Abstract
Contained vascular injuries (CVI) of spleen include pseudoaneurysms (PSA) and arterio-venous fistulae (AV-fistulae), and their reported prevalence varies. Our purpose was to assess the prevalence of early splenic CVI seen on admission CT in patients with splenic trauma admitted to a single level 1 trauma center in 2013–2021, and its detection in different CT protocols. A retrospective, single-center longitudinal cohort study. Nine-year data (2013–2021) of all patients with suspected or manifest abdominal trauma were retrieved. All patients, > 15 years with an ICD code for splenic trauma (S36.0XX) were included. CT and angiographic examinations were identified. Reports and images were reviewed. Splenic CVI CT criterion was a focal collection of vascular contrast that decreases in attenuation with delayed imaging. Number of CVIs and treatment was based on medical records and/or available angioembolization data. Of 2805 patients with abdominal trauma, 313 patients (313/2805; 11.2%) fulfilled the study entry criteria. 256 patients (256/313; 81.8%) had a CT examination. Sixteen patients had splenectomy before CT, and the final study group included 240 patients (240/313; 76.7%). Median New Injury Severity Score (NISS) was 27 and 87.5% of patients had NISS > 15. Splenic CVI was found in 20 patients, which yields a prevalence of 8.3% (20/240; 95% CI 5.2–12.6%). In those cases with both late arterial and venous phase images available, CVI was seen in 14.5% of cases (18/124, 95% CI 8.6–22.0%). None of the patients with CVI died within 30 days of the injury. The prevalence of early splenic CVI in patients with a splenic trauma was 8.3–14.5% (95% CI 5.2–22.0%). Our data suggests that both arterial and venous phase are needed for CT diagnosis. The 30-day outcome in terms of mortality was good.
Similar content being viewed by others
Introduction
Blunt splenic injury occurs in 2% of all trauma admissions, and splenic rupture is the most common cause of major abdominal injury in the vast majority of cases1,2. It is the most vascular organ in the abdomen, and the bleeding is usually arterial causing a major hemoperitoneum and severe blood loss leading to hemodynamic instability and even death3. Therefore, the detection of active splenic hemorrhage and contained vascular injuries is crucial for identifying the need for either subsequent direct intervention (surgery or transcatheter embolization), or if conservative nonsurgical treatment can be applied4,5,6,7,8. Because of the spleen’s immunological function, salvage rather than removal is the desirable treatment option9.
CT with intravenous contrast is currently the imaging method of choice in major blunt trauma10. It can be used to classify abdominal organ trauma according to the AAST (American Association for the Surgery of Trauma) Injury Grading Scale. The most recent (2018) revision of this11 includes vascular injuries such as traumatic splenic pseudoaneurysms (PSA) and arteriovenous (AV) fistulae. These appear on CT as a focal collection of vascular contrast that decreases in attenuation with delayed imaging. Traumatic PSA and an AV-fistula may be impossible to differentiate on CT, and these findings are best described together as those of contained vascular injury (CVI)6,12,13.
To diagnose CVI, special attention has to be addressed to the way contrast agent is administered. The current recommendation is to image the spleen in both arterial and venous phase13,14. If only venous phase is used, these injuries may go unrecognized. Similarly, if the so-called “split bolus” technique is used, it may be impossible to differentiate active bleeding from contained injury15.
In the past, the true prevalence of early splenic CVI has been difficult to establish, but the improvements in CT technology paired with trends toward more comprehensive imaging workups of trauma has led to an increase in CVI detection16. The reported prevalence of CVI in acute phase varies, but it has been reported to occur in up to 20% in patients with splenic trauma14,16. Therefore, the purpose of this study was to find out the prevalence of early splenic CVI seen on admission CT in patients with splenic trauma admitted to a single level 1 trauma center in 2013–2021, and its detection in different CT protocols.
