Abstract
Cough is known as a protective reflex to keep the airway free from harmful substances. Although brain activity during cough was previously examined mainly by functional magnetic resonance imaging (fMRI) with model analysis, this method does not capture real brain activity during cough. To obtain accurate measurements of brain activity during cough, we conducted whole-brain scans during different coughing tasks while correcting for head motion using a restraint-free positron emission tomography (PET) system. Twenty-four healthy right-handed males underwent multiple PET scans with [15O]H2O. Four tasks were performed during scans: “resting”; “voluntary cough (VC)”, which simply repeated spontaneous coughing; “induced cough (IC)”, where participants coughed in response to an acid stimulus in the cough‐inducing method with tartaric acid (CiTA); and “suppressed cough (SC)”, where coughing was suppressed against CiTA. The whole brain analyses of motion-corrected data revealed that VC chiefly activated the cerebellum extending to pons. In contrast, CiTA-related tasks (IC and SC) activated the higher sensory regions of the cerebral cortex and associated brain regions. The present results suggest that brain activity during simple cough is controlled chiefly by infratentorial areas, whereas manipulating cough predominantly requires the higher sensory brain regions to allow top-down control of information from the periphery.
Similar content being viewed by others
Introduction
Coughing is a protective reflex that protects the airways from harmful substances and is necessary to maintain normal airway patency1. Cough consists of inspiration (inspiratory phase), glottal closure and forced expiratory effort (compressive phase), and glottal opening and expiration (expiratory phase). Two types of coughs can be distinguished: voluntary cough (VC), which occurs spontaneously, and induced cough (IC), which occurs reflexively in response to airway stimuli. IC is evoked by mechanical or chemical stimuli applied to the airways, including TRPV-1 agonists such as capsaicin as well as acids, histamine, and other compounds1,2,3. Despite this concept, VC and IC cannot be isolated alone as single components of cough, and IC may have an impulse component to cough.
Cough reflex tests have been used to screen for dysphagia, and capsaicin, citric acid, and tartaric acid have been used as cough inducers in the clinical setting4,5,6. Applying cough induction techniques to dysphagic patients with impaired cough reflexes is an important tool to prevent aspiration. Recently, we found tartaric acid to be useful in cough induction for dysphagic patients with silent aspiration; this method was named the cough‐inducing method with tartaric acid (CiTA)7. Hence, clinical experience also emphasizes the contribution of the brain to cough regulation.
Brain activity related to cough has been evaluated by a model-based fMRI technique as follows: VC activates a wide range of brain regions, such as the sensorimotor, supplementary motor, orbitofrontal, insular and middle cingulate cortices, thalamus, caudate nucleus, putamen and cerebellum8,9. IC also activates the widely distributed brain network, including the somatosensory, insular, middle cingulate, orbitofrontal and supplementary motor cortices, medulla oblongata, and cerebellum9,10. Suppression of cough(SC) is regulated by the middle cingulate, supplementary motor and insular cortices, right inferior frontal gyrus, caudate nucleus9,11. Thus, many brain regions are believed to play important roles in cough control through a top-down system, in which higher brain regions send information to the brainstem9,12. In addition, brainstem activity in the solitary nucleus, paratrigeminal nucleus, trigeminal nucleus13 is also implicated in cough induction. These findings were generated using a sophisticated model-based analysis to eliminate artifacts of cough-related head motion.
A major issue in imaging studies of brain activity during cough is the presence of artifacts caused by head movement. Despite fMRI reports using sophisticated methods such as modeling physiological factors during tasks and correcting head movement computationally14,15, no real brain activity occurring during cough can be successfully captured with existing techniques. To address this limitation, we have recently developed a PET camera equipped with a free-moving function that enables imaging during head motion16,17. The purpose of the present research was to evaluate brain activity changes during different coughing tasks in normal subjects using this restraint-free PET system to clarify the neurophysiology of cough. We hypothesized that brain activity during cough would be more localized than previously reported.
Results
Physiological data during PET measurement
The number of coughs measured during PET measurement was evaluated using Student’s t test. Significant differences were found between the VC (26.1 ± 12.8, mean ± SD) and IC groups (12.4 ± 9.4) using one-way analysis of variance (repeated-measures ANOVA). The number of coughs during the task was found to be consistent across the measurements (Fig. 1).
