Abstract
Text messaging can promote healthy behaviors, like adherence to medication, yet its effectiveness remains modest, in part because message content is rarely personalized. Reinforcement learning has been used in consumer technology to personalize content but with limited application in healthcare. We tested a reinforcement learning program that identifies individual responsiveness (“adherence”) to text message content and personalizes messaging accordingly. We randomized 60 individuals with diabetes and glycated hemoglobin A1c [HbA1c] ≥ 7.5% to reinforcement learning intervention or control (no messages). Both arms received electronic pill bottles to measure adherence. The intervention improved absolute adjusted adherence by 13.6% (95%CI: 1.7%–27.1%) versus control and was more effective in patients with HbA1c 7.5- < 9.0% (36.6%, 95%CI: 25.1%–48.2%, interaction p < 0.001). We also explored whether individual patient characteristics were associated with differential response to tested behavioral factors and unique clusters of responsiveness. Reinforcement learning may be a promising approach to improve adherence and personalize communication at scale.
Similar content being viewed by others
Introduction
Text messages can be delivered at low cost and provide reminders, education, and motivational support for health behaviors on an ongoing basis1. They have demonstrated effectiveness for supporting physical activity, medication adherence, and other daily self-management activities that are guideline recommended for managing chronic diseases, like type 2 diabetes2,3. However, many prior text messaging interventions have used generic message content (i.e., the same messages delivered to all patients)4,5,6.
Yet, a key principle for changing health behaviors is personalization and how information is presented to match an individual’s specific needs, which may also change over time7,8,9,10. Personalization can be based upon simple characteristics, such as name, age, or health metrics11. More detailed personalization could potentially be achieved by incorporating routines or behavioral barriers, and adjusting frequently12,13.
A major obstacle to achieving personalization based on underlying behavioral tendencies is the ability to predict what patients will actually respond to. Traditionally, theory-based assessments, or expert opinion (like barrier elicitation by clinicians), have been used to tailor behavioral messaging particularly at the outset, and sometimes, with updates at intervals14,15,16,17,18. For example, the REACH trial used interactive texts that asked participants directly about their adherence through weekly feedback, and another recent trial used dynamic tailoring based on patients’ implementation intention plan17,18. An alternative approach is to use observations of what content patients actually respond to and use that as the basis for what they will respond to in the future. This process is made feasible by mobile health tools (like electronic pill bottles) that passively measure health behaviors on an ongoing basis. Consistent with this, there is emerging interest in just-in-time adaptive interventions (JITAIs), or an intervention design that adapts support (e.g., type, timing, intensity) over time in response to an individual’s changing status and context19,20.
An efficient approach to achieve such personalized intervention is with the use of reinforcement learning21,22. This machine learning method trains a statistical model based on rewards from actions of the model in an environment. In the context of behavior change, the model observes individual behaviors in response to cues it provides (like text messages) and learns to optimize response (like adherence) through systematic trial-and-error23,24. This technique has technological underpinnings applied in computer gaming and robotics21,25,26,27. In contrast to other approaches to achieving personalization, reinforcement learning uses approaches that predict the effectiveness of different intervention components and also can use latently derived estimates for tailoring (rather than end user input); and, as interventions are deployed, updates the predictions based on their successes and failures (both at the individual and group level)28. That is, the algorithm “learns” to personalize as it experiments, or “adapts”29.
Reinforcement learning has thus far had limited use in health care27,28,30,31,32 and has not been applied to medication adherence, an essential daily activity for most patients with chronic disease, and especially diabetes, which affects 529 million individuals globally2,33. While machine learning generally has been shown to be helpful in measuring suboptimal adherence34,35, there remains much opportunity to explore how it and related techniques can improve adherence. Accordingly, we launched the REinforcement learning to Improve Non-adherence For diabetes treatments by Optimizing Response and Customizing Engagement trial (REINFORCE) to evaluate the impact of a text messaging program tailored using reinforcement learning on medication adherence for patients with type 2 diabetes22.
The trial design has been published22 with expanded details in the Methods. In brief, 60 patients with type 2 diabetes (with their latest glycated hemoglobin A1c [HbA1c] lab value ≥ 7.5% in the past 180 days) were randomized to a reinforcement learning intervention or control (no intervention) based on pre-specified power calculations. In both arms, patients received a separate electronic pill bottle for each of their diabetes medications, with bottles that look like those dispensed by retail pharmacies but with an electronic cap that recorded the dates and times in which participants took their medications. A figure of the infrastructure was previously published22. The reinforcement learning algorithm personalized daily texts based on adherence, patient characteristics, and message history using the following 5 behavioral factors: (1) how the messages are structured (“Framing”; classified as neutral, positive [invoking positive outcomes of medication use], or negative [invoking consequences of medication non-use]), (2) observed feedback (“History”, i.e., including the number of days in the prior week the patient was adherent), (3) social reinforcement (“Social”, i.e., referring to loved ones), (4) whether content was a reminder or informational (“Content”), and (5) whether the text included a reflective question (“Reflective”). Individual messages contained elements from these different factor sets, examples of which have been published previously22. The primary outcome was average pill bottle-measured adherence over a 6-month follow-up. After trial completion, we described the performance of the reinforcement learning algorithm process itself and explored responsiveness to behavioral factors using subgroup analyses and clustering methods, as prior work has suggested that there may be important differences in responsiveness24.