Methods
Approval from the local ethics committee was obtained for this retrospective, longitudinal cohort study (Ethical application number 2019-05643, 2022-02753-02) and due to its retrospective design, informed consent was waived. Nine-year data (2013–2021) of all consecutive patients with suspected or manifest abdominal trauma were retrieved from the local trauma register at the Karolinska University Hospital, a level 1 trauma center with a total catchment area of approximately 2.5 million people. The individual patient data is coded in the registry by trained nurses with several years of experience. The data is registered according to the The Utstein Trauma Template for Uniform Reporting of Data following Major Trauma17. The extraction of data from the register is acknowledged and supervised by the local research council. To ensure that all patients with abdominal trauma could be identified, the initial dataset from the register included all trauma patients admitted with either an AIS-code for body region 5 or an ICD code S3X.XX. All patients, 15 years and older with an ICD code for splenic trauma (S36.0XX) were included. Both blunt and penetrating trauma were included. The following parameters were extracted from the trauma register and/or electronic medical records: age, gender, Injury Severity Score (ISS), New Injury Severity Score (NISS), 30-day mortality, injury mechanism17, surgical and/or radiological interventions (angioembolization), and length of hospital stay (LOS).
The CT images were retrieved from the local picture archiving and communication systems (the Radiological Information System (RIS)/Picture Archiving and Communication System (PACS); SECTRA AB, Linköping, Sweden), and the CT protocol was recorded. Also, patients with CT scans from other hospitals were included.
CT and angiographic examinations from all eligible patients were identified and the images as well as imaging and clinical reports were reviewed by a senior radiologist (SKK) with 30 years of experience. All CT scans had previously been interpreted by at least one radiology resident and a senior radiologist.
The splenic traumas seen on CT were graded according to AAST Injury Grading Scale 1994 and 2018 revisions. For splenic CVI identified in CT, we used the criterion described earlier, i.e., a focal collection of vascular contrast that decreases in attenuation with delayed imaging13. If a CVI was suspected, a second radiologist (ZA) reviewed the cases, and any discrepancy was solved by consensus.
The reference standard for CVI detection was angioembolization data, and in instances where this was unavailable, the reference standard comprised follow-up CT and information from medical records.
CT technique
At our hospital, the multitrauma imaging was conducted on a 256-slice multi-detector CT (MDCT) (Revolution CT, GE Healthcare, Milwaukee, Wisconsin, USA) and a 64-slice MDCT scanner (LightSpeed VCT, GE Healthcare, Milwaukee, Wisconsin, USA). During 2015 the Revolution CT scanner (RevCT) was installed and replaced the LightSpeed VCT scanner (VCT) in our trauma department. Concomitantly our single-phase (venous) standard trauma CT protocol was modified to a multi-phase protocol by including a whole-body CT in arterial phase for high-energy trauma patients. The imaging parameters are presented in Tables 1 and 2.
Statistics
Mann–Whitney U test for independent samples and chi-square test were used to calculate the difference between patients with and without splenic CVI. Confidence intervals were calculated using binomial exact test. Statistical analyses were done using a commercial software package SAS/STAT v.9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
Approval from the local ethics committee was obtained for this retrospective, longitudinal cohort study (Swedish Ethical Review Authority, Ethical application number 2019-05643, 2022-02753-02).
Consent to participate
Informed consent was waived by Swedish Ethical Review Authority due to the retrospective design. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by Karolinska University Hospital’s Internal Review Board.
Results
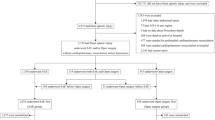
During the nine-year study period, a total of 2805 patients were admitted to the trauma center following abdominal trauma.
A total of 313 patients (313/2805; 11.2%) had splenic trauma, and 256 (256/313; 81.8%) had undergone a CT on admission. Of the 57 cases with no CT, 13 were dead on admission, and in the remaining 44 cases, no CT or angiographic examinations on admission could be found or retrieved. Furthermore, sixteen patients had had a splenectomy before CT, so the final study group included 240 patients (240/313; 76.7%) (Table 3). The main injury mechanism was motor vehicle collision (MVC) in 90 cases (37.5%), followed by fall from height in 62 cases (25.8%) and blunt trauma to the body in 22 cases (9.2%). The yearly prevalence is presented in Table 4. The CT protocol included both arterial and venous phase in 124 (124/240; 51.7%) cases and a venous phase only in 107 (107/240; 44.6%) cases. The remaining 9 (9/240; 3.7%) presented with different variations of CT protocols (Table 5).
