Abstract
Ecological niche models (ENMs) serve as valuable tools in assessing the potential species distribution, identifying crucial habitat components for species associations, and facilitating conservation efforts. The current study aimed to investigate the gastrointestinal nematodes (GINs) infection in sheep, predict and analyze their ecological niches and ranges, and identify the key bioclimatic variables influencing their distribution across three distinct climatic regions in Iran. In a cross-sectional study, a total of 2140 fecal samples were collected from semi-arid (n = 800), arid (n = 500), and humid-subtropical (n = 840) climates in East Azerbaijan, Kerman, and Guilan provinces, respectively. The flotation method was employed to assess stool samples, whereby the fecal egg count (the number of parasite eggs per gram [EPG]) was ascertained for each individual specimen. Employing a presence-only approach, the multi-scale maximum entropy (MaxEnt) method was used to model GINs' habitat suitability using 93 selected points/locations. The findings revealed that Guilan (34.2%) and East Azerbaijan (19.62%) exhibited the utmost proportion of Strongyle-type eggs. East Azerbaijan province also displayed the highest proportion of Marshallagia and Nematodirus, respectively (approximately 40% and 27%), followed by Guilan and Kerman provinces, while Kerman province had the highest proportion of Trichuris (approximately 15%). Ecological niche modeling revealed that the precipitation of the driest quarter (Bio17) exerted the most significant influence on Marshallagia, Nematodirus, Trichuris, and ُSُُُtrongyle-type eggs' presence in East Azerbaijan and Kerman provinces. For Guilan province, the most influential factor defining habitat suitability for Strongyle-type eggs, Marshallagia, and Nematodirus was increasing slope. Additionally, the distribution of Trichuris was most affected by the variable Bio2 in Guilan province. The study highlights the response of GINs to climate drivers in highly suitable regions, providing insights into ecologically favorable areas for GINs. In conclusion, this study provides a better understanding of GINs and the environmental factors influencing their transmission dynamics.
Similar content being viewed by others
Introduction
Gastrointestinal nematodes (GINs) are commonly found in grazing ruminants worldwide and have a detrimental impact on livestock production. These parasites, particularly the trichostrongylid nematode species, are responsible for causing reproductive problems and economic losses in ruminants1,2,3,4,5,6,7,8. The most significant nematode species that greatly impact sheep production in temperate climates are Ostertagia, Teladorsagia, Haemonchus, Trichostrongylus, Nematodirus, and Trichuris9,10,11. Since these parasites have free-living stages, their development, interaction with the host, and transmission dynamics are greatly influenced by different climatic patterns and a variety of environmental situations12,13,14.
In recent years, global warming has posed complex challenges regarding the distribution and severity of certain infectious diseases15. The Intergovernmental Panel on Climate Change (IPCC) predicts that there will be further increases in temperature (ranging from 1 to 4 °C) and changes in precipitation are predicted globally16. These changes have raised concerns from the Food and Agriculture Organization (FAO) regarding the rise of parasitic diseases in livestock and its potential negative impact on future food security17. In many regions, changes in the population dynamics of parasites and the transmission of their free-living stages (such as GINs) are expected due to alterations in land use, the presence of anthelmintic resistance, and extreme climatic changes14,18,19. These changes include shifts in seasonal dynamics, species distribution, and an increased risk of habitat loss. Predicting patterns of parasite risk and gaining a better understanding of GINs epidemiology is essential for managing livestock health, particularly in the context of climate change and the strong reliance of the GIN life cycle on environmental conditions, as well as the inadequacy of current nematode control measures1,6,20,21,22.
Ecological niche models (ENMs) and species distribution models (SDMs) are mathematical tools that utilize occurrence records and environmental data to create a correlative model for predicting the ecological requirements and potential geographic distributions of parasites23,24. One such model is the maximum entropy (MaxEnt) model, which accurately predicts a species' probability distribution and assesses the impact of climate change on species ranges and the risk of species invasion25,26,27. MaxEnt is a relatively simple machine-learning algorithm with accurate prediction model and stable operation that can handle categorical and continuous environmental layers and small sample sizes, offering the advantage of straightforward result interpretation28,29.
