Abstract
Malaria infection leads to hematological abnormalities, including deranged prothrombin time (PT). Given the inconsistent findings regarding PT in malaria across different severities and between Plasmodium falciparum and P. vivax, this study aimed to synthesize available evidence on PT variations in clinical malaria. A systematic literature search was performed in PubMed, Embase, Scopus, Ovid, and Medline from 27 November 2021 to 2 March 2023 to obtain studies documenting PT in malaria. Study quality was evaluated using the Joanna Briggs Institute checklist, with data synthesized through both qualitative and quantitative methods, including meta-regression and subgroup analyses, to explore heterogeneity and publication bias. From 2767 articles, 21 studies were included. Most studies reported prolonged or increased PT in malaria patients compared to controls, a finding substantiated by the meta-analysis (P < 0.01, Mean difference: 8.86 s, 95% CI 5.32–12.40 s, I2: 87.88%, 4 studies). Severe malaria cases also showed significantly higher PT than non-severe ones (P = 0.03, Hedges’s g: 1.65, 95% CI 0.20–3.10, I2: 97.91%, 7 studies). No significant PT difference was observed between P. falciparum and P. vivax infections (P = 0.88, Mean difference: 0.06, 95% CI − 0.691–0.8, I2: 65.09%, 2 studies). The relationship between PT and malaria-related mortality remains unclear, underscoring the need for further studies. PT is typically prolonged or increased in malaria, particularly in severe cases, with no notable difference between P. falciparum and P. vivax infections. The inconsistency in PT findings between fatal and non-fatal cases highlights a gap in current understanding, emphasizing the need for future studies to inform therapeutic strategies.
Similar content being viewed by others
Introduction
Malaria, caused by Plasmodium species infection, is a serious global health challenge. In 2021, over 200 million people were infected, leading to an estimated 619,000 deaths1. Infections by Plasmodium species are transmitted through the bites of infected female Anopheles mosquitoes2. The five most common Plasmodium species infecting humans are Plasmodium falciparum (P. falciparum), Plasmodium malariae (P. malariae), Plasmodium vivax (P. vivax), Plasmodium ovale (P. ovale), and Plasmodium knowlesi (P. knowlesi), with P. falciparum responsible for the most fatal infections. After transmission via mosquito bites, the malaria parasite travels to the liver, replicates in the host's liver cells, and then proceeds to infect red blood cells in the circulation3. Plasmodium infection can lead to a wide range of clinical symptoms, which may include fever, chills, headaches, nausea, vomiting, muscle aches, fatigue, and, in some cases, jaundice due to the destruction of red blood cells. Symptoms can vary from mild, uncomplicated malaria, which involves the aforementioned symptoms without signs of severe organ dysfunction, to severe malaria, characterized by more serious conditions such as impaired consciousness, respiratory distress, multiple convulsions, severe anemia, hemoglobinuria, acute kidney injury, hyperparasitemia, and complications leading to death4. Well-documented hematological changes occur during malaria infection, such as anemia, thrombocytopenia, and lymphocytopenia5,6. Moreover, severe malaria can result in significant liver damage, respiratory distress, renal failure, multi-organ dysfunction, abnormal bleeding, and cerebral malaria—a fatal neurological complication predominantly associated with P. falciparum infection7.
Coagulation, a critical process in hemostasis, involves a complex cascade of events that lead to the formation of a blood clot, preventing excessive bleeding when blood vessels are injured8,9. Initially, the process begins with the vascular injury, triggering the exposure of tissue factor, a key activator of the coagulation cascade. This exposure leads to the activation of the extrinsic pathway, marked by the conversion of prothrombin to thrombin. Thrombin then plays a central role in converting fibrinogen into fibrin, which forms the structural basis of a clot10. Simultaneously, the intrinsic pathway, initiated by contact activation factors within the blood (Factors XII, XI, VIII, IX), converges with the extrinsic pathway to amplify thrombin production. The common pathway, involving both extrinsic and intrinsic pathways, culminates in the stabilization of the fibrin clot alongside activated platelets, effectively sealing the site of vascular injury8,9,10.
Coagulation abnormalities are common laboratory findings in malaria infection, observable across the spectrum from uncomplicated to severe malaria11,12. Previous studies have reported that clinically apparent bleeding and disseminated intravascular coagulation (DIC), a severe coagulopathy, is associated with severe malaria infection and is more common in cases of cerebral malaria13,14. It has been reported that approximately 5–10% of severe malaria cases develop DIC, which is associated with high mortality15. The pathogenesis of Plasmodium species, specifically, P. falciparum is known to cause severe endothelial dysfunction in cases of both uncomplicated and fatal malaria due to its unique ability to sequester in the microvasculature and induce inflammatory responses11. Various procoagulants present during malaria infection, including tissue factor (TF) released from damaged vascular endothelial cells, the induction of TF expression in endothelial cells, exposed phosphatidylserine on infected red blood cells, lysis of activated platelets, macrophage migration inhibitory factor (MIF), and interleukin-6 cytokine (IL-6), are believed to activate the coagulation system16,17,18.