The grading of splenic injuries according to AAST OIS 1994 and 2018 systems are presented in Tables 6 and 7. The most common injury was Grade III using the 1994 revision, whereas Grade III and IV equaled using the 2018 revision.
In 66 cases, an angioembolization was performed with either coils or Amplatz plugs (G II 1; G III 10; G IV 39 and G V 16 cases. Grading according to AAST OIS 2018 revision). In one case a collagen fleece (TachoSil) was applied.
Contained vascular injuries
During this study period, splenic CVI was found in 20 patients (Figs. 1, 2). This yields a prevalence of 8.3% (20/240, 95% CI 5.2–12.6%). In addition to information in medical records, a follow-up CT was available in one case, and subsequent conventional angiography in 14 cases. In our material, all splenic CVIs that were identified on angiogram were previously also identified on CT.
A 55-year-old male involved in a motor vehicle accident with multiple rib fractures and G III kidney injury (not shown), ISS 26. Contrast-enhanced CT scans (A–D). Axial, coronal, and sagittal images in (A–C), arterial and (D) portal venous phases. Images in arterial phase show indistinct area of hyperattenuating foci (arrows). This area washes out at portal venous phase. Patient underwent angiography (E), and contained vascular injury (arrow) was subsequently embolized (not shown).
In 12 patients (12/20; 60%), only one CVI was seen, two in 4 patients and three in 3 patients. In one patient, multiple small CVIs were seen.
In these patients, the injury mechanism was MVC in 8 cases, fall from height in 6 cases, stabbing injury in 3, undefined blunt trauma to torso, fall on the same level and pedestrian struck by car in 1 case each.
Hospital length of stay was shorter in patients with CVI than in patients without CVI (p = 0.0469). No statistically significant differences were found in age, gender, ISS or NISS (Table 8). None of the patients with CVI died within 30 days of the injury.
CT protocol and CVI
In 18 patients, CVI was detected when both late arterial and venous phase was used. In the two patients, when using a venous phase only, the CVI was confirmed either by conventional angiography or follow-up CT angiography. In cases where both late arterial and venous phase images were available, CVI was seen in 14.5% (18/124, 95% CI 8.6–22.0%).
Treatment of CVIs
Angioembolization was done in 13 cases (including two out of three cases with a stabbing injury), splenectomy in one. The remaining 6 cases had a non-operative management.
Discussion
The major aim in this study was to find out the prevalence of early splenic CVI seen on admission CT in a single trauma center’s large unselected material of consecutive patients. In our nine-year series we found a total of 20 patients with splenic CVIs. This yields a prevalence of 8.3%, which is slightly higher than previously reported early splenic CVI prevalence of 3.7–4.7%18. It is known that the CT diagnosis of CVI is protocol dependent13,14. This is also seen in our study, as 18 out of 20 CVIs were found when both arterial and venous phase was used. As only 51.7% of the CT examinations in this study did include both arterial and venous phase, the prevalence was 14.5% when this protocol was used. This is in accordance with previously reported prevalence of 15%14,16. In other words, a substantial number of early CVIs may have been missed because of a suboptimal trauma CT protocol. This might also be the case in the previous study where only venous phase was used18. The same may also apply to institutions using the biphasic “split bolus” technique. In one study it was shown that the sensitivity and specificity to diagnose PSA using this technique was 39% and 71%, respectively15. However, more studies are needed to verify this as their number of patients (n = 36) was rather small. The change in CT scanner in our hospital most likely did not have impact on the results, since the incidences of both early and latent PSA have previously been shown to remain remarkably stable despite advances in CT technology18.