Considering the limited information on the effects of climate change and environmental factors on nematode ranges in Iran, as well as the potential risk of species invasions, we employed MaxEnt and Arc-geographical information system (Arc-GIS) platforms to model the potential geographical distribution of GINs in three different climatic regions of Iran: East Azerbaijan, Kerman, and Guilan provinces, representing semi-arid, arid, and humid-subtropical zones respectively. By understanding the geographical distribution of GINs and identifying the dominant bioclimatic and environmental factors affecting their transmission dynamics, we aimed to offer valuable insights to decision-makers in implementing future national surveillance and control measures. Our objective was to predict suitable habitats for GIN species and identify the critical bioclimatic and environmental/topographic variables impacting their geographic distribution.
Material and method
Study area
In the present study, sampling was carried out in Guilan, East Azerbaijan, and Kerman provinces which are located in the northern, northwestern, and southeastern regions of Iran, and have humid-subtropical, semi-arid, and arid climatic zones, respectively.
Guilan Province (Coordinates: 37.2774° N 49.5890° E) is situated along the southern strip of the Caspian Sea and spans an area of 14,600 km2 with elevations ranging from 15 m below to 300 m above sea level. This province experiences a temperate Caspian climate, with mean monthly temperatures ranging from 6.3 to 29.8 °C, and has the highest rainfall in the region, with a mean annual precipitation of 1506 mm30. The large area of Guilan province is mountainous, and covered with dense vegetation31.
East Azerbaijan province (Coordinates: 38.0766° N 46.2800° E) is located in the northwest of Iran, and covers an area of about 45,650 km2. The climate of this province is characterized by a Mediterranean continental and cold semi-arid climate. The capital city, Tabriz, receives an average annual rainfall of 310 mm32.
Kerman province (Coordinates: 30.2907° N 57.0679° E) with an area of about 180,726 km2 is situated in the south-central part of Iran. It experiences a semi-arid to dry climate33.
Species occurrence data
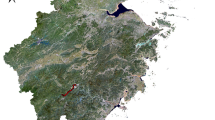
A cross-sectional study was conducted from April to September 2020, using cluster sampling. Fresh fecal samples (6 g) were collected directly from the rectum of native sheep using plastic bags which were stored on ice and then refrigerated at 4 °C. A total of 2140 fecal samples were collected from three different climate zones including East Azerbaijan (n = 800), Guilan (n = 840), and Kerman provinces in Iran (n = 500). The study area consisted of ninety-three points/locations, with ten cities and forty villages selected from East Azerbaijan, seven cities and twenty-eight villages selected from Guilan, and six cities and twenty-five villages selected from Kerman (Table 1 and Fig. 1).
A total of 93 sampling sites were selected as inputs for calibrating the ecological niche models in three provinces (A), points/locations are shown on map as black dots (B), as well as three bioclimatic zones. The spatial patterns of some of environmental variables applied in the MaxEnt model were also shown in the figure (C–E).
Each labeled sample was sent to the Parasitology Department of the Faculty of Veterinary Medicine at the University of Tehran for identification. The identification process was based on the key morphological characteristics of the nematode's eggs. Additionally, the proportion and intensity of GINs were assessed using the fecal egg count (number of parasite eggs per gram [EPG]). Stool samples were examined microscopically using McMaster's method, with flotation in zinc chloride and saturated salt solution (Specific gravity: 1.53).
Ecological niche modeling (ENM)
Bioclimatic and environmental variables
For the ecological niche modeling, a set of 19 bioclimatic variables, 4 environmental/topographic variables (including aspect [direction of slop], slope, and altitude, as well as the normalized difference vegetation index (NDVI) were used (Figs. 1. and 2, Table 2). The bioclimatic data were extracted from the WorldClim database (http://www.worldclim.org/current) with a spatial resolution of 30 s (∼1 km2).
Topographic variables layers of aspect and slope were also derived from the digital elevation model (DEM) of Iran obtained from the WorldClim website using ArcMap 10.3. The NDVI data was generated from the MODIS satellite image in 2016 with a spatial resolution of 30 s (∼1 km2) and adapted to an ASCII standard format.
All bioclimatic, topographic and environmental variables were clipped using a boundary mask for Iran boundaries and then adapted to an ASCII standard format in ArcMap 10.5 for later use in MaxEnt modeling.
Modeling procedure
The maximum entropy modeling (MaxEnt version 3.3.3 k) was applied to estimate the probability of species presence. MaxEnt was chosen for its good performance in testing the predictive ability of the model34. The maxent algorithm is capable of providing the probability distribution of maximum entropy given to empirical constraints such as the spatial distributions of species and environmental conditions, allowing for least-biased, constraint-satisfying probability distribution inference35.