Comparisons of urge-to-cough scores among cough conditions were performed using the Friedman test with multiple comparisons. Significant differences were found between the cough-induced and cough-suppressed conditions relative to the resting and VC conditions (resting vs. induced, p < 0.001, resting vs. suppressed p < 0.001, induced vs. voluntary, p < 0.001 by t-statistics). The median and interquartile range for each task at baseline are as follows: resting (median 1, interquartile [1–2]), voluntary (2 [1–2]), induced (6 [4–7]), and suppressed (5 [4–7]). No difference was found between resting and voluntary conditions.
Imaging data during PET measurement
Brain activation in VC vs. resting
The regions predominantly activated during the VC task were examined (Table 1, Fig. 2A). Activation of the left supplementary motor cortex was found only in the supratentorial region. In the infratentorial area, the cerebellar vermis extending to the pons and the right cerebellar hemisphere were also observed to be significantly activated. In the current analysis, we did not include the number of coughs throughout the study, as the number of coughs was zero in SC, and the factors (scan order, subject's urge to cough, PET-related global signal intensities) was considered as a covariate. In a preliminarily analysis of comparing VC to resting state by incorporating the number of coughs as a covariate, enhanced responses were observed in the bilateral supplementary motor areas (Suppl. Table 1).
Statistical parametric mapping (SPM) results. Brain regions with significant activation in voluntary cough (A), induced cough (B) and suppressed cough (C) compared with rest. See Table 1 for a list of activated areas. The color bar indicates the t values.
Brain activation in IC vs. resting
While no significant responses were found in the cerebral cortical areas, the infratentorial regions, including the pons, medulla oblongata and cerebellar regions, were activated (Table 1, Fig. 2B). Among these regions, the pons and vermis had especially strong activation.
Brain activation in SC vs. resting
Significant activation was found in the superior frontal region covering the sensory cortex and the anterior and middle cingulate cortices. Strong activation was also found in the diencephalon, left anterior insula and right putamen (Table 1, Fig. 2C). Incorporating the parameters of the urge to cough into the analysis, additional responses appeared in the operculum and weakly in the caudate nucleus.
Comparisons of brain activations in IC with VC or SC
Comparison of IC with VC to examine the contribution of the sensory component to cough revealed that the basal forebrain, left thalamus, pons, medulla oblongata, and cerebellum were significantly activated (Table 2, Fig. 3). Specifically, the thalamus and cerebellar vermis were found to be most strongly activated, and the putamen was also activated to some extent. Taking the urge to cough into consideration in the analysis, we found that a wider range of cerebral regions played a role in the regulation of cough under sensory stimulation. Interestingly, compared with SC, only the cerebellar regions were strongly activated, and some weak activation was observed in the medulla (Table 2, Fig. 3), which indicated that these infratentorial regions are implicated in the motor component of cough regulation.
Statistical parametric mapping (SPM) results. Brain regions with significant activation in induced cough vs. voluntary cough and induced cough vs. suppressed cough. See Table 2 for a list of activated areas. The color bar indicates the t values.
Brain activation by tartaric acid stimulation
We investigated the effects of tussive agent (tartaric acid) on the brain by comparing conditions with tartaric acid (IC + SC) and without tartaric acid (VC + rest). This comparison showed significant activation in the right putamen and left thalamus in conditions with tartaric acid (Table 3, Fig. 4A). The pons and cerebellar regions were also activated during the tartaric acid–treated condition. In addition, incorporating the urge-to-cough score into the analysis showed a wider area of significant activation, including the cerebral cortex and diencephalon (Table 3). The brain response in the pons (p < 0.05) at tartaric acid stimulation was higher at NRS grades 1–2 than at NRS at grades 9–10, suggesting that the pons is not directly related to internal emotional changes (Fig. 4B).
(A) Statistical parametric mapping (SPM) results. Brain regions significantly activated by the tartaric acid stimulus, identified by comparing the combination of induced and suppressed conditions with the combination of voluntary and rest conditions. See Table 3 for a list of activated areas. The color bar indicates the t value. (B) There was a significant difference between pontine responses at NRS 1–2 and those at NRS 9–10 in the baseline condition tartaric acid stimulation.