Results
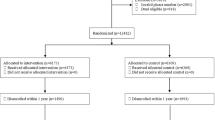
Among 60 patients, 29 and 31 were randomized to the intervention and control arms, respectively, of which 1 intervention and 3 control patients did not complete follow-up (Fig. 1). All 60 patients were included in the intention-to-treat analysis.
In total, 26 patients (43%) were female and 35 (58%) were White (Table 1). Baseline characteristics were slightly different between the arms based on absolute standardized differences but were well-balanced on key metrics including age, sex, baseline HbA1c values, and baseline adherence. Intervention group patients had less formal education (e.g., 24.1% vs. 16.1% having no more than a high school education) and took more oral diabetes medications (31% vs. 19% taking ≥2 medications) versus control patients.
Description of the reinforcement learning algorithm learning process
In total, 5143 text messages were sent to patients in the intervention arm (n = 29) during the 6-month study period. Intervention patients received daily messages; an average of 27.7 (SD: 5.9) unique messages were sent to each patient (Table 2). In aggregate, 514 (10.0%), 2473 (48.1%), and 2058 (40.0%) of text messages contained ≥3, ≥4, and ≥5 behavioral factors respectively.
The reinforcement learning algorithm also adapted its selection of behavioral factors in the text messages; the proportions of intervention arm patients who received the five factors over the trial are shown descriptively in Supplemental Fig. 1 panels. For example, positive framing as a factor (Supplemental Fig. 1a) was initially not frequently selected by the algorithm during the first two months of the trial but became more prevalent later. By contrast, negative framing was more commonly selected at first but decreased over time (Supplemental Fig. 1b). Other plots for receipt of history, social reinforcement, content, and reflection are shown in Supplemental Fig. 1c–f, respectively. More patients were selected to receive social reinforcement, content, and reflection as factors as the trial progressed, while the proportion of patients receiving history (observed feedback) remained relatively equal over time.
Figure 2 shows the change in adjusted R2 of the reinforcement learning algorithm over the trial. This statistic, which describes the extent to which adherence is explained by algorithm predictions for behavior following a message sent to participants, increased over time, indicating that the algorithm learned to send more effective messages to patients.
The adjusted R2 from trial calendar day 31 to 279 (March 13, 2021–December 19, 2021) is plotted from each day’s model. We calculated adjusted R2 from the proportion of variance in daily adherence that is explained by the five intervention factors in the reinforcement learning model. We selected those windows as they each had a minimum of 5 patient observations that day.
The most influential features and interactions from the reinforcement learning algorithm are shown in Fig. 3. Fixed characteristics that carried the most weight within the model were baseline HbA1c, self-reported level of patient activation, number of medications included in electronic pill bottles, concomitant insulin use, and employment status based on their interactions. The behavioral factors with the largest weight included positive framing, observed feedback, and social reinforcement.
This figure shows the model weights from the feature importance score from the reinforcement learning algorithm, which indicates which features were more or less importance to the model. The weights above were the 20 most influential features, ranked from highest to lowest. Abbreviations: HbA1c, glycated hemoglobin A1c; SGLT2, sodium-glucose cotransporter-2.
Effect of the reinforcement learning intervention on the primary outcome
Over the 6-month follow-up, average adherence to medication was 74.3% (SD: 30.8%) in the reinforcement learning intervention arm compared with 67.7% (SD: 29.4%) in the control arm (Fig. 4). After adjusting for the block randomized design and baseline characteristics, average adherence among intervention patients was 13.6% (95%CI: 1.7%, 27.1%, p = 0.047) higher than control (shown in Fig. 4). Sensitivity analyses, including omitting the first two weeks of pill bottle data and censoring patients in both arms after 30 days of pill bottle non-use (3 patient and 1 patient in intervention and control arms, respectively) did not change the results (Supplemental Table 1).
We used generalized estimating equations with an identity link and normally-distributed errors to evaluate the effect of the intervention on adherence to medication measured by pill bottles compared with control. The points on the figure are the point estimates and the error bars are the 95% confidence intervals from the relevant sample sizes. These models were adjusted for baseline characteristics and the block randomized design. The primary outcome is shown at the top. The results of exploratory subgroup analyses by key demographic and clinical characteristics also shown; these were performed by repeating the same models within each subgroup, using interaction p-values to assess between subgroups.
Hypothesis-generating demographic and clinical subgroup analyses that explored interactions between patient characteristics and the intervention’s effectiveness on adherence are also shown in Fig. 4. The strongest interaction between the overall effectiveness of reinforcement learning and adherence was by baseline HbA1c level. Specifically, in patients with HbA1c 7.5- < 9.0%, the intervention improved adherence by 33.6% (95%CI: 15.9%, 51.4%) versus control contrasted with those with baseline HbA1c ≥ 9% (interaction p value: 0.001) in which there was no significant difference compared with control. In patients who were non-adherent at baseline (i.e., self-reported missing >1 medication dose in the 30 days before enrollment), the intervention improved adherence by 33.0% (95%CI: 13.1%, 52.8%) versus control, but this interaction was not significant (interaction p value: 0.214).