Eighty percent of the location points were randomly assigned as model training (model calibration) and the remaining 20% was used to test the model's predictive value with 10 repetitions (model validation), allowing for simple statistical analysis36. Maximum iterations were also set to 500.
Habitat suitability was defined by a logistic threshold value (the 10th percentile training presence [TPTP]), ranging from 0 (unsuitable) to 1 (best habitat suitability), to eliminate biologically irrelevant noise in the model37.
The accuracy of the modeling (model’s goodness-of-fit) was evaluated using a threshold independent receiver-operating characteristic (ROC) which calculated the area under receiver-operating characteristic curve (AUC) values (0.5 = random to 1.0 = highest value) via plotting the model’s sensitivity against 1- specificity38. Following the execution of 10 replicates, the average AUC values were calculated for both the training and test datasets. The contribution of each variable in the model was identified using the percentage contribution of the Jackknife analysis. The ASCII output map of GINs was loaded in ArcGIS 10.5 for the average model.
Results
Proportion and intensity of GINs
The findings of the study revealed the most remarkable proportion of Strongyle-type egg (34.2%) and Trichuris (14.4%) in Guilan and Kerman provinces, respectively (Table 3). East Azerbaijan province records the highest proportions of Marshallagia (39.8%) and Nematodirus (27.5%), (Table 3).
In Guilan province, specifically in 7 cities and 28 villages, the proportion of Strongyle-type egg was by far the highest (34.2%), followed by Trichuris (8.4%), Nematodirus (7.3%), and Marshallagia (4.5%).
East Azerbaijan province exhibited the highest proportion of Marshallagia (39.8%), followed by Nematodirus (27.5%), Strongyle-type egg (19.62%), and Trichuris (1.3%).
Kerman province was found to harbor Trichuris (14.4%) with the highest proportion, followed by Strongyle-type egg (14.2%), Nematodirus (7%), and Marshallagia (4.4%).
The overall intensity of GINs across the different climates was found to be low, ranging from 1 to 8.1 based on EPG (range; 1–8.1), (Table 3).
Species distribution modeling and contribution of variables
Models constructed with variables demonstrated relatively accurate prediction. The plotted ROC curve displayed the AUC values ranging from 0.81 to 0.99 for the training data and from 0.66 to 0.97 for the test data (Fig. 2). The AUC train and AUC test for each species are denoted separately in Fig. 3, while Fig. 4 depicts the probability maps showing species occurrence predictions arranged by the Maxent color scheme, ranging from green (occurrence “0”) to red (occurrence “1”).
MaxEnt habitat suitability maps of GINs in three climatic regions, Iran. Areas depicted as red are of high suitability and areas depicted as blue are of low suitability. Areas depicted as green to red show suitability of regions from 0 (unsuitable habitat) to 1 (highly suitable habitat), visualizing the potential risk of GINs. (A): Azerbaijan province, (B): Kerman province, (C): Guilan province.
Through the MaxEnt model's internal jackknife analysis, it was revealed that three variables of Bio17, Bio14, and Bio18 have the highest mean contributions in predicting the distribution of Marshallagia, Nematodirus, Trichuris, and Strongyle-type eggs in East Azerbaijan and Kerman provinces when used in isolation (Fig. 5). In Guilan province, slope, NDVI, and altitude were the variables with the highest importance contribution when used in isolation for Marshallagia, Nematodirus, and Strongyle-type eggs. Moreover, the jackknife test of variables' contribution indicated that Bio2, Bio16, and NDVI were the most significant predictors of the presence probability of Trichuris in Guilan province (Fig. 5).
The Jackknife test for environmental variables’ contribution in modeling in three climates zone, Iran. Training and AUC gains is based upon the three scenarios including without variable, with only variable and with all variable. Blue bar depicts regularized training gain of individual environmental variable relative to all environmental variables (Red bar). (A) Azerbaijan province, (B) Kerman province, (C) Guilan province.
Response of variables to GINs habitat suitability
Figure 6 presents the response curves of the most important variables to GINs habitat suitability.
Response curves of MaxEnt models for dominant variables; Bio17 (Precipitation of the driest quarter; mm), slope and NDVI contributed remarkably to the suitability model, indicating the most significant influence of variables on presence-only habitat suitability of GINs. (A): East Azerbaijan province, (B): Kerman province, (C): Guilan province.