Discussion
The present study was the first to depict brain activity during coughing using our restraint-free PET system that enables the collection of positron signals per second while head motion was corrected every 4 ms. Throughout this study, cerebellar activation was highlighted during cough irrespective of voluntary and induced conditions, while brain activity in the cerebral cortex was found in more limited conditions. Indeed, suppressing cough activated regions such as the cingulate cortex, central posterior gyrus, left insular cortex and left diencephalon. In the present study, we used tartaric acid to induce cough. As reported before7, there were significant differences in the urge to cough and the number of coughs during the cough-inducing stimulus compared to placebo. Although participants were completely blinded to the administration of tartaric acid, some subjects felt a strong urge to cough even when saline was administered.
Using the current head motion correction method, we found that brain activity during the coughing task was much more localized than previously reported, as we had hypothesized prior to this study. Brain activity during cough has been thought to be controlled in a top-down manner from higher brain regions via the network stimulated by brainstem input8,9,11. Comparing brain activity at rest and during other tasks, significant brain activation during VC was chiefly confined to the cerebellum extending to the pons. In contrast, during IC, significant activation was found in the diencephalon to the brainstem, including the hypothalamus and cerebellum (Table 1, Fig. 2B). These findings indicate that the cerebellum is a key player in regulation of cough.
The cerebellum is involved in coordinating the timing of lingual and oral motor movements during swallowing18. Stimulation of the cerebellum with rTMS has been reported to activate areas of the cerebral cortex involved in swallowing movements. A study examining coughing behavior in cats after cerebellectomy did not reach a conclusion that the cerebellum is tightly associated with coughing, in which muscle activity during cough increased immediately after cerebellectomy and its movement during cough varied randomly over time19. Cerebellar activity in the present study was still observed after head motion correction, suggesting that the cerebellum may contribute to the coordination and planning of coughing movements in the same way that it functions during eating and swallowing.
Suppressing cough is an act of motor inhibition. It has been reported that suppressing a cough activates the dorsomedial prefrontal cortex, middle cingulate cortex, supplementary motor cortex, right lateral inferior frontal gyrus, caudate nucleus, and right insular cortex9,11,20, suggesting the existence of an inhibitory network for coughing. Within this network, the anterior cingulate cortex, insula, and midbrain are also activated by painful stimuli, suggesting a link to the suppression of cough21,22,23. It was reported that nociception and cough show similar peripheral and central mechanisms24,25,26,27. Indeed, the regions associated with cough suppression in the present study were similar to those reported above. Furthermore, we found a response that was even more consistent with previous studies after we incorporated the intensity of the urge to cough into the analysis. As hypothesized, brain activations in the caudate nucleus, operculum, and infratentorial brain regions (cerebellum to medulla oblongata) might occur as a result of a stronger perception of the cough impulse. The fact that cerebellar activation was still observed during cough suppression suggests that cerebellar control may be important in the inhibition of cough generation.
Reflex cough is mediated by vagal afferents from the upper airways and the tracheobronchial tree28,29,30. In the present study, the brainstem was activated in the IC vs. VC and SC vs. rest conditions when the urge to cough incorporated (Fig. 1, Table 1). Both VC and the cough reflex are thought to involve the brainstem. In particular, the medulla oblongata responds to the induction of cough and exhibits different activation depending on the type of coughing9,12. This may indicate that the brainstem is not strongly involved in voluntary (more precisely, “automatic”) cough in the present study. The sensory processing of cough is considered to involve the pathways of the solitary nucleus and the paratrigeminal nucleus in the medulla oblongata30,31,32,33. In the present study, activation in the brainstem during cough induction occurred in the brainstem covering the solitary nucleus, the dorsal nucleus of the vagus nerve, and the hypoglossal nucleus in the medulla oblongata during cough induction. The solitary nucleus projects Aδ fibers that are distributed in the trachea and larynx30,34. In contrast, the paratrigeminal nucleus, which corresponds to the C fibers distributed in the nociceptors of the lung35, was not significantly activated. Therefore, stimulation at the pharyngeal level by cough medicines can effectively induce coughing.