We graded splenic injuries according to AAST OIS 1994 and 2018 systems (Tables 6 and 7). When compared to two other large series19,20, the severity spectrum is quite similar when using the 1994 grading system as the most common (40–49%) Grade was III. Also, when using the 2018 grading system, there was a shift towards more Grades IV and V. This reflects the inclusion of CVIs and active bleeding to these higher grades in the 2018 revision.
Delayed splenic CVI, PSA in particular, is not uncommon with a prevalence of 3–20%18,21,22,23,24. However, if the delayed PSAs really are delayed or simply missed on admission because of a suboptimal CT protocol is still unclear18,25. Also, since CVIs are often multiple, 40% in our study, this may increase the risk of delayed bleeding of initially missed CVI further emphasizing the importance of early detection. Rupture of PSA is a rare but well-known complication of blunt splenic trauma with reported incidence of 6%26,27. In the majority of cases, it occurs within a week28,29, but cases that occur months after trauma have also been reported30. In cases of delayed rupture mortality ranges from 5 to 15%31,32 which is much higher than the 1% seen in acute splenic injury33. On the other hand, it has been shown that regardless of injury grade, early screening leads to a decrease in failure rate emphasizing the importance of early diagnosis27. In our study, thirteen patients (65%; 13/20) underwent angioembolization, one underwent splenectomy, and six had a conservative management. The decision on invasive (splenectomy or angioembolization) or conservative treatment was in some cases the result of repeated clinical assessments and other investigations, such as laboratory tests and imaging studies. Conservative management was chosen for those patients who were identified as not to benefit from invasive treatment of the splenic injury.
In a large multi-center study based on radiology reports and medical charts, CVI was reported in 20% of the patients with blunt splenic trauma16. However, as the authors point out, their study included selection bias because all participating centers are American College of Surgeons–verified level 1 trauma facilities. As such, findings likely reflect patients seen at similar centers and should not be generalized to all splenic injuries. Also, their data was highly selective, as exclusion criteria included for example penetrating trauma to the abdomen and/or pelvis, CT images obtained more than 12 h before or after initial presentation, splenectomy prior to CT imaging, patients with insufficient follow-up or missing key data, and death before definitive treatment of splenic injury. Similarly, in our study the final study group with CT available and no prior splenectomy included 76.7% of patients with splenic trauma. This may have an impact on the true overall prevalence of CVI, thereby affecting the generalization of our results..
The primary limitation of this study is the retrospective study design from a single trauma center. No additional double-reading was performed, so there is a risk that not all CVIs were identified. However, all CT scans had previously been interpreted by two radiologists and we chose this consensus approach because it mirrors the clinical scenario at our trauma unit where double-reading is the standard of care.
Moreover, not all of the patients had undergone a subsequent angiography that could serve as a reference standard. This also means that we could not differentiate PSA from AV. However, catheter angiography is indicated in either case34. In our hospital, all trauma patients with a suspected, or verified, injury to major vessels undergo angiography if it is thought to influence the handling of the patient and if they are clinically fit for the procedure. However, we did not focus on treatment and/or indications for angioembolizations as this is beyond the scope of this retrospective study and treatment protocols vary with time. The use of routine angioembolization for all splenic PSAs has been questioned as there is a high rate of discordance between CT and angiographic identification of splenic PSAs and even when identified at angiogram and embolized, close to half will remain perfused35. Also, the authors say that for most isolated splenic PSAs, the natural history of these lesions may be more benign than previously believed. Comparably, the hospital legth of stay was shorter and all patients with CVI in our study survived regardless of the applied treatment.