Our model demonstrated that precipitation of the driest quarter (Bio17) plays a crucial role in controlling the distribution of Marshallagia, Nematodirus, Trichuris, and Strongyle-type eggs in East Azerbaijan and Kerman provinces. Additionally, slope was identified as a key topographic variable influencing the distribution of Strongyle-type eggs, Marshallagia, and Nematodirus in Guilan province, while Bio2 had the most significant impact on the presence probability of Trichuris in this province. It is noteworthy that NDVI exhibited the largest decrease in value when removed, (lighter blue bars), suggesting that it incorporates valuable information not available in the other variables.
Discussion
Among the three climatic regions under study, the humid-subtropical area (Guilan province) exhibited the highest proportion of Strongyle-type eggs (34.2%). This was followed by the Mediterranean continental and the cold semi-arid climate (East Azerbaijan province) with a proportion of 19.62% and arid area (Kerman province) with a proportion of 14.2%. The EPG, on the other hand, was found to be lower in all climatic zones under investigation, ranging from 1 to 8.1.
Whereas, the East Azerbaijan province showed the highest proportion of Marshallagia (39.8%) and Nematodirus (27.5%), surpassing the other two regions considerably. The remaining two zones, Guilan and Kerman, made manifest a proportion tantamount to less than 8% and 5% of the aforementioned parasites, respectively. Furthermore, the highest proportion of Trichuris eggs in sheep (14.4%) was observed in Kerman province compared to the wet province of Guilan and East Azerbaijan province.
To compare the infection rates of sheep GINs between older reports dating back more than four decades and the present day, it is evident that not only are the rates of GINs on the decline but also EPG is on the decline presently39,40. This decline in GINs is occurring, owing to the increasing implementation of anthelmintic treatments and sheep movement, which suggests a positive trend in herd management strategies.
Ruminant GINs are greatly influenced by environmental and climatic conditions. The main parasitic pathogens of the ruminant’s gastrointestinal tract such as Haemonchus, Ostertagia, Teladorsagia, Trichostrongylus, Nematodirus, Oesophagostomum, and Trichuris play a crucial role in animal health11,41,42.
Climatic variables have the potential to influence the prevalence, intensity, and geographical distribution of GINs, particularly when parasite species exhibit climate-driven spatial variation. Temperature and rainfall, being the foremost influential factors, exert a significant impact on the growth and development of free-living stages, including eggs and various larval stages (namely, L1, L2, L3) of these nematodes 6,11.
Additionally, climate change holds the capacity to exert its influence upon the transmission success of parasites by altering the host immune defenses, alongside altering the density of these hosts, thus irrevocably impacting the spatial distribution of these species43,44. By establishing a correlation model that demonstrates the connection between species and environmental and bioclimatic variables, important insights into species' ecological requirements and habitat suitability can be obtained24.
In the present study, the modeling results signify that GINs are distributed to diverse extents across nearly all territories of East Azerbaijan, except for Trichuris, which is predominantly found in the western regions of the province. The jackknife analysis revealed that Bio17 had the most significant contribution to the model, followed by Bio14 and Bio18, in determining the habitat suitability of GINs in East Azerbaijan. This province experiences a Mediterranean continental and cold semi-arid climate.
In Kerman province, the modeling results revealed that the main distribution of GINs is concentrated in the southern and northern regions of the province. The jackknife analysis predicted Bio17 as the variable with the most contribution in the model for all nematodes under investigation in the province, followed by Bio 14 and Bio18. The southeastern fringes of Iran, including Kerman province, are particularly affected by the monsoon weather phenomenon. With a semi-arid to dry climate characterized by low rainfall and high temperatures, the more arid regions create unfavorable conditions for larval development, survival, and migration out of feces onto herbage. Consequently, these regions provide less environmentally suitable habitats for GINs.
In the province of Guilan, the presence of distribution of GINs spans across nearly all areas, indicating that the conditions are highly favorable for the growth and development of eggs and free-living stages of trichostrongylid species. The distribution of Strongyle-type eggs, Marshallgia, Nematodirus, and Trichuris, does also bear close resemblance. The predicted factors with the most significant influence on Marshallagia, Nematodirus, and Strongyle-type eggs are envisaged to be the slope, followed by NDVI, and altitude. Additionally, we have discovered that three variables, namely Bio2, Bio16, and NDVI, are the most important predictors of the presence probability of Trichuris.