Exploratory analyses of responsiveness to behavioral factors
In hypothesis-generating analyses, we whether responsiveness to the tested behavioral factors (determined by optimal adherence) differed by patient baseline characteristics. As shown in Fig. 5, patients who were aged <65 years (compared with ≥65), were of White race/ethnicity (compared with non-White), had HbA1c < 9% (compared with ≥9%), were of other marital status (compared with married/partnered), and were taking multiple medications (compared with 1) responded better than their counterparts to most or almost all behavioral factors. In contrast, women were more responsive to messages reporting their medication-taking history than men but were less responsive to other factors. Finally, patients who were more non-adherent at baseline (self-reported missing >1 dose, compared to those who reported missing ≤1 doses in the last 30 days) were more responsive to positively-framed messages and less responsive to messages reporting their medication-taking history, but had similar responsiveness to all other factors.
This figure shows the results of these exploratory analyses with the outcome being optimal adherence (adherence=1) for the day after the factor was selected and sent within the text message. We used generalized estimating equations for each behavioral factor with a log link and binary-distributed errors, adjusted for patient baseline characteristics but unadjusted for patient-level clustering. Light red indicates a negative association (Relative risk 0.50–0.99); Light blue indicates a positive association (Relative risk 1.01–1.50); and Dark blue indicates a strong positive association (Relative risk ≥1.50). Abbreviations: CI Confidence interval, HbA1c glycated hemoglobin A1c.
Adherence differed based on whether that behavioral factor had been sent the prior day (Fig. 6). For instance, adherence was highest when negatively-framed messages and messages containing observed medication feedback were sent two days in a row (i.e., red columns). By contrast, no difference in adherence was observed when text messages including and not including the behavioral factor were alternated.
This figure shows the average daily adherence measured by pill bottle over the course of the trial among the 29 intervention arm participants. These results are stratified by the text message sent in the prior day and/or the same day contained that intervention factor (e.g., positive framing). For example, the dark blue bar for “positive framing” indicates the level of adherence if the prior’s day text message contained positive framing but that day’s text message did not.
Using k-means clustering analysis of average adherence given the behavioral factors, we identified three unique patient clusters (Fig. 7). These clusters included: (1) Group 1 (Orange, n = 9) responding best to observed feedback, (2) Group 2 (Yellow, n = 4) responding best to social reinforcement and observed feedback, and (3) Group 3 (Blue, n = 16) responding equally to all message types. Individuals who were married/partnered were more likely to be in Group 1 compared with the other two groups, but most associations were non-significant owing at least in part to small sample size (Table 3).
Each color represents one of the three different patient groups identified from the exploratory k-means clustering analysis for the average pill bottle adherence measured over 6 months (primary outcome). These groups include: (1) Group 1 (Orange, n = 9) was the most adherent in response to observed feedback (“history”), (2) Group 2 (Yellow, n = 4) was the most adherent in response to social reinforcement or observed feedback, and (3) Group 3 (Blue, n = 16) was equally adherent in response to all types of messages. The error bars show the standard error for the cluster based on the underlying sample size (i.e., a threshold of ≥25 observations was applied).
Discussion
In this randomized-controlled trial of a reinforcement learning intervention that personalized text messaging content for patients with diabetes (and HbA1c ≥ 7.5%, above most guideline targets), we found that the intervention improved adherence to medication over a 6-month follow-up. The intervention was particularly effective among patients with HbA1c between 7.5 and 9.0%. Adherence changes of this magnitude have been associated with differences in patient outcomes and health care spending36,37.
Numerous trials have demonstrated that text messages support adherence to medication1,4,5,6,11,38,39,40. However, the effectiveness of many prior approaches has been limited, in part because they have not personalized the content and presentation of the messages patients receive38. To our knowledge, no study has personalized text messages for adherence in real-time on a daily basis through latent measurement of adherence and response, especially using reinforcement learning. Some prior work has personalized text messages for adherence based on simple user characteristics, preferences or self-reported adherence, at pre-specified intervals, or through relatively static “if-then” rules, but have not adapted based on observing what patients respond to11,17,18,19. Reinforcement learning has indicated early promise for other health behaviors. For example, a 3-arm trial of 27 patients tested the impact on physical activity of different text messaging approaches for individuals with type 2 diabetes, finding that text messaging using reinforcement learning resulted in significant more physical activity and lower HbA1c values than non-personalized weekly texting strategies24,27. Reinforcement learning interventions for titrating anti-epilepsy medications and selecting sepsis protocols have also demonstrated effectiveness31,32,41.
The reinforcement learning intervention appears to have learned from patient observations and changed the messages that it selected over time. This was particularly evident in its approach to message framing. The algorithm initially favored negatively framed messages (e.g., highlighting the negative disease consequences of non-adherence to medication) but over time, there was a noticeable shift such that more patients received either a neutral tone or positively framed message (e.g., highlighting positive consequences of adherence). This change was also seen quantitatively with the increasing proportion of variance in daily adherence explained by the behavioral factors in the text messages (i.e., the adjusted R2). By the end of the trial, the adjusted R2 was consistently over 0.40, meaning that much of the difference in adherence on a given day could be explained by the five algorithm factors. Additional features, for example, the interaction between positive framing and observed feedback as well as higher HbA1c and patient activation provided the greatest weight to the model prediction, suggesting that the algorithm incorporated learned information. Together, these findings suggest that the reinforcement learning algorithm not only changed its strategy over time but also improved its performance in predicting what types of messages would improve individuals’ adherence.