Conclusions
In conclusion, the prevalence of early splenic CVI in patients with splenic trauma was 8.3–14.5% (95% CI 5.2–22.0%). Our data suggests that both arterial and venous phase are needed for CT diagnosis. The 30-day outcome in terms of mortality was good.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to sensitive nature of the research and IRB restrictions but are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CVI:
-
Contained vascular injury
- PSA:
-
Pseudoaneurysm
- AV-fistula:
-
Arterio-venous fistula
- ISS:
-
Injury severity score
- NISS:
-
New injury severity score
- LOS:
-
Length of hospital stay
- RIS:
-
Radiological information system
- PACS:
-
Picture archiving and communication system
- MDCT:
-
Multidetector CT
References
Bjerke, S., Pohlman, T., Saywell, R. M. Jr., Przybylski, M. P. & Rodman, G. H. Jr. Evolution, not revolution: Splenic salvage for blunt trauma in a statewide voluntary trauma system—A 10-year experience. Am. J. Surg. 191, 413–417 (2006).
Sladyga, A. & Benjamin, R. An evidence-based approach to spleen trauma: Management and outcomes. In Acute Care Surgery and Trauma: Evidence Based Practice (ed. Cohn, S. M.) 131–137 (Informa, 2009).
Naude, G. et al. (eds) Trauma Secrets: Questions and Answers Reveal the Secrets to Effective Care of the Trauma Patient 2nd edn. (Hanley & Belfus, 2003).
Bessoud, B. et al. Nonoperative management of traumatic splenic injuries: Is there a role for proximal splenic artery embolization?. Am. J. Roentgenol. 186, 779–785 (2006).
Haan, J. M. et al. Western Trauma Association Multi-Institutional Trials Committee Splenic embolization revisited: A multicenter review. J. Trauma 56, 542–547 (2004).
Anderson, S. W. et al. Blunt splenic trauma: Delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology 243, 88–95 (2007).
Hamilton, J. D. et al. Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. RadioGraphics 28, 1603–1616 (2008).
Davis, K.A., Fabian. T.C., Croce. M.A., Gavant, M.L., Flick, P.A., Minard, G., et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J. Trauma 44, 1008–13; discussion 1013–5 (1998).
Di Sabatino, A., Carsetti, R. & Corazza, G. R. Post-splenectomy and hyposplenic states. Lancet 378, 86–97 (2011).
Leidner, B., Adiels, M., Aspelin, P., Gullstrand, P. & Wallén,. Standardized CT examination of the multitraumatized patient. Eur. Radiol. 8, 1630–1638 (1998).
Kozar, R. A. et al. AAST Patient Assessment Committee. Organ injury scaling 2018 update: Spleen, liver, and kidney. J. Trauma Acute Care Surg. 85, 1119–1122 (2018).
Shanmuganathan, K. et al. Nonsurgical management of blunt splenic injury: Use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology 217, 75–82 (2000).
Boscak, A. R. et al. Optimizing trauma multidetector CT protocol for blunt splenic injury: Need for arterial and portal venous phase scans. Radiology 268, 79–88 (2013).
Uyeda, J. W., LeBedis, C. A., Penn, D. R., Soto, J. A. & Anderson, S. W. Active hemorrhage and vascular injuries in splenic trauma: Utility of the arterial phase in multidetector CT. Radiology 270, 99–106 (2014).
Marovic, P., Beech, P. A., Koukounaras, J., Kavnoudias, H. & Goh, G. S. Accuracy of dual bolus single acquisition computed tomography in the diagnosis and grading of adult traumatic splenic parenchymal and vascular injury. J. Med. Imaging Radiat. Oncol. 61, 725–731 (2017).
Lee, J. T. et al. American Society of Emergency Radiology multicenter blunt splenic trauma study: CT and clinical findings. Radiology 299, 122–130 (2021).
Brohi, K. The Utstein template for uniform reporting of data following major trauma: A valuable tool for establishing a pan-European dataset. Scand. J. Trauma Resusc. Emerg. Med. 16, 8 (2008).
Weinberg, J. A. et al. Computed tomography identification of latent pseudoaneurysm after blunt splenic injury: Pathology or technology?. J. Trauma 68, 1112–1116 (2010).