In the northern regions of Iran, specifically the Caspian and Wet Mediterranean zones, a humid-subtropical climate prevails, with mild rainy winters and humid hot summers. These areas tend to experience fewer dry periods, thus creating suitable environmental conditions for the development of eggs and free-living stages of GIN species.
During winter, third-stage larvae (L3) of most species are capable of residing in the rangelands, resulting in the infection of ruminants, particularly newborn lambs, during the spring season45. The monthly rainfall in Rasht and Bandar-e Anzali within Guilan province, excluding the summer season, bestows abundant moisture essential for both the robust development of trichostrongylid larvae in fecal pellets and the successful outward migration of L3 larvae from feces. However, it is not conducive to the vertical migration of L3 onto herbage31,45,46. This condition may also be present, to a lesser extent, in East Azerbaijan province during the rainy season (autumn, winter, and spring). On the other hand, this condition is not widespread in climate regions such as Kerman province, characterized by ''Hot Dry Desert and Hot Semi-Desert'' climates, thereby limiting the possibilities of migration out of feces and subsequent survival.
In the analysis of species' spatial distribution and their climate-driven spatial variations, it is of utmost importance to refrain from disregarding the finer-scale geographic variations, including the impact of altitude. The Caspian region proved altitude's substantial role as a determining factor for the model of Marshallagia and Nematodirus.
Eggs and free-living stages of trichostrongylid infections such as Trichostrongylus, Ostertagia, Nematodirus, and Cooperia showcase an ability to survive at very low temperatures (e.g., above 4 °C for the development of L3 larvae). However, Haemonchus requires temperatures above 8 °C for L3 larvae development. As temperatures rise in spring, pasture contamination with L3 larvae of most species increases throughout the summer, extending into autumn for certain species. Milder winters can facilitate the over-winter survival of Haemonchus L321. Conversely, extremely high temperatures may pose unsuitability for larval survival, hindering their feeding and increasing metabolism6,13,21,47. Warm temperatures have been associated with a significant decrease in Ostertagia spp. The eggs and free-living larvae of Ostertagia and Nematodirus exhibit resistance to cold temperatures, and snow cover plays a protective role for infective larvae, however, severe winters are connected to a decrease in endemic species47. Drier summers could reduce grass growth and availability of summer grazing, inhibiting larvae development6. It is worth noting that the shedding of eggs by adult worms during the periparturient egg rise period results in the widespread distribution of large quantities of eggs in the pasture after immunity to trichostrongylid infection. Nematodirus spp. infection is not related to the periparturient egg rise in ewes; instead, lambs are responsible for the contamination of pastures. Furthermore, Nematodirus eggs have been found to require warmth in spring after prolonged chilling (below 11 °C) for their development47,48.
From a local perspective, bioclimatic variables such as temperature, rainfall, and their variations within and between years, along with topographic/vegetation variables as well as their interactions with husbandry and management practices (farm-level risk factors), are all implicated in larval availability and the geographical distribution of the parasites, however, a strategic dosing regimen, consisting of frequent administration of anthelmintic drugs, has been widely employed in the country over the past decades to reduce infection rates. This approach, devoid of comprehensive understanding of GINs population dynamics, may precipitate the manifestation of anthelmintic resistance. Instead, a more regionally attuned strategy, acknowledged as targeted treatment, ought to be adopted. At a regional level, ecological niche models hold great value in predicting primary infection periods, assessing the impact of intervention impacts, and understanding the influence of environmental and bioclimatic factors. These models aid in the formulation of rational strategies such as judicious use of anthelmintics, to effectively reduce parasite transmission and alleviate the burden on animals, while mitigating economic damage.
Conclusions and further recommendations
In the present study, MaxEnt employed an ecological niche modeling technique to generate a habitat suitability map for GINs in the provinces of East Azerbaijan, Guilan and Kerman, Iran. The objective was to elucidate the primary predictors of GINs presence probability across various host environments, incorporating bioclimatic and environmental variables.
