Abstract
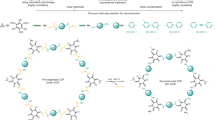
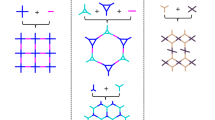
Covalent organic frameworks (COFs) are typically synthesized using solvothermal conditions (>120 °C, >72 hours) in harmful organic solvents. Here we report a strategy to rapidly (<60 minutes) synthesize imine-linked COFs in aqueous acetic acid using sonochemistry and thus avoid most of the disadvantages of solvothermal methods. Using the sonochemical method, we synthesized to our knowledge previously unreported COFs. The crystallinity and porosity of these COFs is comparable to or better than those of the same materials made by established solvothermal routes. The sonochemical method even works in sustainable solvents, such as food-grade vinegar. The generality of the method is shown in the preparation of a 2D COF with pendant functionalization and of a COF with 3D connectivity. Finally, a COF synthesized sonochemically acts as an excellent photocatalyst for the sacrificial hydrogen evolution from water, showing a more sustained catalytic performance compared with that of its solvothermal analogue. The speed, ease and generality of this sonochemical method together with improved material quality makes the use of sound an enabling methodology for the rapid discovery of functional COFs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source Data are provided with this paper. All other data supporting the finding of this study are available within this article and its Supplementary Information. The experimental procedures and characterization of all the COFs are provided in the Supplementary Information.
References
Wang, Z., Zhang, S., Chen, Y., Zhang, Z. & Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 49, 708–735 (2020).
Rodríguez-San-Miguel, D., Montoro, C. & Zamora, F. Covalent organic framework nanosheets: preparation, properties and applications. Chem. Soc. Rev. 49, 2291–2302 (2020).
Song, Y., Sun, Q., Aguila, B. & Ma, S. Opportunities of covalent organic frameworks for advanced applications. Adv. Sci. 6, 1801410 (2019).
Geng, K. Y. et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020).
Campbell, N. L., Clowes, R., Ritchie, L. K. & Cooper, A. I. Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem. Mater. 21, 204–206 (2009).
Matsumoto, M. et al. Rapid, low temperature formation of imine-linked covalent organic frameworks catalyzed by metal triflates. J. Am. Chem. Soc. 139, 4999–5002 (2017).
Zhu, D. et al. Rapid, ambient temperature synthesis of imine covalent organic frameworks catalyzed by transition-metal nitrates. Chem. Mater. 33, 3394–3400 (2021).
Kandambeth, S. et al. Selective molecular sieving in self-standing porous covalent–organic-framework membranes. Adv. Mater. 29, 1603945 (2017).
Karak, S. et al. Constructing ultraporous covalent organic frameworks in seconds via an organic terracotta process. J. Am. Chem. Soc. 139, 1856–1862 (2017).
Karak, S., Kumar, S., Pachfule, P. & Banerjee, R. Porosity prediction through hydrogen bonding in covalent organic frameworks. J. Am. Chem. Soc. 140, 5138–5145 (2018).
Biswal, B. P. et al. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 135, 5328–5331 (2013).
Das, G., Shinde, D. B., Kandambeth, S., Biswal, B. P. & Banerjee, R. Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 50, 12615–12618 (2014).
Emmerling, S. T. et al. In situ monitoring of mechanochemical covalent organic framework formation reveals templating effect of liquid additive. Chem 7, 1639–1652 (2021).
Feriante, C. H. et al. Rapid synthesis of high surface area imine-linked 2D covalent organic frameworks by avoiding pore collapse during isolation. Adv. Mater. 32, 1905776 (2019).
Sick, T. et al. Switching on and off interlayer correlations and porosity in 2D covalent organic frameworks. J. Am. Chem. Soc. 141, 12570–12581 (2019).
Thote, J. et al. Constructing covalent organic frameworks in water via dynamic covalent bonding. IUCrJ 3, 402–407 (2016).
Lu, J. et al. Large-scale synthesis of azine-linked covalent organic frameworks in water and promoted by water. New J. Chem. 43, 6116–6120 (2019).
Martín-Illán, J. Á. et al. Green synthesis of imine-based covalent organic frameworks in water. Chem. Commun. 56, 6704–6707 (2020).