Routine laboratory coagulation tests, such as prothrombin time (PT) and activated partial thromboplastin time (APTT), are commonly used to investigate blood coagulation changes. PT reflects the activity of the extrinsic (factor VII) and common (factors V, X, II, and I) coagulation pathways, while APTT monitors the competency of the intrinsic (Factors XII, XI, VIII, IX) and common pathways19. Prior research indicates that the extent of coagulation derangement often corresponds with the severity and activity of the disease process20. Some studies indicate that patients with high parasitemia often experience alterations in coagulation tests, as high parasitemia is known to enhance fibrin formation and activate plasminogen, thus disturbing the coagulation system21,22,23. Notably, prolonged PT has been observed in P. falciparum infections characterized by high parasitemia22, suggesting that PT could potentially serve as a predictive marker for the clinical progression of Plasmodium infection. Nevertheless, the results of PT in malaria remain inconsistent across different studies. Therefore, this systematic review and meta-analysis aims to investigate differences in PT between malaria patients and uninfected controls, between varying degrees of disease severity, between different Plasmodium species, and between fatalities and survivors. Such a data collection would help in monitoring and predicting disease progression and could aid in preventing life-threatening complications of malaria infection through early detection and proper management of the hypercoagulable state.
Methods
Protocol and registration
The protocol of systematic review was registered at PROSPERO with a registration number CRD42022346003. The systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines24.
Data sources and searches
A comprehensive search was conducted in databases such as PubMed, Embase, Scopus, Ovid, and Medline from their inception up to March 2, 2023 (the final date of searches), to identify studies reporting on PT in malaria cases. To construct the search strategy, the search terms were combined with Boolean operators (AND, OR): (coagulation OR "blood clotting" OR "prothrombin time" OR prothrombin OR "russell viper venom time" OR "russells viper venom time" OR "thrombotest" OR "quick test"). Language restrictions were applied to include only English-language articles, without constraints on the year of publication. The details of the searches in all databases are listed in Table S1.
Definitions
Severe P. falciparum malaria is characterized by the presence of P. falciparum asexual parasitaemia alongside one or more complications as specified by the WHO criteria for severe malaria such as impaired consciousness, prostration, severe malarial anemia, jaundice, acidosis, renal impairment, significant bleeding, shock, multiple convulsions, hypoglycemia, and hyperparasitemia25. Severe P. vivax malaria follows the same definition as severe P. falciparum malaria, except that it doesn't require any specified parasite density thresholds. Non-severe malaria, on the other hand, is identified by the detection of asexual Plasmodium parasitemia, yet with the absence of any complications stipulated by the WHO's criteria for severe malaria.
Outcomes
The outcomes of the systematic review were the difference in PT between the following groups of participants: (i) malaria cases and uninfected controls, (ii) severe and non-severe malaria cases, (iii) different Plasmodium species, and (iv) deaths and survivors.
Eligibility criteria
The PICo (P: population, I: Outcome of interest, Co: context) approach was used to include eligible studies. (i) P: patients with malaria in all clinical severity (asymptomatic, uncomplicated, severe, or fatal malaria). (ii) I: prothrombin time in qualitative (prolonged or normal) and quantitative using mean ± standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for data not normally distributed. Co: worldwide or global. The inclusion criteria are: (i) original studies that investigated prothrombin time in patients with malaria; (ii) study designs that could be cross-sectional, cohort, or case–control studies; (iii) PT assessments conducted upon admission, before treatment. The exclusion criteria were: case reports/case series, clinical trials without baseline PT measurements, letters, communications, conference abstracts, book series, in vitro studies, articles not in English, and reviews/systematic reviews.
Study selection and data extraction
Study selection was performed by two review authors (MK and SS) independently using EndNote 20 for reference management. First, duplicate studies were excluded, and then titles and abstracts of the remaining studies were screened. Second, studies with irrelevant titles and abstracts were removed. Third, potentially relevant studies were examined for full-text, and ineligible studies were removed with specific reasons. Fourth, studies that met the eligibility criteria were included in the systematic review. After the studies were selected based on the eligibility criteria, the following data were extracted from each study using a pilot-tested, standard datasheet: name of the first author, publication year, study design, study location (year of conduction), characteristics of participants enrolled, Plasmodium spp., age range, PT in malaria and other groups, method for malaria detection, and method for PT. Data from each study were extracted by two authors (SS and MK). Any disagreements regarding study selection and data extraction between the two authors were resolved through discussion to reach a consensus.
Quality assessment
Two review authors (SS and TD) independently assessed the quality of included studies using the Joanna Briggs Institute (JBI) critical appraisal checklist for observational studies26. The checklist contains a set of questions that determine the internal and external validity of a study. These questions cover various aspects of study design, conduct, and reporting, including: sampling of study participants, sample size, study subjects and the setting, data analysis, methods used for the identification of the condition, a standard, reliable way for measurement of the condition, statistical analysis, confounding factors, identification of subpopulations, and measurement of outcome. Each item is scored as "yes," "no," "unclear," or "not applicable". The total score defines the overall quality of the study in which low, moderate, and the high quality if the total score were ≤ 50%, 51–74%, and ≥ 75%, respectively.
Data synthesis
Data synthesis was performed using qualitative and quantitative approaches. The qualitative involved the narrative description of the results of an individual study; meanwhile, the quantitative synthesis involved the pooling of results from several studies that reported the same outcome data. In the present study, the pooled mean difference (MD) or standardized mean difference (Hedge’s g) was used as the pooled effect estimates of PT between groups of participants including (i) malaria cases and uninfected controls, (ii) severe and non-severe malaria cases, (iii) and different Plasmodium species. The heterogeneity of the effect estimates were determined using the I2 for inconsistency in which I2 values between 25–50%, 51–75%, and > 75% indicated low, moderate, and significant heterogeneity, respectively27. In the meta-analysis comparing PT between groups of participants, the meta-regression and subgroup analyses were conducted to identify the source of the heterogeneity. These analyses were stratified based on several factors such as publication year, study design, country, continent, Plasmodium species, age groups, methods for malaria diagnosis, and methods for PT assessment. For any comparison involving more than ten studies, the potential publication bias and the influence of small-study effects were assessed using the funnel plots, contour-enhanced funnel plots, and Egger’s regression test28. All the computations were performed using Stata software version 17.0 (StataCorp LLC, College Station, TX).