Regarding all nematodes under examination (Marshallagia, Nematodirus, Trichuris, and Strongyle-type eggs), the variable Bio17 exhibited the most substantial contribution to the model in East Azerbaijan and Kerman provinces, followed closely by Bio14 and Bio18. In Guilan province, the variables with the greatest impact on Marshallagia, Nematodirus, and Strongyle-type eggs' presence probability were slope, subsequently, followed by NDVI, and altitude. In the humid-subtropical climate of Guilan province, the paramount determinants showcasing the presence probability of Trichuris are evidently Bio2, Bio16, and NDVI.
Further, it is of paramount significance to engage in comprehensive research pertaining to the ecological niche of ruminant GINs not solely within the particular areas being investigated, but also spanning diverse regions nationwide. Such investigations should specifically prioritize the analysis of seasonal dynamics and herd-level management factors in relation to the predictive model.
Data availability
All data generated are included in the current article.
Abbreviations
- GINs:
-
Gastrointestinal nematodes
- SDMs:
-
Species distribution models
- MaxEnt:
-
Maximum entropy
- Arc-GIS:
-
Arc-geographical information system
- EPG:
-
Number of parasite eggs per gram
- DEM:
-
Digital elevation model
- ROC:
-
Receiver-operating characteristic
- AUC:
-
Area under receiver-operating characteristic curve
- FAO:
-
Food and agriculture organization
- FEC:
-
Fecal egg count
- ENMs:
-
Ecological niche models
- NDVI:
-
Normalized difference vegetation index
References
Charlier, J. et al. Climate-driven longitudinal trends in pasture-borne helminth infections of dairy cattle. Int. J. Parasitol. 46(13–14), 881–888. https://doi.org/10.1016/j.ijpara.2016.09.001 (2016).
Rose, H., Hoar, B., Kutz, S. J. & Morgan, E. R. Exploiting parallels between livestock and wildlife: Predicting the impact of climate change on gastrointestinal nematodes in ruminants. Int. J. Parasitol. Parasit. Wildl. 3(2), 209–219. https://doi.org/10.1016/j.ijppaw.2014.01.001 (2014).
Wang, T. et al. Seasonal epidemiology of gastrointestinal nematodes of cattle in the northern continental climate zone of western Canada as revealed by internal transcribed spacer-2 ribosomal DNA nemabiome barcoding. Parasit. Vectors 14(1), 604. https://doi.org/10.1186/s13071-021-05101-w (2021).
Watson, M. J. What drives population-level effects of parasites? Meta-analysis meets life-history. Int. J. Parasitol. Parasit. Wildl. 2, 190–196. https://doi.org/10.1016/j.ijppaw.2013.05.001 (2013).
Morgan, E. et al. Global change and helminth infections in grazing ruminants in Europe: Impacts, trends and sustainable solutions. Agriculture 3(3), 484–502. https://doi.org/10.3390/agriculture3030484 (2013).
Morgan, E. R. & van Dijk, J. Climate and the epidemiology of gastrointestinal nematode infections of sheep in Europe. Vet. Parasitol. 189(1), 8–14. https://doi.org/10.1016/j.vetpar.2012.03.028 (2012).
Nurcahyo, R. W. et al. Occurrence of gastrointestinal parasites in cattle in Indonesia. In IOP Conference Series: Earth and Environmental Science 686(1), 012063. https://doi.org/10.1088/1755-1315/686/1/012063 (2021).
von Samson-Himmelstjerna, G., Harder, A. & Schnieder, T. Quantitative analysis of ITS2 sequences in trichostrongyle parasites. Int. J. Parasitol. 32(12), 1529–1535. https://doi.org/10.1016/S0020-7519(02)00163-7 (2002).
Soulsby, E. J. L. Helminths, arthropods & protozoa of domesticated animals 7th edn. (Bailliere Tindall, 1986).
Gharekhani, J., GeramiSadeghian, A. & Yousefi, M. Parasitic helminth infections in native sheep (Mehraban) in Hamedan, Iran. J. Adv. Vet. Anim. Res. 2(2), 115. https://doi.org/10.5455/javar.2015.b59 (2015).
Rinaldi, L. et al. Haemonchus contortus: Spatial risk distribution for infection in sheep in Europe. Geospatial Health 9(2), 325. https://doi.org/10.4081/gh.2015.355 (2015).
Altizer, S., Ostfeld, R. S., Johnson, P. T. J., Kutz, S. & Harvell, C. D. Climate change and infectious diseases: From evidence to a predictive framework. Sci. 341(6145), 514–519. https://doi.org/10.1126/science.1239401 (2013).