Margari, S. et al. Emergency CT for assessment and management of blunt traumatic splenic injuries at a Level 1 Trauma Center: 13-year study. Emerg. Radiol. 25, 489–497 (2018).
Morell-Hofert, D. et al. Validation of the revised 2018 AAST-OIS classification and the CT severity index for prediction of operative management and survival in patients with blunt spleen and liver injuries. Eur. Radiol. 30, 6570–6581 (2020).
Weinberg, J.A., Magnotti, L.J., Croce, M.A., Edwards, N.M., Fabian, T.C. The utility of serial computed tomography imaging of blunt splenic injury: Still worth a second look? J. Trauma 62, 1143–7; discussion 1147–8 (2007).
Wallen, T. E. et al. Delayed splenic pseudoaneurysm identification with surveillance imaging. J. Trauma Acute Care Surg. 93, 113–117 (2020).
Muroya, T. et al. Delayed formation of splenic pseudoaneurysm following nonoperative management in blunt splenic injury: Multi-institutional study in Osaka, Japan. J. Trauma Acute Care Surg. 75, 417–420 (2013).
Poletti, P. A. et al. Blunt splenic trauma: Can contrast enhanced sonography be used for the screening of delayed pseudoaneurysms?. Eur. J. Radiol. 82, 1846–1852 (2013).
Davis, K.A., Fabian, T.C., Croce, M.A., Gavant, M.L., Flick, P.A., Minard, G., et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J. Trauma 44, 1008-13; discussion 1013-5 (1998).
Sabe, A. A., Claridge, J. A., Rosenblum, D. I., Lie, K. & Malangoni, M. A. The effects of splenic artery embolization on nonoperative management of blunt splenic injury: A 16-year experience. J. Trauma 67, 565–572 (2009).
Leeper, W. R. et al. Delayed hemorrhagic complications in the nonoperative management of blunt splenic trauma: Early screening leads to a decrease in failure rate. J. Trauma Acute Care Surg. 76, 1349–1353 (2014).
Gavant, M. L. et al. Predicting clinical outcome of nonsurgical management of blunt splenic injury: using CT to reveal abnormalities of splenic vasculature. Am. J. Roentgenol. 168, 207–212 (1997).
Santorelli, J. E. et al. Readmission after splenic salvage: How real is the risk?. Surgery 171, 1417–1421 (2022).
Maria, C., Nickolaos, T., George, G. & Michail, K. Delayed splenic rupture 4 months following minor blunt abdominal trauma. Glob. J. Rare Dis. 6, 001–003 (2021).
Kluger, Y. et al. Delayed rupture of the spleen—Myths, facts, and their importance: Case reports and literature review. J. Trauma 36, 568–571 (1994).
Foster, R. P. Delayed haemorrhage from the ruptured spleen. Br. J. Surg. 57, 189–192 (1970).
Cogbill, T. H. et al. Nonoperative management of blunt splenic trauma: A multicenter experience. J. Trauma 29, 1312–1317 (1989).
Fleiter, T. R. & Archer-Arroyo, K. Blunt abdominal and retroperitoneal trauma. In Problem Solving in Emergency Radiology E-Book (eds Mirvis, S. E. et al.) 244–316 (Elsevier Health Sciences, 2014).
Radding, S. et al. A pseudo-dilemma: Are we over-diagnosing and over-treating traumatic splenic intraparenchymal pseudoaneurysms?. J. Trauma Acute Care Surg. https://doi.org/10.1097/TA.0000000000004117 (2023).
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.K.K., Z.A., A.E., A.K. Methodology: S.K.K., Z.A., A.E., A.K. S.K.K. wrote the main manuscript text and carried out the data analysis. S.K.K. and A.K. prepared and collected the data. S.K.K. and Z.A. reviewed the imaging analysis procedure. All authors reviewed, read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koskinen, S.K., Alagic, Z., Enocson, A. et al. The prevalence of early contained vascular injury of spleen. Sci Rep 14, 7917 (2024). https://doi.org/10.1038/s41598-024-58626-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58626-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.