Results
Search results
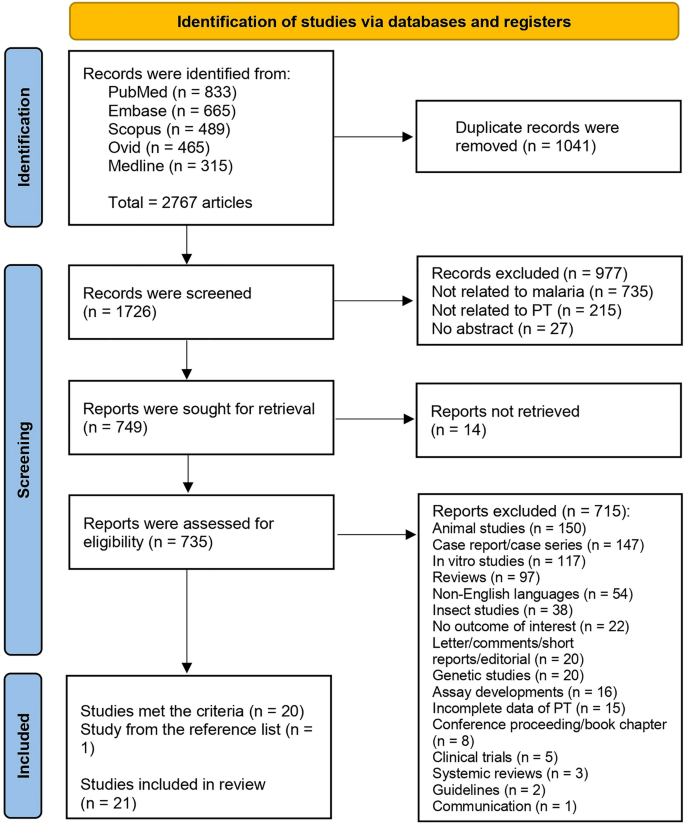
From the identified 2767 articles across databases including PubMed, Embase, Scopus, Ovid, and Medline, 1041 duplicates were removed. This resulted in 1726 unique records, which were screened for relevance. Subsequently, 977 records not relevant to malaria, PT, or lacking abstracts were eliminated. Of the 749 reports marked for retrieval, 14 were inaccessible, leaving 735 for eligibility assessment. After rigorous evaluation, 715 were further excluded due to a variety of reasons including irrelevance to the study's focus, being animal/in vitro studies, reviews, non-English language studies, among others. Ultimately, 20 studies that met the selection criteria were identified. An additional study, identified from a reference list, was included, totaling 21 studies for review29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 (Fig. 1).
Characteristics of the included studies
Table 1 summarize the characteristics of the 21 studies included in the review. Out of 21 studies analyzed, the majority (52.38%) were published between 2010 and 2019, with the common study design being retrospective (33.33%). Most research was conducted in Asia (61.90%), specifically India (38.1%). The Plasmodium species most studied was P. falciparum (42.86%). Study participants were mainly adults (33.33%) or unspecified (33.33%). Microscopy (52.38%) was the dominant method for malaria detection, while the majority of studies (66.67%) did not specify their PT measurement method (Table 1). Details of all included studies were demonstrated in Table S2.
Quality of the included studies
For case–control studies, two studies29,33 showed issues in group comparability regarding the presence or absence of disease (Table S3). Despite meeting the criteria for appropriate matching of cases and controls, the same identification criteria, and standard exposure measurement, both studies lacked the identification of and strategies for dealing with confounding factors. For cross-sectional studies, one study was included in the appraisal despite not clearly defining inclusion criteria, failing to identify confounding factors, and lacking appropriate statistical analysis31. One study, on the other hand, met all appraisal criteria35. Two studies43,48, while failing to fully address confounding factors, were also included due to their adherence to the remaining criteria.
For cohort studies, a significant number of studies all demonstrated a similar pattern30,32,34,36,37,38,40,41,42,46,47,49. They met most of the appraisal criteria but failed to identify or state strategies to handle confounding factors and did not employ strategies to address incomplete follow-up. Despite these shortcomings, they were all included in the appraisal. One study managed to identify and address confounding factors but did not manage to address incomplete follow-up39. One study did not adequately address confounding factors or incomplete follow-up and failed to use appropriate statistical analysis, yet was included44. Lastly, one study45 faced a unique issue with unclear exposure measurement but was included despite this and the usual issues with confounding factors and incomplete follow-up.