O’Connor, L. J., Walkden-Brown, S. W. & Kahn, L. P. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet. Parasitol. 142(1–2), 1–15. https://doi.org/10.1016/j.vetpar.2006.08.035 (2006).
Rose, H., Wang, T., van Dijk, J. & Morgan, E. R. GLOWORM-FL: A simulation model of the effects of climate and climate change on the free-living stages of gastro-intestinal nematode parasites of ruminants. Ecol. Model. 297, 232–245. https://doi.org/10.1016/j.ecolmodel.2014.11.033 (2015).
Garrett, K. A. et al. The effects of climate variability and the color of weather time series on agricultural diseases and pests, and on decisions for their management. Agric. For. Meteorol. 170, 216–227. https://doi.org/10.1016/j.agrformet.2012.04.018 (2013).
IPCC, Climate Change: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri, R. K. and Meyer, L. A. (eds.)]. IPCC, Geneva, Switzerland, 151 (2014).
FAO. Climate change and food security: risks and responses. Food and Agriculture Organization of the United Nations, Rome, Italy. (2015).
Pickles, R. S. A., Thornton, D., Feldman, R., Marques, A. & Murray, D. L. Predicting shifts in parasite distribution with climate change: A multitrophic level approach. Glob. Change Biol. 19(9), 2645–2654. https://doi.org/10.1111/gcb.12255 (2013).
Titcomb, G. et al. Water sources aggregate parasites with increasing effects in more arid conditions. Nat. Commun. 12(1), 7066. https://doi.org/10.1038/s41467-021-27352-y (2021).
Mas-Coma, S., Valero, M. A. & Bargues, M. D. Effects of climate change on animal and zoonotic helminthiases. Rev. Sci. Tech. 27(2), 443–457 (2008).
van Dijk, J., David, G. P., Baird, G. & Morgan, E. R. Back to the future: Developing hypotheses on the effects of climate change on ovine parasitic gastroenteritis from historical data. Vet. Parasitol. 158(1–2), 73–84. https://doi.org/10.1016/j.vetpar.2008.08.006 (2008).
Verschave, S. H., Charlier, J., Rose, H., Claerebout, E. & Morgan, E. R. Cattle and nematodes under global change: Transmission models as an ally. Trends Parasitol. 32(9), 724–738. https://doi.org/10.1016/j.pt.2016.04.018 (2016).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190(3–4), 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 (2006).
Warren, D. L. & Seifert, S. N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 21(2), 335–342. https://doi.org/10.1890/10-1171.1 (2011).
Liu, G. et al. An ecological network perspective in improving reserve design and connectivity: A case study of Wuyishan nature reserve in China. Ecol. Model. 306, 185–194. https://doi.org/10.1016/j.ecolmodel.2014.10.004 (2015).
Tang, X., Yuan, Y., Li, X. & Zhang, J. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 12, 652500. https://doi.org/10.3389/fpls.2021.652500 (2021).
Araújo, M. B. & Peterson, A. T. Uses and misuses of bioclimatic envelope modeling. Ecol 93(7), 1527–1539. https://doi.org/10.1890/11-1930.1 (2012).
Xiong, D., Yu, H. & Liu, Q. Tagging complex NEs with MaxEnt models: Layered structures versus extended tagset. In International conference on natural language processing (eds Carbonell, J. G. & Siekmann, J.) 537–544 (Springer, Berlin, Heidelberg, 2004).
Yuan, H.-S., Wei, Y.-L. & Wang, X.-G. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 17, 140–145. https://doi.org/10.1016/j.funeco.2015.06.001 (2015).
Dinpashoh, Y., Singh, V. P., Biazar, S. M. & Kavehkar, S. Impact of climate change on streamflow timing (case study: Guilan Province). Theor. Appl. Climatol. 138, 65–76. https://doi.org/10.1007/s00704-019-02810-2 (2019).
Halimi, M., Farajzadeh, M., Delavari, M. & Arbabi, M. Developing a climate-based risk map of fascioliasis outbreaks in Iran. J. Infect. Public Health 8(5), 481–486. https://doi.org/10.1016/j.jiph.2015.04.024 (2015).
Sihag, P., Esmaeilbeiki, F., Singh, B. & Pandhiani, S. M. Model-based soil temperature estimation using climatic parameters: The case of Azerbaijan Province, Iran. Geol. Ecol. Landsc. 4(3), 203–215. https://doi.org/10.1080/24749508.2019.1610841 (2020).