In our daily clinical practice, we encourage dysphagic patients to promote voluntary coughing and occasionally use CiTA to induce coughing. We compared brain activity during induced coughing by the CiTA protocol with voluntary or suppressed coughing to examine the sensory (IC > VC) and motor (IC > SC) components of the cough. In IC compared with SC, the motor component showed responses in the cerebellum, excluding the vermis. The current analyses suggest that the cerebellar vermis might be associated with the sensory control component (Table 2, Figs. 3, 5), whereas the lateral regions of the cerebellar hemispheres might be important in the motor control component (Table 2, Figs. 3, 5). Brain responses in the pons and cerebellar hemispheres may be related to coughing behavior itself, irrespective of the internal urge to cough (Figs. 4B, 5). It has been reported that both motor and sensory components related to coughing are derived from networks involving extensive brain activity9. The activated regions in both components in the present study were narrower than the range reported before. An animal experiment with rats showed that cough-inducing stimuli activated a pathway between the brainstem and the subthalamic nucleus and that the input of nociceptive stimuli to the brainstem is under descending control of central origin. The present results highlighted the forebrain, thalamus, and brainstem activity as the basis of the sensory component. As suggested elsewhere, it is likely that the input from induced stimuli is perceived in the diencephalon and brainstem, while the basal forebrain and thalamus are involved in making adjustments to the sensory input36. It was reported that the forebrain network was less active during cough suppression in patients with chronic cough (persistent natural induction of cough), while it was more active in healthy subjects20. The present response in the healthy subjects was compatible with this observation. Based on previous literature, we also made comparisons between tasks with CiTA and tasks without CiTA to examine the effect of tartaric acid in the context of cough. This comparison showed significant brain activation in the putamen, thalamus, operculum, pons, and cerebellum (Fig. 5, Table 3), suggesting that CiTA may be an effective tool to activate the sensory and perceptual control system. With the urge to cough incorporated, broader regions in the cerebral cortex were included as regions with significant activation in the CiTA-related condition, possibly because the dorsomedial prefrontal cortex became responsive when the coughing impulse component was added. In the current study, the tussive agent was continuously delivered in short suspensions (10 s) for a total of 40 s to induce a sufficient number of coughs while avoiding respiratory stress. In preliminary experiments, stress was frequently observed due to tidal respiration. So, the outcomes might have been different if the tidal breathing of tussive agent was introduced.
A hypothesized schematic brain network for cough. Brain regions that significantly contribute to cough regulation in each cough condition are illustrated with different colors. The pons and brainstem may be the most important regions in the control of stimulated cough. SMA supplemental motor area, V cerebellar vermis, Cer cerebellar hemisphere, Put putamen, Tha thalamus, BF basal forebrain, EC entorhinal cortex, P pons, MO medulla oblongata, SMC sensorimotor cortex, Opm operculum, DCN diencephalon.
The limitations of this study are as follows. Current cough tasks do not allow clear separation of cough components in terms of central nervous system mechanisms. While VC and IC may be motor- and sensory-dominant, respectively, the presence of cough and reflex impulses cannot be completely excluded. The subjects were instructed to sit on a reclining chair while coughing but not to hit their head against the internal wall of the gantry. This indicates that cough in the current situation differs from cough in the real-world situation. Although we used 10% tartaric acid, similar to the concentration used at the bedside, the content was not optimized for each participant. Therefore, the number and magnitude of coughs varied depending on their responsiveness. It may be necessary to set the concentration of cough-inducing substances administered based on the guidelines of the European Respiratory Society (ERS). Additionally, respiratory status during coughing may be a potential confounding factor, as respiratory rhythm can influence emotion and cognition in brain physiology37. This may be a common black box in brain mapping studies. The current study was exploratory in nature and considered the whole brain, including the brainstem, to be part of search area; accordingly, the intensity and extent of the signal were thresholded without correction for multiple comparisons. In addition, this whole-brain analysis is not ideal for exploring the specific areas of activation within the brainstem because PET has lower spatial resolution than fMRI.