Difference in PT between malaria patients and uninfected controls
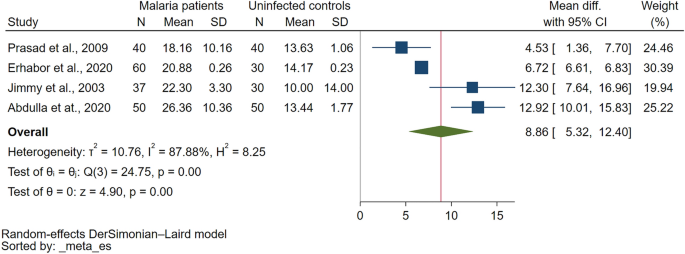
Comparison of PT between malaria patients and uninfected controls were reported in eight studies29,31,33,34,36,45,46,47. Six studies demonstrated that PT was significantly higher in malaria patients than uninfected controls29,33,34,36,46,47 while two studies reported that PT was prolonged in uncomplicated malaria (11/47 cases) as compared to normal uninfected controls31. PT was prolonged only in cerebral malaria but normal in non-cerebral severe malaria, uncomplicated malaria, non-malaria, and healthy controls45. The meta-analysis using the data from four studies that reported quantitative data of PT29,33,36,47 showed a significantly higher PT in malaria patients as compared to uninfected controls (P < 0.01, Mean difference: 8.859 s, 95% CI 5.315–12.403 s, I2: 87.88%, 4 studies Fig. 2).
Difference in PT between severe and non-severe malaria
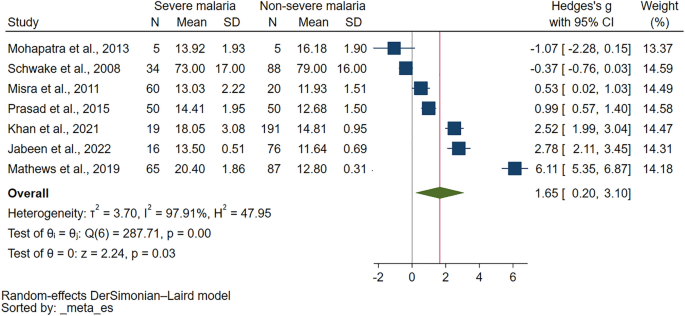
Comparison of PT in severe and non-severe malaria were reported in 11 studies31,35,37,40,41,42,43,44,45,48,49. Out of 11 studies examining PT in severe and non-severe malaria, varied results were reported. Several studies35,37,40,42,48 found PT to be higher in severe malaria than non-severe cases. One study found no significant difference in PT between severe and non-severe malaria49, while another reported normal PT levels in both conditions41. Interestingly, one study reported normal PT in severe malaria but prolonged PT in non-severe malaria31. Another study found prolonged PT in cerebral malaria but normal in non-cerebral severe malaria and non-severe malaria45, whereas one found prolonged PT in cerebral severe malaria but normal in non-severe malaria43. Finally, one study reported prolonged PT in both severe and non-severe malaria44. The meta-analysis using the data from seven studies that reported quantitative data of PT35,37,40,42,44,48,49 showed a significantly higher PT in severe malaria than non-severe malaria (P = 0.03, Hedges's g: 1.65, 95% CI 0.20–3.10, I2: 97.91%, 7 studies Fig. 3).
The meta-regression analysis was further performed to identify the source of heterogeneity of the effect estimate. The results showed that publication years, study design, country, continent, age group, Plasmodium species, diagnostic method for malaria, or method for PT measurement did not affect the pooled effect estimate (Table S4). Although these covariates did not affect the pooled effect estimate, the R2 values indicated that the covariate, diagnostic method for malaria, explains 11.15% of the variation in PT differences between severe and non-severe malaria cases, indicating some level of explanatory power, though still relatively low. The subgroup analysis has been further performed to identify the source of heterogeneity of the effect estimate (Table 2). The subgroup analysis based on publication years showed no difference in PT for studies published from 2010 to 2019 (P = 0.18), while those from 2000 to 2009 reported a significant increase in PT (P < 0.01). The subgroup analysis based on study design showed that cross-sectional study subgroup yielded a significant increase in PT (P = 0.04), while prospective studies subgroup showed no difference in PT (P = 0.12). The subgroup analysis based on continent found a significant increase in PT for severe malaria cases in Asia (P = 0.01). The subgroup analysis based on age showed a trend toward higher PT in adults with severe malaria, but no conclusive results for unspecified age or all age groups due to insufficient data. In the context of Plasmodium species, studies reporting on P. falciparum indicated a non-significant difference in PT (P = 0.38). P. vivax studies revealed a non-significant difference in PT (P = 0.48). Subgroup analysis based on diagnostic methods demonstrated a significant increase in PT for severe malaria when Microscopy/RDT was used (P = 0.03), but not when Microscopy alone was used (P = 0.65). Lastly, the method of PT measurement subgroup analysis showed a significant increase in PT in severe malaria for studies where the PT measurement method was not specified (P = 0.01). The manual and optical clotting methods could not generate P-values due to insufficient data.
Difference in PT between different Plasmodium species
Comparison of PT in different Plasmodium species were reported in six studies30,32,35,37,39,41. There appears to be variance in the PT based on the Plasmodium species involved in the malaria infection. For instance, one study suggested PT prolongation in both P. vivax and P. falciparum malaria32. Contrarily, another study found PT to be prolonged in P. falciparum malaria, but normal in P. vivax malaria and mixed infections39. In mixed infection cases, PT was found to be significantly higher than in P. vivax mono-infection cases30. However, another investigation reported normal PT for both P. falciparum and P. vivax malaria41. Two additional studies did not provide qualitative results comparing PT between different Plasmodium species, but they provided quantitative data for the meta-analysis 35,37. There was no significant difference in PT between patients with P. falciparum and those with P. vivax mono-infection (P = 0.88, Mean difference: 0.06, 95% CI − 0.691–0.8, I2: 65.09%, 2 studies, Supplementary Fig. 1). There was no significant difference in PT between patients with P. falciparum/P. vivax mixed infections and those with P. vivax mono-infection (P = 0.35, Mean difference: 4.37 s, 95% CI − 4.77–13.52 s, I2: 99.21%, 2 studies, Supplementary Fig. 2).
