Abstract
In 70 patients with KIT D816V positive systemic mastocytosis (SM) including 36 patients with advanced SM (AdvSM), we correlated the extent of reported mucosal mast cell ([m]MC) infiltration of the upper and/or lower gastrointestinal tract (UGIT, n = 63; LGIT, n = 64; both, n = 57) with symptoms and markers of MC burden/subtype. GI symptoms were reported by all patients (mean 2.1 number of symptoms). A strong mMC infiltration was identified in 24 patients (UGIT, 17/63, 27%; LGIT, 19/64, 30%). Concurrent involvement of UGIT and LGIT (n = 12) correlated with female gender (75%) and a higher symptom burden (mean 2.7) but not with MC burden or subtype. Significant differences between non-AdvSM and AdvSM were reported regarding food intolerance (54% vs. 17%), cramping (54% vs. 22%) and weight loss (0% vs. 64%). KIT D816V was identified in 54/56 (96%) available biopsies. In 46 patients, digital PCR revealed a correlation with low albumin levels (r = − 0.270, P = 0.069) and the KIT D816V VAF in peripheral blood (r = 0.317, P = 0.036) but not with the extent of mMC infiltration or markers of MC burden/subtype. Although MC mediator triggered GI symptoms have a substantial impact on the quality of life, correlation to objective disease parameters is lacking thus making its systematic assessment challenging.
Similar content being viewed by others
Introduction
Systemic mastocytosis (SM) is a rare myeloid neoplasm characterized by variable infiltration and multifocal accumulation of neoplastic mast cells (MC) in bone marrow (BM), skin and visceral organ systems1,2,3,4,5. According to the International Consensus Classification (ICC)/World Health Organization Classification (WHO-5), the major diagnostic criterion for SM is the presence of MC aggregates (defined as 15 or more MCs) in BM or other extracutaneous organs, including the gastrointestinal tract (GIT)4,5. Minor diagnostic criteria include co-expression of CD25/CD2/CD30 by neoplastic MCs, 25% of MCs with a spindle-shaped or atypical morphology, the presence of an activating point mutation at codon 816 of KIT (in ≥ 90% KIT D816V, driver mutation), and a serum total tryptase > 20 ng/mL (ICC: in absence of a myeloid neoplasm; WHO-5: adjusted in case of hereditary alpha-tryptasemia). Advanced SM (AdvSM) comprises the subtypes aggressive SM (ASM), SM with an associated myeloid neoplasm (SM-AMN), and MC leukemia (MCL)4. Indolent phases of the disease include indolent SM (ISM), bone marrow mastocytosis (BMM; low BM MC infiltration and tryptase and absence of cutaneous involvement) and smoldering SM (BM MC infiltration > 30% and serum tryptase > 200 µg/L)1,5,6.
MC mediator release, e.g. through histamines, leukotrienes, and organ infiltration lead to manifold symptoms including life-threatening complications7,8. GI symptoms are present in up to 50–70% of SM patients and include, but are not limited to food intolerance, nausea, emesis, cramping, and diarrhea, ultimately causing malabsorption/weight loss representing a C-finding for diagnosis of ASM1,9,10,11,12. Although GI involvement constitutes a fundamental factor for morbidity and quality of life, only little is known about its association with the overall clinical, morphological and genetic features.
We therefore sought to investigate the presence and extent of reported gastrointestinal mucosal MC infiltration and analyze the correlation with symptoms, markers of disease burden and subtype in 70 SM patients.
Methods
Patients
Initially, a total of 246 KIT D816V positive SM patients with any signs of GI symptoms (presence or absence of food intolerance, nausea/emesis, cramping, diarrhea, weight loss > 10%) were identified in the ‘German Registry on Disorders of Eosinophils and Mast Cells’ (GREM). For further analysis, the mean number of symptoms per patient was assessed and correlated to laboratory and histopathological findings. Diagnosis and subtyping of SM were carried out according to the ICC criteria4. The study design adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of the Medical Faculty of Mannheim, Heidelberg University (Heidelberg, Germany). All patients gave written informed consent.
