Abstract
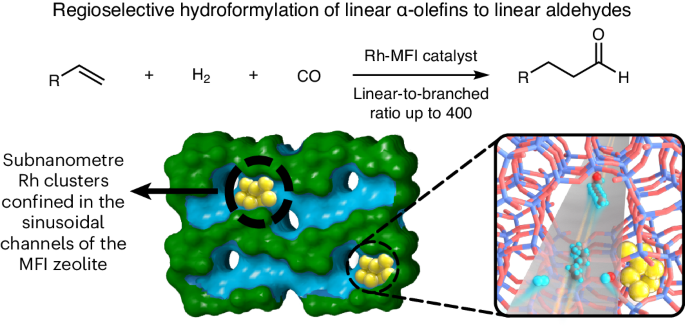
Achieving the regioselective hydroformylation of linear α-olefins to linear aldehydes using solid catalysts with regioselectivities comparable to the corresponding homogeneous process is a great challenge in the chemical industry. Despite the tremendous efforts devoted to this research topic, most of the reported heterogeneous metal catalysts still give considerably lower regioselectivities than well-established homogeneous metal catalysts. Here we show the design of efficient Rh-zeolite catalysts, in which subnanometre Rh clusters are selectively confined in the sinusoidal ten-membered-ring channels of MFI zeolite, for the hydroformylation of long-chain linear α-olefins (C6–C12) into linear aldehydes with very high linear-to-branched aldehyde ratios (up to 400). The exceptional catalytic performances result from the involvement of the MFI zeolite framework as a rigid solid ligand that accommodates subnanometre Rh clusters in the sinusoidal channels of the MFI zeolite.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and Supplementary Information or from the corresponding authors upon reasonable request. The optimized structures of all reaction intermediates and transition states derived from theoretical calculations are provided as Supplementary Data.
References
Franke, R., Selent, D. & Borner, A. Applied hydroformylation. Chem. Rev. 112, 5675–5732 (2012).
Pospech, J., Fleischer, I., Franke, R., Buchholz, S. & Beller, M. Alternative metals for homogeneous catalyzed hydroformylation reactions. Angew. Chem. Int. Ed. 52, 2852–2872 (2013).
Hood, D. M. et al. Highly active cationic cobalt(II) hydroformylation catalysts. Science 367, 542–548 (2020).
Linear Alpha-Olefins—Chemical Economics Handbook (S&P Global, 2023); https://ihsmarkit.com/products/linear-alpha-olefins-chemical-economics-handbook.html
Klein, H., Jackstell, R., Wiese, K.-D., Borgmann, C. & Beller, M. Highly selective catalyst systems for the hydroformylation of internal olefins to linear aldehydes. Angew. Chem. Int. Ed. 40, 3408–3411 (2001).
Seayad, A. et al. Internal olefins to linear amines. Science 297, 1676–1678 (2002).
Broussard, M. E. et al. A bimetallic hydroformylation catalyst: high regioselectivity and reactivity through homobimetallic cooperativity. Science 260, 1784–1788 (1993).
Rodrigues, F. M. S., Carrilho, R. M. B. & Pereira, M. M. Reusable catalysts for hydroformylation‐based reactions. Eur. J. Inorg. Chem. 2021, 2294–2324 (2021).
Hanf, S., Rupflin, L. A., Gläser, R. & Schunk, S. A. Current state of the art of the solid Rh-based catalyzed hydroformylation of short-chain olefins. Catalysts 10, 510 (2020).
Amsler, J. et al. Prospects of heterogeneous hydroformylation with supported single atom catalysts. J. Am. Chem. Soc. 142, 5087–5096 (2020).
Sun, Q. et al. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: synergistic effect of high ligand concentration and flexible framework. J. Am. Chem. Soc. 137, 5204–5209 (2015).
Li, C. et al. Single atom dispersed Rh-biphephos&PPh3@porous organic copolymers: highly efficient catalysts for continuous fixed-bed hydroformylation of propene. Green. Chem. 18, 2995–3005 (2016).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 55, 16054–16058 (2016).
Zhang, J. et al. Enhancing regioselectivity via tuning the microenvironment in heterogeneous hydroformylation of olefins. J. Catal. 387, 196–206 (2020).
Riisager, A., Fehrmann, R., Haumann, M. & Wasserscheid, P. Supported ionic liquid phase (SILP) catalysis: an innovative concept for homogeneous catalysis in continuous fixed‐bed reactors. Eur. J. Inorg. Chem. 2006, 695–706 (2006).
Madhavan, N., Jones, C. W. & Weck, M. Rational approach to polymer-supported catalysts: synergy between catalytic reaction mechanism and polymer design. Acc. Chem. Res. 41, 1153–1165 (2008).
Hübner, S., de Vries, J. G. & Farina, V. Why does industry not use immobilized transition metal complexes as catalysts? Adv. Synth. Catal. 358, 3–25 (2016).
Davis, R. J., Rossin, J. A. & Davis, M. E. Hydroformylation by rhodium zeolite A catalysts. J. Catal. 98, 477–486 (1986).
Shang, W. et al. Efficient heterogeneous hydroformylation over zeolite-encaged isolated rhodium ions. CCS Chem. 5, 1526–1539 (2023).
Liu, L. & Corma, A. Confining isolated atoms and clusters in crystalline porous materials for catalysis. Nat. Rev. Mater. 6, 244–263 (2020).
Sun, Q., Wang, N. & Yu, J. Advances in catalytic applications of zeolite-supported metal catalysts. Adv. Mater. 33, e2104442 (2021).
Goel, S., Wu, Z., Zones, S. I. & Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 134, 17688–17695 (2012).
Wang, H., Wang, L. & Xiao, F. S. Metal@zeolite hybrid materials for catalysis. ACS Cent. Sci. 6, 1685–1697 (2020).