The intervention was also particularly effective in patients with HbA1c between 7.5 and 9.0%. The reason for this can be explained in two ways; first, patients further from guideline targets may need treatment intensification in addition to better adherence to their existing medications2,3. Second, a prior trial also suggested that individuals have varying preferences for how to escalate diabetes care at different levels of HbA1c values; those with HbA1c between 7.5 and 9.0% were more interested in adherence support than other interventions16. While less pronounced and not statistically significant, patients reporting worse adherence at baseline also tended to respond more to the intervention. This may also have been due to the fact that individuals who report missing multiple doses in the last 30 days most likely have substantial non-adherence42 and are an ideal target population for an adherence intervention.
Supporting the potential benefits of personalization and for generating future hypotheses, we explored characteristics of patients who responded differently to different message types. The most notable was that women responded better to receiving observed feedback about their medication-taking than men but responded less well to positive framing, social messaging, informational messaging rather than reminders, and messages that were intended to provoke reflection. One explanation could be that some women are already aware of how their own health can benefit loved ones and may prefer more straightforward reminders and feedback about their medication-taking performance24,43, although future work should explore further within larger sample sizes.
In our exploratory analyses, there were clusters of patients who responded to different types of messages. In specific, one group responded best to observed feedback, and a second group responded best to social reinforcement and observed feedback, while a third responded equally to all types of messages. We also found higher adherence when negatively-framed messages and messages that contained observed feedback were provided two days in a row, perhaps reflecting the need to reinforce these types of messages, but not others. The fact that the algorithm de-prioritized negative framing on average over time but that it was effective in combination with observed feedback is also worthy of consideration. This could in part be explained by underlying heterogeneity of the patient population in their responsiveness, and in how the information was sequenced, emphasizing the potential impact of personalization but should be explored further.
Future work could extend these findings in several ways. First, researchers should test the added impact of using reinforcement learning with non-personalized text messages. Second, the impact of a reinforcement learning intervention should be tested on long-term clinical outcomes and in a larger and more diverse sample to confirm some of the exploratory analyses about responsiveness to different behavioral factors. Finally, this work could be applied in other ways, for example to other disease states or related guideline-recommended daily activities such as physical activity or diet.
Several limitations should be acknowledged. First, electronic pill bottles could have influenced adherence, especially during the initial period of observation; however, they have been shown to correlate strongly with actual pill consumption44,45, and we minimized this observer bias by using pill bottles in both arms. While we powered the study to detect a 10% difference in adherence, the standard deviations were wider than anticipated, likely owing to the small sample size and overall heterogeneity in medication-taking than previously observed46. The findings may also not generalize to patients with pre-diabetes or gestational diabetes or those without reliable access to a smartphone. The subgroup and responsiveness analyses were also limited by small sample sizes and should be considered exploratory. It is also currently technologically less feasible to passively measure adherence to injectable agents in a scalable manner, and oral diabetes medications are the cornerstone of first and second-line type 2 diabetes treatments. Finally, we also chose not to have a “generic” text messaging arm, in part to test the highest possible efficacy of the intervention so we cannot assess the incremental benefit of personalization versus generic messaging with this design.
In conclusion, the reinforcement learning intervention led to improvements in adherence to oral diabetes medication and was particularly effective in patients with HbA1c between 7.5 and 9.0%. This trial provides insight into how reinforcement learning could be adapted at scale to improve other self-management interventions and provides promising evidence for how it could be improved and tested in a wider population.
Methods
Study design
Trial design details have been previously published22. The protocol was designed, written, and executed by the investigators (Fig. 1). Study enrollment began in February 2021 and completed in July 2021. Follow-up of all patients ended in January 2022; the final study database was available in March 2022.
Study population and randomization
The trial was conducted at Brigham and Women’s Hospital (BWH), a large academic medical center in Massachusetts, USA. Potentially-eligible patients were individuals 18–84 years of age diagnosed with type 2 diabetes and prescribed 1–3 daily oral diabetes medications, with their most recent glycated hemoglobin A1c (HbA1c) level ≥7.5% (i.e., above guideline targets)47. These criteria were assessed using BWH electronic health record (EHR) data. To be included, patients also had to have a smartphone with ability to receive text messages, have working knowledge of English, not be enrolled in another diabetes trial at BWH, not use a pillbox or switch to using electronic pill bottles for their diabetes medications for the study, and be independently responsible for taking medications. Smartphone connectivity was essential to measure daily adherence, but they have been widely adopted, even among patients from socioeconomically disadvantaged backgrounds48,49. Patients using insulin or other diabetes injectables in addition to their oral medication were allowed to be included to enhance generalizability.