In conclusion, the cerebellum plays a role in the regulation of cough not only through motor coordination but also through the perceptual control of cough. The brainstem is especially involved in the manipulation of cough (Fig. 5). We discuss our future outlook. The current study partly clarified the underlying mechanism of CiTA, which allows clinicians to predict whether it will be effective before attempting it in patients with brain lesions who have sustained damage to the regions related to cough control. To confirm this, the current study is worth extending into a future study in dysphagic patients.
Materials and methods
Participants
Twenty-four healthy male volunteers (27.4 ± 4.0) were recruited. Male-only participation was adopted because in women there were confounding effects such as hormonal fluctuations and pregnancy. All participants were right-handed nonsmokers who drank occasionally; had no neuropsychiatric, respiratory or allergic disorders; and did not use medication that could affect cognitive function. Prior to participation, the subjects underwent respiratory function tests using a spirometer (Minato Medical Science Co., Ltd., Osaka, Japan) to confirm that they had no lung function impairment, and they inhaled a 10% solution of l-tartaric acid with a nebulizer (Shinei Kogyo Co., Ltd., Saitama, Japan) to assess whether their cough reflex was adequate for the planned study. Participants were excluded if no cough reflex was generated within 30 s after nebulizer inhalation. Data from one participant were excluded because he had difficulty completing all scans due to a sensation of fatigue. The study was conducted from September 2021 to July 2022 in compliance with the Declaration of Helsinki and with the approval of the clinical ethics committees of Hamamatsu University School of Medicine [20-328], Hamamatsu City Rehabilitation Hospital [21-09], and Hamamatsu Photonics K.K. (H-184). Written informed consent was obtained from all participants.
MRI data acquisition
We performed MRI to exclude brain abnormalities and to conduct anatomic normalization using an Optima MR360 Advance 1.5 T scanner (GE Healthcare) with a 12 channel head coil. Structural T1-weighted images were acquired in the sagittal plane (124 slices, 1.4 mm slice thickness, 0.98 × 0.98 mm2 in-phase resolution, echo time (TE) 5.1 ms, repetition time (TR) 12.2 ms, flip angle 25°) in 5 min 0.4 s of scanning time.
PET data acquisition and tasks
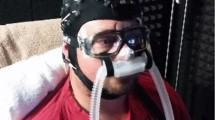
We used a brain PET system (HITS655000: Hamamatsu Photonics K.K., Hamamatsu, Japan)16. [15O]H2O was used to evaluate brain activity with the head unrestrained. Participants were fitted with masks for cough measurement (Fig. 1). The mask was connected to the previously mentioned spirometer and nebulizer.
[15O]H2O experiments consisted of four tasks: resting, VC (simple repetition of cough), IC (using CiTA) and SC (suppressing cough against stimulation by CiTA). In the VC condition, subjects were instructed to cough as much as possible within their ability. Due to the counterbalance of tasks performed, the number of coughs in the VC might vary as seen in Fig. 1C. For CiTA, a 10% solution of l-tartaric acid was used in the IC and SC tasks, and saline was used as a placebo during the resting and VC tasks. No information about task content was given during the study. These sessions were counterbalanced across the study. The route for [15O]H2O administration was secured in the right forearm. Two-minute PET scans were performed after tracer injection (list-mode data acquired every second). The task was initiated 20 s after tracer injection and continued for one minute, in which the timing of tracer entry into the brain was optimally determined during the task. The tussive agent (tartaric acid) was delivered continuously through the mask connected with the nebulizer via a nylon tube. The nebulizer was turned on for 20 s and off for 10 s for a total of two cycles to avoid subject stress due to continuous inhalation of tartaric acid (Fig. 1). We used 60 s of [15O]H2O data reconstructed after the increase in the PET count of the brain. The dose of [15O]H2O injected was 2.5 MBq/kg per scan. During the [15O]H2O experiment, the number of coughs and the intensity of coughing (cough peak flow) were measured (see the waveforms measured by spirometry in Fig. 1). At the end of each scan, the urge to cough during the task was checked on a 10-point scale using the numerical rating scale (NRS). For each task, those indices were included in the analysis.
