the original idea for the study and collected the clinical data. D.R.H., I.H., P.V.L. and A.N. supported software development and modeling. L.A.-R. performed the physiological simulations, supported by T.N. and D.T. L.A.-R. led the statistical analysis. All authors were involved in drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial conflicts of interest. P.D.M., J.P.G., P.J.L. and D.R.H. are named as inventors on a University of Sheffield patent application describing the CFD method (‘VIRTU-Q: a method for determining volumetric blood flow’; patent application no. 1813170.6).
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Data 1
Underlying clinical data for all cases
Supplementary Data 2
Underlying hemodynamic data for all cases
Source data
Source Data Fig. 1
Source data columns for the plots
Source Data Fig. 2
Source data columns for the plot
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aubiniere-Robb, L., Gosling, R., Taylor, D.J. et al. The Complementary Value of Absolute Coronary Flow in the Assessment of Patients with Ischaemic Heart Disease. Nat Cardiovasc Res 1, 611–616 (2022). https://doi.org/10.1038/s44161-022-00091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00091-z