As previously described22, potentially eligible patients with a recent or upcoming diabetes clinic visit were identified from the EHR on a biweekly basis. Once identified, the patients’ endocrinologists were contacted for permission to include their patient(s) in the study. Patients approved for enrollment were sent a letter on their endocrinologist’s behalf inviting them to participate and were then contacted by telephone. Patients who agreed provided their written informed consent captured through REDCap electronic data capture tools50,51, completed a baseline survey containing measures including demographics, self-reported adherence42, health activation52, and automaticity53 of medication-taking, and were mailed a separate Pillsy® electronic pill bottle for each of their eligible diabetes medications (i.e., each patient received between 1–3 pill bottles). Electronic pill bottles have been widely used in prior research and have shown high concordance with other measurement methods44,54. The data from the pill bottles were transmitted through the patients’ smartphones via an app that otherwise had no features enabled for the app or pill bottles (i.e., any latent adherence reminders through the pill bottle were turned off). A figure of the infrastructure has been previously published22.
After receiving the pill bottles, patients were randomized in a 1:1 ratio to intervention or control using block randomization based on baseline level of self-reported adherence (i.e., ≤1 dose or >1 doses missed in the last 30 days42) and (2) baseline HbA1c of <9.0% or ≥9.0%2. Patients were asked to use these devices instead of regular pill bottles or pillboxes for their eligible oral diabetes medications. After randomization, patients were followed for 6 months for outcomes. At the end of follow-up, patients were contacted to complete a follow-up survey and ensure complete synchronization of their pill bottles. Both arms received a $50 gift card for participation.
Intervention
The intervention was a reinforcement learning text messaging program that personalized daily text messages based on the electronic pill bottle data. Messages were selected by the Microsoft Personalizer® algorithm22,24, a reinforcement learning system which aimed to achieve the highest possible sum of “rewards” over time, and which adapted over time by monitoring the success of each message to nudge patients to adhere to their medications.
The messages were based on behavioral science principles of how content influences patient behavior55,56,57. Based on qualitative interviews58, we selected 5 behavioral factors for the messages: (1) framing (classified as neutral, positive [invoking positive outcomes of medication use], or negative [invoking consequences of medication non-use]), (2) observed feedback (“History”, i.e., including the number of days in the prior week the patient was adherent), (3) social reinforcement (“Social”), (4) whether content was reminder or informational (“Content”), and (5) whether the text included a reflective question (“Reflective”)7,8,24,59,60,61. We designed ≥2 text messages for each unique set of factors (i.e., 47 unique sets across 128 text messages); examples of the factors sets contributing to the reinforcement learning model have been published22.
Every day, adherence from the prior day was measured by the electronic pill bottles, with values ranging from 0 to 1 based on the fraction of daily doses taken across their diabetes medications, averaging if they are taking multiple medications22. These served as the “reward” events used to provide feedback to Microsoft Personalizer®. The algorithm learned to predict which factors should have been included in the message on a given day to maximize the rewards that the algorithm received (i.e., adherence).
The algorithm used several attributes to predict which factors to select. These included patient baseline characteristics (e.g., age, sex, race/ethnicity, number of medications, concomitant insulin use, self-reported patient activation, education level, employment status, marital status, and therapeutic class), the number of days since each factor had last been sent, and whether the medication had already been taken before the algorithm was run for that day. The algorithm was trained to predict whether or not to include each aspect separately using a “contextual bandit” framework62,63,64. The specific message to be sent was randomly selected from messages matching the required aspects.
The text messages were sent on a daily basis to patients using a third-party SMS platform, including an introductory text and simple reminder text to synchronize their pill bottles if they had not been connected for ≥7 days.
Patients in the control arm received the same introductory and simple reminder text to synchronize their pill bottles if they had not been connected for ≥7 days but otherwise received no intervention.
Study outcomes
The trial’s primary outcome was medication adherence assessed in the 6 months after randomization using the average daily adherence for each patient (which already averaged across multiple medications)22. While other secondary outcomes were measured, we focus on the primary outcome and related analyses in this manuscript.
Statistical analysis
The overall trial was powered to detect a 10% difference in average adherence over the 6-month follow-up, assuming a SD = 12.5%. We reported key sociodemographic and clinical pre-randomization variables separately for intervention and control using absolute standardized differences (imbalance as a difference >0.1)65. Intention-to-treat principles were used for all randomized patients, with a two-sided hypothesis tested at α = 0.05. We used SAS 9.4 (Cary, NC) for analyses.
The process and performance of the reinforcement learning algorithm were descriptively examined. The average proportion of patients who received each behavioral factor was estimated and plotted over time for each individual patient. To explore the extent to which adherence was explained by algorithm predictions each day, the adjusted R2 based on the algorithm predictions was estimated for each day over the trial. Specifically, we calculated the proportion of variance in daily adherence that was explained by just the five intervention factors. We also explored the most influential features selected by the model when predicting which messages to send for the entire follow-up period; higher scores indicates more influence to the model.