PET data reconstruction
During the PET scans, the participants wore a head cap with four LED markers. The locations of these markers were tracked every 4 ms by two high-speed cameras (HAMAMATSU Intelligent Vision System, IVS) mounted at the back of the gantry. Using information about head movements monitored during the scans, we corrected the line of response (LOR) of list-mode data and reconstructed the image using a 3D list-mode dynamic row-action maximum likelihood algorithm (DRAMA) by applying a quaternion algorithm to variations of LED markers. The accuracy of this method was reported in detail in our previous paper using phantom studies using the quaternion formula17. The PET images were reconstructed with a matrix of 128 × 128 × 42 voxels (voxel size: 2.6 × 2.6 × 3.4 mm3). Images were corrected for attenuation and scattered events with emission-based segmentation attenuation correction (ESAC) and single scatter stimulation (SSS), respectively. For the image reconstruction of each 2-min [15O]H2O PET scan, we selected 60-s time frames that started 10 kcps after the onset of the scan. Head movements were corrected every 4 ms, whereas PET data were acquired every 1 s. Since head movements within 4 ms can be accurately corrected, it is possible that the movement and image inhomogeneity have a combined effect. However, statistical procedures involving image normalization and smoothing of images minimize such combined effects on brain mapping at the macroscopic level.
PET data analyses
We performed whole brain analyses of [15O]H2O PET data with Statistical Parametric Mapping software (SPM12; Wellcome Trust Center for Neuroimaging, London, UK) running on MATLAB software (MathWorks, Natick, MA). We realigned the reconstructed [15O]H2O PET images to the mean image of each participant. Structural images of each participant were coregistered to the mean image of the realigned [15O]H2O PET volumes. The coregistered structural images were spatially normalized to the standard brain space as defined by the Montreal Neurological Institute (MNI) using the unified segmentation algorithm with light regularization, which is a generative model that combines tissue segmentation, bias correction, and spatial normalization in the inversion of a single unified model38. After spatial normalization, the resultant deformation field was applied to the realigned [15O]H2O PET imaging data, which were resampled every 2 mm using seventh-degree B-spline interpolation. All normalized functional images were then smoothed using an isotropic Gaussian kernel of 8 mm full width at half maximum (FWHM). We assumed that the scan-to-scan variability within a PET session and the session-by-contrast interactions were approximately equal in our [15O]H2O PET measurements. We thus adopted a fixed-effect analysis with a multiparticipant and multi-condition full factorial design and included all scans of all participants into a single general linear model in SPM. Condition-specific effects were estimated with a general linear model, whereas the order of PET image acquisition, subjects’ urge to cough, and global signal intensities were used as covariates in the design matrix to account for the effect of these variables and global signal normalization. The motion parameters as well as the order of PET image acquisition was treated as a covariate so that the influence of the body motion would not be all or nothing and would be affected by time. Comparison of adjusted mean reginal cerebral blood flow between conditions was performed on a voxel-by-voxel basis with t-statistics. The resultant set of voxel values for each contrast constituted a t-statistic SPM{t}, which was then transformed to a unit normal distribution (SPM{z}) map39. The current whole brain statistical maps were thresholded at a peak level of p < 0.001 (Z value > 2.97) without correction for multiple comparisons in the cluster-level p value because of the exploratory nature of this study.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the absence of agreement from the participants but are available from the corresponding authors on reasonable request.
References
Widdicombe, J. & Fontana, G. Cough: What’s in a name?. Eur. Respir. J. 28(1), 10–15. https://doi.org/10.1183/09031936.06.00096905 (2006).
Kollarik, M. & Undem, B. J. Sensory transduction in cough-associated nerves. Respir. Physiol. Neurobiol. 152, 243–254. https://doi.org/10.1016/j.resp.2005.12.008 (2006).
Magni, C. et al. Voluntary and reflex cough: Similarities and differences. Pulm. Pharmacol. Ther. 24, 308–311. https://doi.org/10.1016/j.pupt.2011.01.007 (2011).
Hutchings, H. A., Morris, S., Eccles, R. & Jawad, M. S. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir. Med. 87(5), 379–382 (1993).
Addington, W. R., Stephens, R. E., Gilliland, K. & Rodriguez, M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch. Phys. Med. Rehabil. 80, 150–154. https://doi.org/10.1016/S0003-9993(99)90112-0 (1999).