Dehshiri, S. S. & Firoozabadi, B. A new application of measurement of alternatives and ranking according to compromise solution (MARCOS) in solar site location for electricity and hydrogen production: A case study in the southern climate of Iran. Energy 261, 125376. https://doi.org/10.1016/j.energy.2022.125376 (2022).
Slater, H. & Michael, E. Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS One 7(2), e32202. https://doi.org/10.1371/journal.pone.0032202 (2012).
De Martino, A. & De Martino, D. An introduction to the maximum entropy approach and its application to inference problems in biology. Heliyon 4(4), e00596. https://doi.org/10.1016/j.heliyon.2018.e00596 (2018).
Phillips, S. J. A brief tutorial on Maxent. AT&T Res. 190(4), 231–259 (2005).
Kriticos, D. J. et al. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 90(4), 1033–1043. https://doi.org/10.1007/s10340-017-0869-5 (2017).
Marini, M. A., Barbet-Massin, M., Martinez, J., Prestes, N. P. & Jiguet, F. Applying ecological niche modelling to plan conservation actions for the red-spectacled Amazon (Amazona pretrei). Biol. Conserv. 143(1), 102–112. https://doi.org/10.1016/j.biocon.2009.09.009 (2010).
Eslami, A. & Nabavi, L. Species of gastrontestinal nematodes of sheep from Iran. Bull. Soc. Pathol. Exot. 69, 92–95 (1976).
Nabavi, R. et al. Study on the prevalence, Intensity, seasonal dynamics of abomasum helminths in sheep from different climatic zones of Iran. World Appl. Sci. J. 12(4), 441–445 (2011).
Cringoli, G. et al. Gastrointestinal strongyle faecal egg count in goats: Circadian rhythm and relationship with worm burden. Vet. Res. Commun. 32(1), S191–S193. https://doi.org/10.1007/s11259-008-9163-64 (2008).
Charlier, J., Voort, M. V., Kenyon, F., Skuce, P. & Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 30(7), 361–367. https://doi.org/10.1016/j.pt.2014.04.009 (2014).
Lafferty, K. D. & Holt, R. D. How should environmental stress affect the population dynamics of disease?. Ecol. Lett. 6, 654–664 (2003).
Morley, N. J. & Lewis, J. W. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 30, 221–227 (2014).
Shadman, M. et al. Mapping habitat suitability for gastrointestinal nematodiasis of ruminants in southern Caspian Sea littoral: A predicted risk pattern model based on the MaxEnt. Trop. Anim. Health Prod. 52, 3843–3854. https://doi.org/10.1007/s11250-020-02423-2 (2020).
Langrova, I., Jankovska, I., Borovsky, M. & Fiala, T. Effect of climatic influences on the migrations of infective larvae of cyathostominae. Vet. Med. 4(1–2), 18–24. https://doi.org/10.17221/5745-VETMED (2003).
Constable, P. D., Hinchcliff, K. W., Done, S. & Gruenberg, W. Diseases of the alimentary tract–ruminant in veterinary medicine (Eleventh Edition), 436–621. (2017).
Morgan, E. R. & van Dijk, J. The influence of temperature on the development, hatching and survival of Nematodirus battus larvae. Parasitol. 35(2), 269–283. https://doi.org/10.1017/S0031182007003812 (2008).
Acknowledgements
The authors express their deep gratitude and appreciation to all staff of the Faculty of Veterinary Medicine, University of Tehran who contributed to the laboratory work in collecting the data for this study.
Author information
Authors and Affiliations
Contributions
B.M. conceived the study and assisted with the study design; A.A.H.B., M.S., S.F., and G.M. were responsible for the data collection and analysis; M.S., G.M., S.F., and K.M. wrote the original draft; B.M. critically reviewed the manuscript and ensured the accuracy of the findings. All authors engaged in discussions regarding the findings and made valuable contributions to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meshgi, B., Hanafi-Bojd, A.A., Fathi, S. et al. Multi-scale habitat modeling framework for predicting the potential distribution of sheep gastrointestinal nematodes across Iran’s three distinct climatic zones: a MaxEnt machine-learning algorithm. Sci Rep 14, 2828 (2024). https://doi.org/10.1038/s41598-024-53166-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53166-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.