For the primary outcome, we evaluated the effect of the reinforcement learning intervention on adherence using generalized estimating equations with an identity link function and normally distributed errors. These models were adjusted for the block-randomized design, and given imbalances in some important covariates, also controlled for differences in measured baseline characteristics. Some of these imbalances included: more patients in the intervention arm with no more than a high school education (24.1% vs. 16.1%), fewer intervention patients who were married/or partnered (44.8% vs. 54.8%), and more intervention patients taking multiple diabetes medications (31.0% vs. 19.4%). Each of these characteristics have been shown previously to influence adherence66,67. Exploratory subgroup analyses were performed according to key demographic/clinical subgroups including age, sex, race/ethnicity, marital status, baseline HbA1c, number of years using oral diabetes medications, baseline self-reported adherence, and number of pill bottle medications. There was no missing data for the primary outcome. Several other sensitivity analyses were also conducted, including omitting the first 14 days for observer effects44 and censoring patients in both arms when the pill bottles were not connected for ≥30 days.
Additional exploratory and descriptive analyses of adherence in response to the intervention factors were also conducted for future hypothesis generation. First, the associations between key baseline characteristics and optimal adherence (i.e., adherence value = 1 for the subsequent day) by behavioral factor for intervention patients were explored. To do so, we used generalized estimating equations for each behavioral factor (e.g., positive framing) with a log link and binary-distributed errors with optimal adherence, including all patient baseline characteristics but unadjusted for patient-level clustering due to sample size. Then, we described adherence to different behavioral factors based on the sequence of delivered text messages. Finally, patients were clustered by their average response to different text message factors using k-means clustering analysis, using a threshold of ≥25 responses; smaller numbers were replaced by average variable values. Using these clusters, we explored the bivariate association between key baseline patient demographic/clinical characteristics and membership in each group using multinomial logistic regression (Referent: Group 3). Together, these findings may provide a more accurate starting point for future programs.
Data availability
De-identified data necessary to reproduce results reported here are posted on the Harvard Dataverse, an open access repository for research data, at https://dataverse.harvard.edu/. Some additional data, specifically dates such as for example dates of medication use, will be available upon reasonable request and execution of appropriate data use agreements, because dates are Protected Health Information under 45 CFR §164.154(b).
Code availability
Code necessary to reproduce results reported here are available in the Harvard Dataverse at https://dataverse.harvard.edu/.
References
Lauffenburger, J. C. & Choudhry, N. K. Text messaging and patient engagement in an increasingly mobile world. Circulation 133, 555–556 (2016).
ElSayed, N. A. et al. Glycemic targets: standards of care in diabetes-2023. Diabetes Care 46, S97–S110 (2023).
ElSayed, N. A. et al. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care 46, S140–S157 (2023).
Hamine, S., Gerth-Guyette, E., Faulx, D., Green, B. B. & Ginsburg, A. S. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J. Med. Internet Res. 17, e52 (2015).
Hartz, J., Yingling, L. & Powell-Wiley, T. M. Use of mobile health technology in the prevention and management of diabetes mellitus. Curr. Cardiol. Rep. 18, 130 (2016).
Dobson, R., Whittaker, R., Pfaeffli Dale, L. & Maddison, R. The effectiveness of text message-based self-management interventions for poorly-controlled diabetes: a systematic review. Digit. Health 3, 2055207617740315 (2017).
Keller, P. A. Affect, framing, and persuasian. J. Mark. Res. 40, 54–64 (2003).
Gong, J. et al. The framing effect in medical decision-making: a review of the literature. Psychol. Health Med. 18, 645–653 (2013).
Yokum, D., Lauffenburger, J. C., Ghazinouri, R. & Choudhry, N. K. Letters designed with behavioural science increase influenza vaccination in Medicare beneficiaries. Nat. Hum. Behav. 2, 743–749 (2018).
Petty R. E. & Cacioppo J. T. The Elaboration Likelihood Model of Persuasion, (Springer Series in Social PsychologyL, 1986).
Thakkar, J. et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern. Med. 176, 340–349 (2016).
Garofalo, R. et al. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 20, 1049–1059 (2016).
Sahin, C., Courtney, K. L., Naylor, P. J. & Rhodes, R. E. Tailored mobile text messaging interventions targeting type 2 diabetes self-management: a systematic review and a meta-analysis. Digit. Health 5, 2055207619845279 (2019).
Choudhry, N. K. et al. Effect of a remotely delivered tailored multicomponent approach to enhance medication taking for patients with hyperlipidemia, hypertension, and diabetes: the STIC2IT cluster randomized clinical trial. JAMA Intern. Med. 178, 1182–1189 (2018).
Choudhry, N. K. et al. Rationale and design of the Study of a Tele-pharmacy Intervention for Chronic diseases to Improve Treatment adherence (STIC2IT): A cluster-randomized pragmatic trial. Am. heart J. 180, 90–97 (2016).
Lauffenburger, J. C. et al. Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: the ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial. PloS one 14, e0214754 (2019).
Kassavou, A. et al. A highly tailored text and voice messaging intervention to improve medication adherence in patients with either or both hypertension and Type 2 diabetes in a UK primary care setting: feasibility randomized controlled trial of clinical effectiveness. J. Med. Internet Res. 22, e16629 (2020).
Nelson, L. A. et al. Effects of a tailored text messaging intervention among diverse adults with Type 2 diabetes: evidence from the 15-Month REACH randomized controlled trial. Diabetes Care 44, 26–34 (2021).