Difference in PT between fatal malaria and non-fatal malaria
The comparison of PT in fatal and non-fatal malaria cases has been addressed in two studies31. One study noted a significantly higher PT in fatal malaria cases compared to surviving cases38. Contrarily, another study reported PT as normal in instances of fatal malaria31.
Publication bias
Publication bias was not assessed using funnel plots, contour-enhanced funnel plots, or Egger’s regression test because the meta-analysis included fewer than ten studies.
Sensitivity analysis
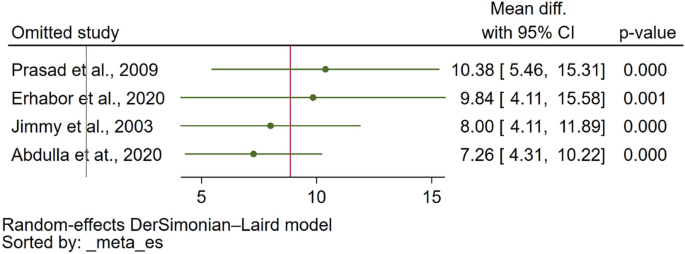
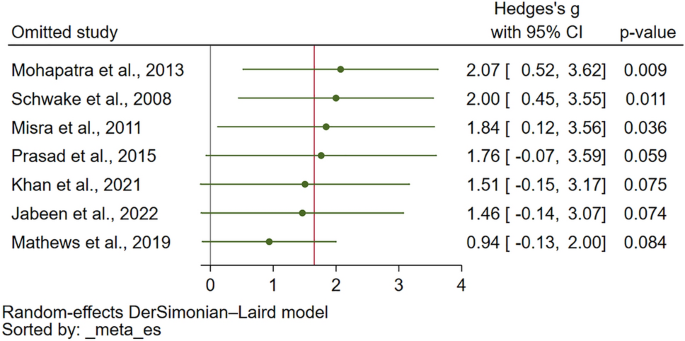
The sensitivity analysis was conducted to assess the robustness of the meta-analysis findings. For assessing PT differences between malaria patients and uninfected controls, the meta-analysis demonstrated stability under both the fixed effect model and when calculating the standard mean difference (SMD) (Supplementary Figs. 3 and 4, respectively). Furthermore, the leave-one-out sensitivity analysis did not identify any single study as an outlier that significantly influenced the overall pooled effect estimate (Fig. 4), affirming the consistency of these results. Similarly, the stability of the meta-analysis comparing PT between severe and non-severe malaria cases was evaluated. When the fixed effect model was applied, the analysis remained stable (Supplementary Fig. 5). However, the leave-one-out sensitivity analysis revealed the presence of study outliers that had a notable impact on the pooled effect estimate (Fig. 5). This highlights certain studies' influence on the overall meta-analysis outcome for PT differences between severe and non-severe malaria cases.
Discussion
Malaria's impact on blood coagulation, evidenced by alterations in prothrombin time (PT), underscores a complex interplay between the disease's pathophysiology and coagulation pathways11. The meta-analysis results also supported the finding from individual study in which PT was statistically increased in malaria patients as compared to uninfected controls. Furthermore, the meta-analysis result was stable when the statistical model or the pooled effect estimate were changed, indicating a certain degree of coagulation activation that causes the alteration of PT during Plasmodium infections. In uncomplicated malaria, prolonged PT could be observed between 3.77 and 16.7% in different studies39,50,51. The presence of a prolonged PT, along with clinical indications such as bleeding manifestations and standard coagulation laboratory tests such as APTT, D-dimers, and fibrinogen degradation products, can suggest the onset of DIC. Consequently, DIC can trigger both microvascular and macrovascular clotting, impairing blood circulation, which can ultimately result in the development of Multiple Organ Dysfunction Syndrome (MODS)52.
The systematic review revealed that patients suffering from severe malaria exhibited a prolonged PT in comparison to those with non-severe malaria. This finding was statistically significant, with PT levels being higher in severe malaria patients than in uninfected controls. Such observations align with the existing literature that links severe malaria, especially cases involving P. falciparum, to significant disruptions in coagulation pathways47. Specifically, P. falciparum-infected erythrocytes are known to exhibit elevated levels of tissue factor16, a key initiator of the coagulation cascade, which can lead to increased PT. The activation of the coagulation system, coupled with the impairment of anticoagulant mechanisms, contributes to a prothrombotic state11, thereby explaining the prolonged PT observed in severe malaria cases. This activation of the coagulation system, along with impaired anticoagulant mechanisms, likely contributes to a prothrombotic state, elucidating the extended PT observed in cases of severe malaria. Numerous studies have documented a trend towards more pronounced PT alterations in patients with severe malaria compared to those presenting with uncomplicated forms of the disease20,32,43,47,53,54,55.