Wakasugi, Y. et al. Usefulness of a handheld nebulizer in cough test to screen for silent aspiration. Odontology 102(1), 76–80. https://doi.org/10.1007/s10266-012-0085-y (2014).
Ohno, T. et al. Cough-inducing method using a tartaric acid nebulizer for patients with silent aspiration. Dysphagia 37(3), 629–635. https://doi.org/10.1007/s00455-021-10313-4 (2022).
Simonyan, K. et al. Functional neuroanatomy of human voluntary cough and sniff production. J. Neurosci. 37(2), 401–409. https://doi.org/10.1016/j.neuroimage.2007.05.021 (2007).
Mazzone, S. B., Cole, L. J., Egan, G. F. & Farell, M. J. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J. Neurosci. 31(8), 2948–2958. https://doi.org/10.1523/jneurosci.4597-10.2011 (2011).
Mazzone, S. B. et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 176, 327–332. https://doi.org/10.1164/rccm.200612-1856OC (2007).
Farell, M. J. et al. Functionally connected brain regions in the network activated during capsaicin inhalation. Hum. Brain Mapp. 35, 5341–5355. https://doi.org/10.1002/hbm.22554 (2014).
Chung, K. F. et al. Cough hypersensitivity and chronic cough. Nat. Rev. Dis. Primers 8, 45. https://doi.org/10.1038/s41572-022-00370-w (2022).
Bautista, T. G., Leech, J., Mazzone, S. B. & Farrell, M. J. Regional brainstem activations during capsaicin inhalation using functional magnetic resonance imaging in humans. J. Neurophysiol. 121, 1171–1182. https://doi.org/10.1152/jn.00547.2018 (2018).
Woolrich, M. W., Ripley, B. D. & Smith, S. M. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage 14(6), 1370–1386. https://doi.org/10.1006/nimg.2001.0931 (2001).
Birn, R. M., Murphy, K., Handwerker, D. A. & Bandettini, P. A. fMRI in the presence of task-correlated breathing variations. Neuroimage 47, 1092–1104. https://doi.org/10.1016/j.neuroimage.2009.05.030 (2009).
Watanabe, M. et al. Performance evaluation of a high-resolution brain PET scanner using four-layer MPPC detectors. Phys. Med. Biol. 62(17), 7148–7166. https://doi.org/10.1088/1361-6560/aa82e8 (2017).
Inubushi, T. et al. Neural correlates of head restraint: Unsolicited neuronal activation and dopamine release. Neuroimage 224, 117434. https://doi.org/10.1016/j.neuroimage.2020.117434 (2021).
Rangarathnam, B., Kamarunas, E. & McCullough, G. H. Role of cerebellum in deglutition and deglutition disorders. Cerebellum 13(6), 767–776. https://doi.org/10.1007/s12311-014-0584-1 (2014).
Musselwhite, M. N. et al. Differential effects of acute cerebellectomy on cough in spontaneously breathing cats. PLoS One 16(6), e0253060. https://doi.org/10.1371/journal.pone.0253060 (2021).
Ando, A. et al. Neural correlates of cough hypersensitivity in humans: Evidence for central sensitization and dysfunctional inhibitory control. Cough 71, 323–329. https://doi.org/10.1136/thoraxjnl-2015-207425 (2016).
Zambreanu, L. et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 114(3), 397–407. https://doi.org/10.1016/j.pain.2005.01.005 (2005).
Jansen, K. B. et al. Brain activations during pain: A neuroimaging meta-analysis of patients with pain and healthy controls. Pain 157, 1279–1286. https://doi.org/10.1097/j.pain.0000000000000517 (2016).
Uddin, L. Q. et al. Structure and function of the human insula. J. Clin. Neurophysiol. 34(4), 300–306. https://doi.org/10.1097/wnp.0000000000000377 (2017).
Barnes, P. J. The problem of cough and development of novel antitussives. Pulm. Pharmacol. Ther. 20, 416–422. https://doi.org/10.1016/j.pupt.2006.11.001 (2007).
Canning, B. J. Central regulation of the cough reflex: Therapeutic implications. Pulm. Pharmacol. Ther. 22, 75–81. https://doi.org/10.1016/j.pupt.2009.01.003 (2009).