Hornstein, S., Zantvoort, K., Lueken, U., Funk, B. & Hilbert, K. Personalization strategies in digital mental health interventions: a systematic review and conceptual framework for depressive symptoms. Front. Digit. Health 5, 1170002 (2023).
Tong, H. L. et al. Personalized mobile technologies for lifestyle behavior change: a systematic review, meta-analysis, and meta-regression. Prev. Med. 148, 106532 (2021).
Trella A. L., et al. Designing Reinforcement Learning Algorithms for Digital Interventions: Pre-Implementation Guidelines. Algorithms. Aug 2022;15 https://doi.org/10.3390/a15080255.
Lauffenburger, J. C. et al. REinforcement learning to improve non-adherence for diabetes treatments by Optimising Response and Customising Engagement (REINFORCE): study protocol of a pragmatic randomised trial. BMJ open 11, e052091 (2021).
Jordan S. M., Chandak Y., Cohen D., ZHang M. & Thomas P. S. Evaluating the performance of reinforcement learning algorithms. In Proc. Thirty-Seventh International Conference on Machine Learning. 2020 https://proceedings.mlr.press/v119/jordan20a/jordan20a.pdf.
Yom-Tov, E. et al. Encouraging physical activity in patients with diabetes: intervention using a reinforcement learning system. J. Med. Internet Res. 19, e338 (2017).
Piette, J. D. et al. The potential impact of intelligent systems for mobile health self-management support: Monte Carlo simulations of text message support for medication adherence. Ann. Behav. Med. 49, 84–94 (2015).
Liu, D., Yang, X., Wang, D. & Wei, Q. Reinforcement-learning-based robust controller design for continuous-time uncertain nonlinear systems subject to input constraints. IEEE Trans. Cyber. 45, 1372–1385 (2015).
Hochberg, I. et al. Encouraging physical activity in patients with diabetes through automatic personalized feedback via reinforcement learning improves glycemic control. Diabetes Care 39, e59–e60 (2016).
Liao P., Greenewald K., Klasnja P. & Murphy S. Personalized HeartSteps: A Reinforcement Learning Algorithm for Optimizing Physical Activity. Proc ACM Interact Mob Wearable Ubiquitous Technol. Mar 2020;4 https://doi.org/10.1145/3381007.
Liu, X., Deliu, N. & Chakraborty, B. Microrandomized trials: developing just-in-time adaptive interventions for better public health. Am. J. Public Health 113, 60–69 (2023).
Guez, A., Vincent, R. D., Avoli, M. & Pineau, J. Treatment of epilepsy via batch-mode reinforcement learning. In Proc. Twenty-Third AAAI Conference on Artificial Intelligence. 2008:1671–1678 https://cdn.aaai.org/IAAI/2008/IAAI08-008.pdf.
Klasnja, P. et al. Micro-randomized trials: an experimental design for developing just-in-time adaptive interventions. Health Psychol. 34, 1220–1228 (2015).
Komorowski, M., Celi, L. A., Badawi, O., Gordon, A. C. & Faisal, A. A. The artificial intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 24, 1716–1720 (2018).
Collaborators GBDD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. Jun 2023; https://doi.org/10.1016/S0140-6736(23)01301-6.
Kanyongo, W. & Ezugwu, A. E. Feature selection and importance of predictors of non-communicable diseases medication adherence from machine learning research perspectives. Inform. Med. Unlocked. 38, 101132 (2023).
Kanyongo, W. & Ezugwu, A. E. Machine learning approaches to medication adherence amongst NCD patients: A systematic literature review. Inform. Med. Unlocked. 38, 101210 (2023).
Cutler, R. L., Fernandez-Llimos, F., Frommer, M., Benrimoj, C. & Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ open 8, e016982 (2018).
Bitton, A., Choudhry, N. K., Matlin, O. S., Swanton, K. & Shrank, W. H. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am. J. Med. 126, 357 e7–357.e27 (2013).
Arambepola, C. et al. The impact of automated brief messages promoting lifestyle changes delivered via mobile devices to people with Type 2 diabetes: a systematic literature review and meta-analysis of controlled trials. J. Med. Internet Res. 18, e86 (2016).
Bobrow, K. et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation 133, 592–600 (2016).
Pandey, A., Krumme, A., Patel, T. & Choudhry, N. The impact of text messaging on medication adherence and exercise among postmyocardial infarction patients: randomized controlled pilot trial. JMIR Mhealth Uhealth 5, e110 (2017).
Paredes P G-BR, Czerwinski M., Roseway A., Rowan K. & Hernandez J. PopTherapy: coping with stress through pop-culture. 109–117 (2014) https://dl.acm.org/doi/10.4108/icst.pervasivehealth.2014.255070.
Lauffenburger, J. C. et al. Comparison of a new 3-item self-reported measure of adherence to medication with pharmacy claims data in patients with cardiometabolic disease. Am. heart J. 228, 36–43 (2020).
Shrank, W. H. et al. Are caregivers adherent to their own medications? J. Am. Pharmacists Assoc 51, 492–498 (2011).
Mehta S. J. et al. Comparison of pharmacy claims and electronic pill bottles for measurement of medication adherence among myocardial infarction patients. Med. care. https://doi.org/10.1097/MLR.0000000000000950.