The PT prolongation in severe malaria, observed in the range of 5.74–22.7%56,57,58,59, could be attributed to several mechanisms. One such mechanism might include the release of tissue factors from damaged vascular endothelial cells or the expression of activated tissue factor on endothelial cells, leading to consumptive coagulopathy due to the activation of the extrinsic coagulation pathway16,60. Nevertheless, certain studies showed that the proportion of prolonged PT was similar in both severe and uncomplicated malaria, or there was no significant difference in PT between the two clinical malaria groups41,44,49,61. Inconsistency was observed in the meta-analysis results, as alterations in the statistical model or the pooled effect estimate led to instability in the outcomes. However, based on the available evidence, it can be concluded that patients with severe malaria are more likely to exhibit prolonged or increased PT.
The systematic review highlighted the limited evidence differentiating PT between Plasmodium species. According to the meta-analysis, there were observed similarities in PT between patients with P. falciparum mono-infection and those with P. vivax mono-infection, as well as between patients with P. falciparum/P. vivax mixed infections and those with P. vivax mono-infection. These findings suggest that an increase or prolongation in PT can occur in P. falciparum, non-P. falciparum malaria, and mixed Plasmodium infections. Interestingly, prolonged PT was observed in patients suffering from both severe P. falciparum and severe P. vivax malaria. In a study that involved both P. vivax and P. falciparum59, there was a minimal difference in the proportion of prolonged PT, with severe P. falciparum malaria having a slightly higher proportion of prolonged PT than severe P. vivax malaria (10.6 vs. 5.74%). The slight variance in PT prolongation rates between these two Plasmodium species suggests that underlying mechanisms, such as differences in endothelial activation and microvascular dysfunction62, may contribute to this discrepancy. Nonetheless, the specific factors driving these subtle differences remain to be fully elucidated, highlighting an area for future research.
The systematic review of studies involving patients with both fatal and non-fatal malaria demonstrated varied results. The discrepancies in these findings may be attributable to the multifaceted nature of the factors contributing to the risk of death among malaria patients. For instance, those suffering from fatal malaria could potentially experience a higher consumption of coagulation factors than patients with non-fatal malaria63. This enhanced consumption of coagulation factors in fatal malaria cases may be linked to a heightened degree of coagulation activation. This heightened activation, in turn, could be driven by systemic endothelial activation, a common occurrence in severe or fatal malaria64. This scenario proposes a complex interplay between coagulation factors and the pathophysiology of malaria, suggesting that severe or fatal malaria cases exhibit a hyperactive coagulation state, leading to excessive consumption of coagulation factors, subsequently resulting in a more prolonged PT. However, it is important to underscore that while the correlation between fatal malaria and heightened coagulation activation seems plausible, the evidence is not wholly conclusive. The heterogeneous nature of these findings underscores the need for more in-depth investigations. Future studies should aim to elucidate the precise mechanisms by which malaria influences the coagulation pathway and the subsequent variations in PT, as well as the potential discrepancies in these effects between fatal and non-fatal cases.
The systematic review and meta-analysis of PT in malaria patients have several limitations. First, the significant heterogeneity observed across studies may compromise the validity of the findings. Despite the subgroup analysis and meta-regression performed to identify the source of heterogeneity, the variance remained largely unexplained, which implies that other factors unaccounted for in this study might be contributing to this heterogeneity. Second, the studies used for comparison were not homogenous in terms of patient characteristics, methods of PT measurement, diagnostic methods for malaria, and Plasmodium species, which could affect the comparison of PT among different studies. Moreover, most of the included studies used cross-sectional designs, limiting the ability to establish causality between malaria infection and increased PT. Finally, the limited number of studies and their geographic concentration also pose challenges in generalizing the findings globally.
For future research implications, it is recommended to perform more robust studies, such as randomized controlled trials or prospective cohort studies, which can help to provide a more accurate assessment of the PT in malaria patients and reduce bias. It would also be beneficial to include a more diverse sample, both in terms of geographical distribution and characteristics of patients, to allow for a broader understanding of the relationship between malaria and PT. There is also a need to investigate the exact biological mechanisms driving the observed differences in PT in patients with malaria, as this could provide a more in-depth understanding of the disease's pathophysiology and possibly lead to the development of novel therapeutic interventions.
Conclusion
The systematic review and meta-analysis revealed PT prolongation in malaria infections caused by P. falciparum and P. vivax, particularly in severe cases, yet found no significant difference in PT between the two species. The ambiguous results concerning PT in fatal versus non-fatal cases highlight existing research gaps. Future studies should focus on refining PT measurement methods, exploring the impact of treatment and comorbidities on PT, and unraveling the molecular mechanisms of coagulation alterations induced by malaria. Advancements in these areas promise to inform targeted therapies for malaria-associated coagulopathy, aiming to improve patient outcomes.
Data availability
All data relating to the present study are available in this manuscript and supplementary files.
References
WHO. World Malaria Report (World Health Organization, 2022).
Miller, L. H., Ackerman, H. C., Su, X. Z. & Wellems, T. E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 19(2), 156–167 (2013).
Baer, K. et al. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 9(2), 397–412 (2007).
Severe Falciparum Malaria. World Health Organization, communicable diseases cluster. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl 1), S1-90 (2000).
Kotepui, M., Phunphuech, B., Phiwklam, N., Chupeerach, C. & Duangmano, S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malar. J. 13, 218 (2014).
Maina, R. N. et al. Impact of Plasmodium falciparum infection on haematological parameters in children living in Western Kenya. Malar. J. 9, S4 (2010).