Mutolo, D. Brainstem mechanisms underlying the cough reflex and its regulation. Respir. Physiol. Neurobiol. 243, 60–76. https://doi.org/10.1016/j.resp.2017.05.008 (2017).
O’Neill, J., McMahon, S. B. & Undem, B. J. Chronic cough and pain: Janus faces in sensory neurobiology?. Pulm. Pharmacol. Ther. 26, 476–485. https://doi.org/10.1016/j.pupt.2013.06.010 (2013).
Miller, A. D. & Yates, B. J. Evaluation of role of upper cervical inspiratory neurons in respiration, emesis and cough. Brain Res. 606(1), 143–147. https://doi.org/10.1016/0006-8993(93)91582-D (1993).
Widdicombe, J. G. Afferent receptors in the airways and cough. Respir. Physiol. 114(1), 5–15. https://doi.org/10.1016/S0034-5687(98)00076-0 (1998).
Mazzone, S. B. & Undem, B. J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96, 975–1024. https://doi.org/10.1152/physrev.00039.2015 (2016).
Nassenstin, C. et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J. Physiol. 588(Pt 23), 4769–4783. https://doi.org/10.1113/jphysiol.2010.195339 (2010).
McGovern, A. E., Davis-Poynter, N., Farrell, M. J. & Mazzone, S. B. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. J. Neurosci. 207, 148–166. https://doi.org/10.1016/j.neuroscience.2012.01.029 (2012).
McGovern, A. E. et al. Evidence for multiple sensory circuits in the brain arising from the respiratory system: An anterograde viral tract tracing study in rodents. Brain Struct. Funct. 220, 3683–3699. https://doi.org/10.1007/s00429-014-0883-9 (2015).
Canning, B. J. et al. Identification of the tracheal and laryngeal afferent neurons mediating cough in anaesthetized guinea-pigs. J. Physiol. 557(2), 543–558. https://doi.org/10.1113/jphysiol.2003.057885 (2004).
Kim, S. H. et al. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5-HT3, and Tac1 expression. eNeuro https://doi.org/10.1523/ENEURO.0494-19.2020 (2020).
Ando, A., Mazzone, S. B. & Farrell, M. J. Altered neural activity in brain cough suppression networks in cigarette smokers. Eur. Respir. J. 54, 1900362. https://doi.org/10.1183/13993003.00362-2019 (2019).
Ashhad, S., Kam, K., Del Negro, C. A. & Feldman, J. L. Breathing rhythm and pattern and their influence on emotion. Annu. Rev. Neurosci. 45, 223–247. https://doi.org/10.1146/annurev-neuro-090121-014424 (2022).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851. https://doi.org/10.1183/13993003.00362-2019 (2005).
Friston, K. J. et al. Statistical parametric mapping in functional imaging: A general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Acknowledgements
We would like to thank Messrs. Hideki Kaiya of SHI Accelerator Service, Takeki Nagamine and Shuya Kato of Hamamatsu City Rehabilitation Hospital and staff of Promotion Center for Medical Collaboration and Intellectual Property at Hamamatsu University School of Medicine for their technical and operational support. This study was supported by HUSM Grant-in-Aid, and partly by a research grant from the JSPS KAKENHI (Grant Number: JP16H06402 [Willdynamics] to Ya.O.).
Author information
Authors and Affiliations
Contributions
T.S., T.O., T.S., Y.T., and Y.O. conceived and designed the present study. T.S., Y.O., S.H., Y.O., R.T., T.K., and E.Y. acquired the data. C.S., and Y.M. radiosynthesized [15O] H2O. T.S., T.I., T.O., T.I., and Y.O. analyzed the data. T.S. and Y.O. drafted the manuscript. I.F. and Y.O. supervised the project and oversaw quality control. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
T.I., Yu.O., T.I., and E.Y. are research employees of Hamamatsu Photonics K.K.; The company had no control over the interpretation, writing, or publication of this work. Remaining authors has no competing interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sugi, T., Inubushi, T., Ohno, T. et al. Neural substrates of cough control during coughing. Sci Rep 14, 758 (2024). https://doi.org/10.1038/s41598-024-51477-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51477-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.