Arnsten, J. H. et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin. Infect. Dis. 33, 1417–1423 (2001).
Franklin, J. M. et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med. Care 51, 789–796 (2013).
Garber, A. J. et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive TYPE 2 diabetes management algorithm - 2018 executive summary. Endocr. Pr. 24, 91–120 (2018).
Baptista, S. et al. User experiences with a Type 2 diabetes coaching app: qualitative study. JMIR Diabetes 5, e16692 (2020).
Aguilera, A. et al. mHealth app using machine learning to increase physical activity in diabetes and depression: clinical trial protocol for the DIAMANTE Study. BMJ Open 10, e034723 (2020).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 95, 103208 (2019).
Harris, P. A. et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Wolf, M. S. et al. Development and validation of the consumer health activation index. Med. Decis. Mak. 38, 334–343 (2018).
Gardner, B., Abraham, C., Lally, P. & de Bruijn, G. J. Towards parsimony in habit measurement: testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. Int. J. Behav. Nutr. Phys. Act. 9, 102 (2012).
Volpp, K. G. et al. Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: the heartstrong randomized clinical trial. JAMA Intern. Med. 177, 1093–1101 (2017).
Bandura, A. Self-efficacy: toward a unifying theory of behavioral change. Psychol. Rev. 84, 191–215 (1977).
Gintis, H. A framework for the unification of the behavioral sciences. Behav. Brain Sci. 30, 1–16 (2007).
Tzeng, O. C. & Jackson, J. W. Common methodological framework for theory construction and evaluation in the social and behavioral sciences. Genet. Soc. Gen. Psychol. Monogr. 117, 49–76 (1991).
Lauffenburger, J. C. et al. Preferences for mHealth technology and text messaging communication in patients with Type 2 diabetes: qualitative interview study. J. Med. Internet Res. 23, e25958 (2021). Jun.
Baron, R. M. Social reinforcement effects as a function of social reinforcement history. Psychol. Rev. 73, 527–539 (1966).
Lauffenburger J. C., Khan N. F., Brill G., Choudhry N. K. Quantifying social reinforcement among family members on adherence to medications for chronic conditions: a us-based retrospective cohort study. J. General Intern. Med. https://doi.org/10.1007/s11606-018-4654-9.
Viswanathan, M. et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann. Intern. Med. 157, 785–795 (2012).
Krakow, E. F. et al. Tools for the precision medicine era: how to develop highly personalized treatment recommendations from cohort and registry data using Q-learning. Am. J. Epidemiol. 186, 160–172 (2017).
Laber, E. B., Linn, K. A. & Stefanski, L. A. Interactive model building for Q-learning. Biometrika 101, 831–847 (2014).
Nahum-Shani, I. et al. Q-learning: a data analysis method for constructing adaptive interventions. Psychol. Methods 17, 478–494 (2012).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Lauffenburger, J. C. et al. Prevalence and impact of having multiple barriers to medication adherence in nonadherent patients with poorly controlled cardiometabolic disease. Am. J. Cardiol. 125, 376–382 (2020).
Easthall, C., Taylor, N. & Bhattacharya, D. Barriers to medication adherence in patients prescribed medicines for the prevention of cardiovascular disease: a conceptual framework. Int. J. Pharm. Pract. 27, 223–231 (2019).
Acknowledgements
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number P30AG064199 to BWH (N.K.C. PI). J.C.L. was supported by a career development grant (K01HL141538) from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to thank the Digital Care Transformation team at BWH, the team responsible for managing Microsoft Dynamics 365 SMS Texting.
Author information
Authors and Affiliations
Contributions
All authors meet International Committee of Medical Journal Editors (ICMJE) criteria. JCL had overall responsibility for the trial design and drafted the trial protocol and manuscript. NKC is the co-principal investigator, had overall responsibility for the trial design and trial protocol, and helped draft the trial protocol and manuscript. EYT, PAK, MEM, LGB, CPF, ESS, KC, GB, KH, and NH contributed meaningfully to trial or intervention design and implementation as well as the manuscript. All authors contributed to the refinement of the study protocol and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
At the time this study was conducted, E.Y.-T. was an employee of Microsoft. N.K.C. serves as a consultant to Veracity Healthcare Analytics and holds equity in RxAnte and DecipherHealth; unrelated to the current work, N.K.C. has also received unrestricted grant funding payable to Brigham and Women’s Hospital from Humana. N.H. has received personal fees from Cerebral unrelated to the current work. The remainder of the authors report no conflicts of interest.
Ethical approval
The trial was approved by the institutional review board (IRB) of Mass General Brigham and registered with clinicaltrials.gov (NCT04473326). The authors were responsible for performing study analyses, writing the manuscript, substantive edits, and submitting final contents for publication. Patients were not blinded due to the nature of the interventions. No data monitoring committee was deemed necessary by the IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lauffenburger, J.C., Yom-Tov, E., Keller, P.A. et al. The impact of using reinforcement learning to personalize communication on medication adherence: findings from the REINFORCE trial. npj Digit. Med. 7, 39 (2024). https://doi.org/10.1038/s41746-024-01028-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-024-01028-5