Evaluation of biopsies
Only patients with a histopathological documentation of their GI biopsies were enrolled in this study. The median number of biopsies conducted per patient was 11 (range 3–26). For descriptive analyses, we semi-quantified the extent of MC infiltration in GI biopsies by categorizing them into “strong” MC infiltration (if histologic reports described either compact or diffuse dense infiltrates) versus “minor” MC infiltration (diffuse scattered infiltrates described in the reports). A diagnostic work-up of biopsies was considered to be complete, if a full immunohistochemical staining including KIT, CD25 and MC tryptase was performed and the MC count per high-power field or percentage of MC infiltration in relation to the evaluated area were indicated. Evaluations were carried out at local site or by reference pathologists from the European Competence Network on Mastocytosis (ECNM; H.-P. Horny and K. Sotlar). All BM biopsies and BM smears were evaluated by the ECNM reference pathologists.
Chip-based digital PCR
Measurements of the KIT D816V VAF on DNA, derived from peripheral blood (PB) and biopsies, were performed using the QuantStudio™ three-dimensional (3D) dPCR system in combination with the Applied Biosystems ProFlex PCR System (ThermoFisher Scientific, Waltham, MA, USA). Per sample, a 15 µL reaction volume was prepared. The volume included 7.1 µL of 10 ng/µL DNA, 7.5 µL of QuantStudio™ 3D Digital PCR Master Mix v2 (ThermoFisher Scientific, Waltham, MA, USA) and 0.4 µL of KIT D816V specific Taqman gene expression assay (ID: Hs000000039_rm, ThermoFisher Scientific Waltham, MA, USA).
Statistics
Clinical, laboratory, morphological and molecular data were collected at time of diagnosis. The Mann–Whitney U-test was used to compare continuous variables and medians of distributions. For categorical variables, Fisher’s exact test was carried out. The Spearman rank correlation was applied as a nonparametric measure of rank correlation. P values of < 0.05 (two-sided) were considered statistically significant. Data management and statistical analyses were performed with SPSS (SPSS version 20.0; IBM Corporation, Armonk, NY, USA) and GraphPad Prism software (version 8, GraphPad, La Jolla, CA, USA).
Results
Patients and GI infiltration pattern
We identified 70 patients with KIT D816V positive SM (ISM, n = 26, 37%; SSM, n = 8, 11%; ASM, n = 6, 9%; SM-AHN, n = 20, 29%; MCL ± AHN, n = 10, 14%), GI symptoms and reported mucosal MC infiltration of the upper (UGIT, n = 63) and/or lower GIT (LGIT, n = 64; Fig. 1). UGIT infiltration was observed in 19/30 (63%) ISM/SSM and 25/33 (76%) AdvSM patients. Stomach (ISM/SSM, 11/30, 37%; AdvSM 17/33, 52%) and duodenum (ISM/SSM, 16/30, 53%; AdvSM 24/33, 73%) were frequently involved, while no esophageal infiltration was not reported in any subtype. LGIT infiltration was prominent in both ISM/SSM (31/33, 97%) and AdvSM (29/31, 97%) patients and mostly affected the terminal ileum (ISM/SSM, 29/33, 88%; AdvSM 29/31, 94%) while colon involvement was observed in 16/33 (48%) ISM/SSM and 20/31 (65%) AdvSM patients (Table 1).
Patient flowchart. AdvSM advanced systemic mastocytosis, ASM aggressive systemic mastocytosis, GI gastrointestinal, GREM German Registry on Disorders of Eosinophils and Mast Cells, ISM indolent systemic mastocytosis, MCL ± AHN mast cell leukemia with/without an associated hematologic neoplasm, SM systemic mastocytosis, SM-AHN systemic mastocytosis with an associated hematologic neoplasm, SSM smoldering systemic mastocytosis.