Anghan, H., Sethi, P., Soneja, M., Mahajan, S. & Wig, N. Clinical and laboratory features associated with acute kidney injury in severe malaria. Indian J. Crit. Care Med. 22(10), 718–722 (2018).
Palta, S., Saroa, R. & Palta, A. Overview of the coagulation system. Indian J. Anaesth. 58(5), 515–523 (2014).
Chaudhry, R., Usama, S. M., Babiker, H. M.: Physiology, Coagulation Pathways. StatPearls Treasure Island (FL) (2023).
Norris, L. A. Blood coagulation. Best Pract. Res. Clin. Obstet. Gynaecol. 17(3), 369–383 (2003).
Angchaisuksiri, P. Coagulopathy in malaria. Thromb. Res. 133(1), 5–9 (2014).
Grobusch, M. P. & Kremsner, P. G. Uncomplicated malaria. Curr. Top. Microbiol. Immunol. 295, 83–104 (2005).
Milner, D. A. Jr. et al. The systemic pathology of cerebral malaria in African children. Front. Cell Infect. Microbiol. 4, 104 (2014).
Sailo, L., Pradhan, D., Nongthombam, R. & Bhattacharyya, P. Disseminated intravascular coagulation in malaria: A case report. Niger. J. Med. 55(2), 171–172 (2014).
Francischetti, I. M., Seydel, K. B. & Monteiro, R. Q. Blood coagulation, inflammation, and malaria. Microcirculation 15(2), 81–107 (2008).
Francischetti, I. M. et al. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J. Thromb. Haemost. 5(1), 155–165 (2007).
Kern, P., Hemmer, C. J., Van Damme, J., Gruss, H. J. & Dietrich, M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87(2), 139–143 (1989).
Bridges, D. J. et al. Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115(7), 1472–1474 (2010).
Chaudhry, R., Usama, S. M. & Babiker, H. M. Physiology, Coagulation Pathways. StatPearls Treasure Island (FL) (2022).
Pukrittayakamee, S. et al. Activation of the coagulation cascade in falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 83(6), 762–766 (1989).
Horstmann, R. D. & Dietrich, M. Haemostatic alterations in malaria correlate to parasitaemia. Blut 51(5), 329–335 (1985).
Rojanasthien, S., Surakamolleart, V., Boonpucknavig, S. & Isarangkura, P. Hematological and coagulation studies in malaria. J. Med. Assoc. Thai. 75(Suppl 1), 190–194 (1992).
Bashir, B. A. & Ahmed, M. S. Laboratory blood coagulation in Sudanese with falciparum malaria: a glance of change outcomes. Int. J. Blood Res. Disord. 7, 1–5 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 18(3), e1003583 (2021).
WHO. WHO Guidelines for malaria. Geneva: World Heath Organization; 2023. Available from: https://www.who.int/publications/i/item/guidelines-for-malaria. Accessed 9 Mar 2024.
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., Currie, M., Qureshi, R., Mattis, P., Lisy, K. & Mu, P.-F. Chapter 7: Systematic reviews of etiology and risk: JBI; 2020. Available from: https://synthesismanual.jbi.global. Accessed 22 Dec 2023.
Higgins, J. P. T., Chandler, J., Cumpston, M., Li, T., Page, M. J. & Welch, V. A. (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022. Available from: www.training.cochrane.org/handbook. Accessed 22 Dec 2023.
Cumpston, M. et al. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.ED000142 (2019).
Abdullah, S. J. et al. A severe case of falciparum malaria, 10 years after malaria eradication: A case report. Yale J. Biol. Med. 94(2), 277–284 (2021).
Akhlaq, A. et al. Neurological complications in patients with plasmodium vivax malaria from Karachi, Pakistan. J. R. Coll. Physicians Edinb. 48(3), 198–201 (2018).
Amano, H., Sano, A., Araki, T. & Inoki, S. Fibrin-degradation products in falciparum malaria. Zentralbl Bakteriol Mikrobiol Hyg. A Med. Mikrobiol. Infekt. Parasitol. 250(1–2), 242–247 (1981).
Dasgupta, A., Rai, S. & Das, G. A. Persistently elevated laboratory markers of thrombosis and fibrinolysis after clinical recovery in malaria points to residual and smouldering cellular damage. Indian J. Hematol. Blood Transfus. 28(1), 29–36 (2012).
Erhabor, O., Abdulrahaman, A. & Erhabor, T. Effects of malaria parasitaemia on some haemostatic parameters among pregnant women of African descent in Specialist Hospital Sokoto, Nigeria. Hum. Antibodies 28(1), 21–28 (2020).
Huson, M. A. M. et al. Impact of HIV infection on the haemostatic response during sepsis and malaria. Br. J. Haematol. 173(6), 918–926 (2016).
Jabeen, S. et al. Alteration of coagulation profile in malaria patients and its correlation with the degree of parasitemia. Pak. Armed Forces Med. J. 72(2), 513–517 (2022).
Jimmy, E. O., Saliu, I. & Ademowo, O. Fibrinopeptide-A and fibrinogen interactions in acute, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 97(8), 879–881 (2003).
Khan, M., Nisar, H. & Mushahid, N. Clinical and lab profile of severe and uncomplicated malaria: A prospective study from Khuzdar Balochistan. Pak. J. Med. Sci. 37(7), 1918–1923 (2021).
Khan, R., Quaiser, S. & Haque, S. F. Malarial acute kidney injury: Prognostic markers. Ann. Trop. Med. Public Health 6(3), 280–284 (2013).