Suslick, K. S. Sonochemistry. Science 247, 1439–1445 (1990).
Yang, S. T., Kim, J., Cho, H. Y., Kim, S. & Ahn, W. S. Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv. 2, 10179 (2012).
Yoo, J. et al. In situ synthesis of covalent organic frameworks (COFs) on carbon nanotubes and graphenes by sonochemical reaction for CO2 adsorbents. Chem. Lett. 44, 560–562 (2015).
Xu, H., Gao, J. & Jiang, D. L. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Zhi, Y. F. et al. Covalent organic frameworks as metal-free heterogeneous photocatalysts for organic transformations. J. Mater. Chem. A 5, 22933–22938 (2017).
Nguyen, H. L. et al. A porous covalent organic framework with voided square grid topology for atmospheric water harvesting. J. Am. Chem. Soc. 142, 2218–2221 (2020).
Bai, L. Y., Gao, Q. & Zhao, Y. L. Two fully conjugated covalent organic frameworks as anode materials for lithium ion batteries. J. Mater. Chem. A 4, 14106–14110 (2016).
Ascherl, L. et al. Solvatochromic covalent organic frameworks. Nat. Commun. 9, 3802 (2018).
EL-Mahdy, A. F. M. et al. Strategic design of triphenylamine- and triphenyltriazine-based two-dimensional covalent organic frameworks for CO2 uptake and energy storage. J. Mater. Chem. A 6, 19532–19541 (2018).
Auras, F. et al. Synchronized offset stacking: a concept for growing large-domain and highly crystalline 2D covalent organic frameworks. J. Am. Chem. Soc. 138, 16703–16710 (2016).
de la Peña Ruigómez, A. et al. Direct on-surface patterning of a crystalline laminar covalent organic framework synthesized at room temperature. Chem. Eur. J. 21, 10666–10670 (2015).
Yang, J. et al. Protonated imine-linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 60, 19797–19803 (2021).
Nalesso, S., Bussemaker, M. J., Sear, R. P., Hodnett, M. & Lee, J. A review on possible mechanisms of sonocrystallisation in solution. Ultrason. Sonochem. 57, 125–138 (2019).
Smith, B. J., Overholts, A. C., Hwang, N. & Dichtel, W. R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 52, 3690–3693 (2016).
Li, H. et al. Nucleation and growth of covalent organic frameworks from solution: the example of COF-5. J. Am. Chem. Soc. 139, 16310–16318 (2017).
Stewart, D. et al. Stable and ordered amide frameworks synthesised under reversible conditions which facilitate error checking. Nat. Commun. 8, 1102 (2017).
Zhang, J., Han, X., Wu, X., Liu, Y. & Cui, Y. Multivariate chiral covalent organic frameworks with controlled crystallinity and stability for asymmetric catalysis. J. Am. Chem. Soc. 139, 8277–8285 (2017).
Lan, Y. et al. Materials genomics methods for high-throughput construction of COFs and targeted synthesis. Nat. Commun. 9, 5274 (2018).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Aitchison, C. M. et al. Photocatalytic proton reduction by a computationally identified molecular hydrogen-bonded framework. J. Mater. Chem. A 8, 7158–7170 (2020).
Vyas, V. S. et al. A tunable azine covalent organic framework platform for visible light induced hydrogen generation. Nat. Commun. 6, 8508 (2015).
Bai, Y. et al. Accelerated discovery of organic polymer photocatalysts for hydrogen evolution from water through the integration of experiment and theory. J. Am. Chem. Soc. 141, 9063–9071 (2019).
Wang, P. et al. Exceptional iodine capture in 2D covalent organic frameworks. Adv. Mater. 30, 1801991 (2018).
Zhu, D. & Verduzco, R. Ultralow surface tension solvents enable facile COF activation with reduced pore collapse. ACS Appl. Mater. Interfaces 12, 33121–33127 (2020).
Mullangi, D. et al. Highly stable COF-supported Co/Co(OH)2 nanoparticles heterogeneous catalyst for reduction of nitrile/nitro compounds under mild conditions. Small 14, 1801233 (2018).