Babucci, M., Guntida, A. & Gates, B. C. Atomically dispersed metals on well-defined supports including zeolites and metal–organic frameworks: structure, bonding, reactivity, and catalysis. Chem. Rev. 120, 11956–11985 (2020).
Liu, L., Lopez-Haro, M., Calvino, J. J. & Corma, A. Tutorial: structural characterization of isolated metal atoms and subnanometric metal clusters in zeolites. Nat. Protoc. 16, 1871–1906 (2021).
Liu, L. et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 18, 866–873 (2019).
Fontaine-Gautrelet, C. et al. CO- and N2-FTIR characterisation of oxidised Rh species supported on Ce0.68Zr0.32O2. Phys. Chem. Chem. Phys. 8, 3732–3740 (2006).
Magee, J. W., Palomino, R. M. & White, M. G. Infrared spectroscopy investigation of Fe-promoted Rh catalysts supported on titania and ceria for CO hydrogenation. Catal. Lett. 146, 1771–1779 (2016).
Fang, C.-Y. et al. Synthesis of Rh6(CO)16 in supercages of zeolite HY: reaction network and kinetics of formation from mononuclear rhodium precursors via Rh4(CO)12 facilitated by the water gas shift half-reaction. J. Phys. Chem. C 124, 2513–2520 (2019).
Liu, L. & Corma, A. Identification of the active sites in supported subnanometric metal catalysts. Nat. Catal. 4, 453–456 (2021).
Tsekov, R. & Smirniotis, P. G. Resonant diffusion of normal alkanes in zeolites: effect of the zeolite structure and alkane molecule vibrations. J. Phys. Chem. B 102, 9385–9391 (1998).
Smit, B. & Maesen, T. L. M. Commensurate ‘freezing’ of alkanes in the channels of a zeolite. Nature 374, 42–44 (1995).
Dubbeldam, D., Calero, S., Maesen, T. L. & Smit, B. Understanding the window effect in zeolite catalysis. Angew. Chem. Int. Ed. 42, 3624–3626 (2003).
Maginn, E. J., Bell, A. T. & Theodorou, D. N. Sorption thermodynamics, siting, and conformation of long n-alkanes in silicalite as predicted by configurational-bias Monte Carlo integration. J. Phys. Chem. 99, 2057–2079 (2002).
Yuan, J. et al. Thermal resistance effect on anomalous diffusion of molecules under confinement. Proc. Natl Acad. Sci USA 118, e2102097118 (2021).
Kirkland, E. J. Advanced Computing in Electron Microscopy (Springer, 2010)
Bernal, S. et al. The interpretation of HREM images of supported metal catalysts using image simulation: profile view images. Ultramicroscopy 72, 135–164 (1998).
Simonelli, L. et al. CLÆSS: the hard X-ray absorption beamline of the ALBA CELLS synchrotron. Cogent Phys. https://doi.org/10.1080/23311940.2016.1231987 (2016)
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Acknowledgements
L.L. is grateful for the financial support from the National Natural Science Foundation of China (22272087), Tsinghua University (Initiative Scientific Research Program (20233080016), the International Joint Mission on Climate Change and Carbon Neutrality (20233080033), the Dushi Program (20231080010)) and the National Key R&D Program of China (2022YFA1503901). Z.C. acknowledges the National Natural Science Foundation of China (numbers 21972161 and 22172186), the Autonomous Research Project of the State Key Laboratory of Coal Conversion (number 2022BWZ009), the Inner Mengolia Science & Technology Project Plan (number 2021GG0311), the Major Science and Technology Project of Ordos (2022EEDSKJZDZX001), the Institute of Coal Chemistry, Chinese Academy of Sciences and Synfuels China, Co., Ltd for financial support. L.Z acknowledges the National Natural Science Foundation of China (22103047) and the Young Scholars Program from the School of Vehicle and Mobility of Tsinghua University. M.L.-H. is grateful for financial support from MCIN/AEI/10.13039/501100011033 (PID2019-110018GA-I00) and the support from the DME-UCA Node of the Spanish Singular Infrastructure for Electron Microscopy of Materials (ICTS ELECMI). The in situ XAS experiments were performed at the CLÆSS (BL22) beamline of ALBA Synchrotron with collaboration of the ALBA staff. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy Office of Science by Argonne National Laboratory, and was supported by the US Department of Energy under contract number DE-AC02-06CH11357, as well as the Canadian Light Source and its funding partners. We thank R. Zong for his help in the electron microscopy characterization of the Rh-zeolite catalysts at Tsinghua University.
Author information
Authors and Affiliations
Contributions
L.L. and Z.C. conceived the project. L.Z. designed the theoretical studies. X.D. carried out the materials synthesis and part of the structural characterization. T.Y. performed the hydroformylation reaction tests and data analyses. L.Q. performed the theoretical calculations. H.H. conducted the in situ infrared and XPS investigations and helped with analysis of the obtained XAS data. M.L.-H. characterized the Rh-MFI catalysts using the iDPC-STEM imaging technique and carried out the image simulations. L.L. analysed the iDPC-STEM images. C.M. and G.A. measured the Rh-zeolite materials via in situ XAS and analysed the data. D.M.M. contributed to the spectroscopic characterization of the Rh-zeolite materials via XAS. X.Z. participated in the catalytic studies and data analysis. L.L., Z.C. and L.Z. wrote the manuscript with input from all of the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Marco Ranocchiari and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–50, Tables 1–26, Notes 1–7 and refs. 1–94.
Supplementary Data
Optimized structures of all reaction intermediates and transition states derived from theoretical calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dou, X., Yan, T., Qian, L. et al. Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01155-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01155-y