Kueh, Y. K. & Yeo, K. L. Haematological alterations in acute malaria. Scand. J. Haematol. 29(2), 147–152 (1982).
Mathews, S. E., Bhagwati, M. M. & Agnihotri, V. Clinical spectrum of Plasmodium vivax infection, from benign to severe malaria: A tertiary care prospective study in adults from Delhi, India. Trop. Parasitol. 9(2), 88–92 (2019).
Meltzer, E., Keller, S., Shmuel, S. & Schwartz, E. D-dimer levels in non-immune travelers with malaria. Travel Med. Infect. Dis. 27, 104–106 (2019).
Misra, D. P., Das, S., Pattnaik, M., Singh, S. C. & Jena, R. K. Relationship of hepatic and renal dysfunction with haemorrheological parameters in Plasmodium falciparum malaria. J. Assoc. Physicians India 59, 552–556 (2011).
Mohanty, D. et al. Fibrinolysis, inhibitors of blood coagulation, and monocyte derived coagulant activity in acute malaria. Am. J. Hematol. 54(1), 23–29 (1997).
Mohapatra, S., Samantaray, J. C., Arulselvi, S. & Ghosh, A. Disseminated intravascular coagulation following malaria due to Plasmodium vivax: A thromboelastography-based study. Malar. J. 12, 336 (2013).
Moxon, C. A. et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 13(9), 1653–1664 (2015).
Moxon, C. A. et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122(5), 842–851 (2013).
Prasad, R. et al. Coagulation status and platelet functions in children with severe falciparum malaria and their correlation of outcome. J. Trop. Pediatr. 55(6), 374–378 (2009).
Prasad, R. & Mishra, O. P. Acute kidney injury in children with Plasmodium falciparum malaria: Determinants for mortality. Perit. Dial. Int. 36(2), 213–217 (2016).
Schwake, L. et al. Early treatment of imported falciparum malaria in the intermediate and intensive care unit setting: an 8-year single-center retrospective study. Crit. Care 12(1), R22 (2008).
Butler, T., Tong, M. J., Fletcher, J. R., Dostalek, R. J. & Robbins, T. O. Blood coagulation studies in Plasmodium falciparum malaria. Am. J. Med. Sci. 265(1), 63–67 (1973).
Beale, P. J., Cormack, J. D. & Oldrey, T. B. Thrombocytopenia in malaria with immunoglobulin (IgM) changes. Br. Med. J. 1(5796), 345–349 (1972).
Costello, R. A., Nehring, S. M. Disseminated intravascular coagulation. StatPearls, Treasure Island (FL) (2023).
Ali, H. et al. Parasite density and the spectrum of clinical illness in falciparum malaria. J. Coll. Phys. Surg. Pak. 18(6), 362–368 (2008).
Moxon, C. A. et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 14(2), 1653–1664 (2016).
Moxon, C. A. et al. Loss of protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria. J. Thromb. Haemost. 11, 4 (2013).
Clemens, R. et al. Activation of the coagulation cascade in severe falciparum malaria through the intrinsic pathway. Br. J. Haematol. 87(1), 100–105 (1994).
Holst, F. G. E. et al. Low levels of fibrin-stabilizing factor (factor XIII) in human Plasmodium falciparum malaria: Correlation with clinical severity. Am. J. Trop. Med. Hyg. 60(1), 99–104 (1999).
Naqvi, R., Ahmad, E., Akhtar, F., Naqvi, A. & Rizbi, A. Outcome in severe acute renal failure associated with malaria. Nephrol. Dial. Transplant. 18(9), 1820–1823 (2003).
Zubairi, A. B. S. et al. Severe Plasmodium vivax malaria in Pakistan. Emerg. Infect. Dis. 19(11), 1851–1854 (2013).
Srichaikul, T. Hemostatic alterations in malaria. Southeast Asian J. Trop. Med. Public Health 24(Suppl 1), 86–91 (1993).
Srichaikul, T., Puwasatien, P., Puwasatien, P., Karnjanajetanee, J. & Bokisch, V. Complement changes and disseminated intravascular coagulation in Plasmodium falciparum malaria. Lancet 305(7910), 770–772 (1975).
Woodford, J. et al. Early endothelial activation precedes glycocalyx degradation and microvascular dysfunction in experimentally induced Plasmodium falciparum and Plasmodium vivax infection. Infect. Immun. 88(5), e00895-e919 (2020).
Duangchan, T., Kotepui, M., Sukati, S., Rattanapan, Y. & Wangdi, K. A systematic review and meta-analysis of the proportion estimates of disseminated intravascular coagulation (DIC) in malaria. Trop. Med. Infect. Dis. 8(6), 289 (2023).
Turner, G. D. et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145(5), 1057–1069 (1994).
Author information
Authors and Affiliations
Contributions
M.K., S.S., T.W., and T.D. carried out the study design, study selection, data extraction, and statistical analysis. M.K., S.S., and T.D. drafted the manuscript. K.U.K., F.R.M., C.P.T. performed critical review and revision of the manuscript. All authors read and approved the final version of the manuscript. All authors consent to the publication of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sukati, S., Wannatung, T., Duangchan, T. et al. Alteration of prothrombin time in Plasmodium falciparum and Plasmodium vivax infections with different levels of severity: a systematic review and meta-analysis. Sci Rep 14, 9816 (2024). https://doi.org/10.1038/s41598-024-60170-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60170-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.