Bai, L. et al. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 52, 4128–4131 (2016).
Dong, J., Wang, Y., Liu, G., Cheng, Y. & Zhao, D. Isoreticular covalent organic frameworks for hydrocarbon uptake and separation: the important role of monomer planarity. CrystEngComm 19, 4899–4904 (2017).
Meng, Y., Lin, G., Ding, H., Liao, H. & Wang, C. Impregnation of sulfur into a 2D pyrene-based covalent organic framework for high-rate lithium-sulfur batteries. J. Mater. Chem. A 6, 17186–17191 (2018).
Li, X. et al. Molecular engineering of bandgaps in covalent organic frameworks. Chem. Mater. 30, 5743–5749 (2018).
Shan, Z. et al. Dynamic transformation between covalent organic frameworks and discrete organic cages. J. Am. Chem. Soc. 142, 21279–21284 (2020).
Romero Muñiz, I. et al. Unveiling the local structure of palladium loaded into imine-linked layered covalent organic frameworks for cross-coupling catalysis. Angew. Chem. Int. Ed. 59, 13013–13020 (2020).
Garzón Tovar, L. et al. A MOF@COF composite with enhanced uptake through interfacial pore generation. Angew. Chem. Int. Ed. 58, 9512–9516 (2019).
Acknowledgements
We acknowledge funding from the Leverhulme Trust via the Leverhulme Research Centre for Functional Materials Design and the Engineering and Physical Sciences Research Council (EPSRC). P.Y., H.C., L.L. and B.L. thank the China Scholarship Council for PhD studentships. We thank the Materials Innovation Factory (MIF) team for help with instrument training. We thank M. Lightowler of Stockholm University for testing the feasibility of using 3D electron diffraction to solve the COF structures. The TEM analysis was performed in the Albert Crewe Centre for Electron Microscopy, a University of Liverpool Shared Research Facility.
Author information
Authors and Affiliations
Contributions
W.Z. synthesized and characterized the materials, performed photocatalytic experiments and analysed the photocatalysis results with H.Y. P.Y. performed the scanning electron microscopy, time-correlated single photon counting and FTIR measurements. H.Y. performed the ultraviolet measurements. R.C. performed thermogravimetric analysis. A.M.J. and W.Z. performed gas adsorption measurements. H.C. and L.L. performed electrochemical measurements. M.B. and N.D.B. performed TEM measurements. W.Z. conceived the modelling strategy. B.L. and Z.P. provided useful advice in the structural simulation of COFs. Y.W. and J.W.W. conceived the project. A.I.C., Y.W. and J.W.W. directed the research. Data were interpreted by all the authors and the manuscript was prepared by A.I.C., Y.W., J.W.W. and W.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Synthesis thanks Donglin Jiang, Qihua Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Alison Stoddart was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 A comparison of BET surface areas and total pore volumes of sonoCOFs synthesized in aqueous and organic solvents.
The Brunauer–Emmett–Teller (BET) surface area (dark shading) and pore volume (light shading) of all the synthesized sonoCOFs in aqueous solvent (blue bars) are greater than or equal to the equivalent sonoCOFs synthesized in organic solvent (pink bars).
Supplementary information
Source data
Source Data Fig. 5
Raw data for Fig. 5a,b.
Source Data Fig. 5
Full-size TEM images supporting Fig. 5d,e.
Source Data Extended Data Fig. 1
Raw data for Extended Data Fig. 1.
Rights and permissions
About this article
Cite this article
Zhao, W., Yan, P., Yang, H. et al. Using sound to synthesize covalent organic frameworks in water. Nat Synth 1, 87–95 (2022). https://doi.org/10.1038/s44160-021-00005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-021-00005-0
This article is cited by
-
Sub-second ultrafast yet programmable wet-chemical synthesis
Nature Communications (2023)
-
Growth of single-crystal imine-linked covalent organic frameworks using amphiphilic amino-acid derivatives in water
Nature Chemistry (2023)
-
A general large-scale synthesis approach for crystalline porous materials
Nature Communications (2023)
-
Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality
Nature Synthesis (2023)
-
Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks
Nature Catalysis (2023)