GI symptoms
Symptoms were reported by all patients with a mean number of 2.1 symptoms (range 1–5) per patient: food intolerance (25/70, 36%), nausea/emesis (20/70, 29%), cramping (25/70, 36%), diarrhea (57/70, 81%) and weight loss (23/70, 33%). There was no statistically significant association between GI symptoms and any particular location of GI involvement (P > 0.500). The comparisons of symptoms in relation to minor vs. strong mucosal infiltration, ISM vs. AdvSM and various levels of KIT D816V VAF are summarized in Tables 2, 3 and 4.
Histological and immunohistochemical evaluation
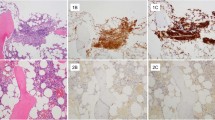
A complete immunohistochemical staining (tryptase, CD117, CD25) was performed in 76/127 biopsies (UGIT, 35/63, 56%; LGIT, 41/64, 64%). An exact quantitative assessment of MC density (MC count per high-power field or percentage of MC infiltration) was available for 18/127 (14%) biopsies only (Table 5).
Compact infiltrates were defined according to the ICC classification4, either being micronodular or band-like in a subepithelial position. Band-like infiltrates were present in the mucosal layer of the GI tract in the patients with positive biopsies.
Diffuse MC infiltrates usually showed the following features:
-
1.
A significant increase in MCs throughout the lamina propria mucosae, > 25% of them with a spindle-shaped appearance.
-
2.
A reduced expression of tryptase but (usually weak) CD25 (or CD 30).
Because of the frequent occurrence of numerous intermingled eosinophils, scattered diffuse infiltrates were difficult to identify without immunohistochemistry.
Severity of GI involvement
A strong MC infiltration (compact or diffuse dense infiltrates) was identified in 24 patients (UGIT, 17/63, 27%; LGIT, 19/64, 30%). It was concurrently observed in UGIT and LGIT in 12/57 (21%) patients of which 5 and 7 patients had non-AdvSM (ISM, n = 4; SSM, n = 1) or AdvSM, respectively (Table 6). If present at one site, the probability to also detect it at the other site was 71% and 63%, respectively (Table 5). The concurrent strong MC infiltration was associated with female gender (9/12, 75%) and a higher number of symptoms (mean 2.7).
Qualitative and quantitative (chip-based) PCR for KIT D816V
Qualitative PCR on DNA extracted and purified from UGIT and/or LGIT GI biopsies was performed for 56/70 (80%) patients of which 54/56 (96%) were KIT D816V positive (Table 5). Chip-based quantitative dPCR was carried out for 46 patients. Neither the median KIT D816V VAF nor the grouping of the KIT D816V VAF (< 5% [n = 18, 39%]), 5–10% [n = 9, 20%], > 10% [n = 19, 41%]) indicated an association with symptoms (Table 4), mucosal MC infiltration (strong vs. minor, P = 0.806; Fig. 2B), parameters of MC burden (Table 7) or subtype (non-AdvSM vs. AdvSM, P = 0.501; Fig. 2A). Spearman correlations revealed a positive correlation of the KIT D816V VAF from GI biopsies with the KIT D816V VAF in PB (ρ = 0.317, P = 0.036) and a negative correlation with the albumin level (ρ = − 0.270, P = 0.069) (Table 8).
Discussion
Beside hepatic MC infiltration with impaired liver function and splenomegaly, mucosal MC infiltration of the GI tract mediates a heterogeneous clinical scenario of mild to severe GI symptoms including food intolerance, nausea, vomiting, abdominal cramping, diarrhea and anaphylaxis. Most severe cases are characterized by malabsorption and significant weight loss > 10% which are established C-findings. Several studies have reported a highly variable GI symptom burden in up to 50–70% of SM patients9,10,11,12. However, the real frequency may be underestimated, because many patients do not undergo an adequate diagnostic work-up on basis of a multi-disciplinary approach. Causes for this may include non-performance of endoscopy, non-detection of microscopic MC infiltration in macroscopically non-suspicious mucosa or the misdiagnosis of eosinophilic colitis due to the inadequate use of immunohistochemistry9,10,13,14,15,16.
In our series, diarrhea was the most commonly reported subtype-independent symptom, while weight loss was most frequently observed in AdvSM and food intolerance and abdominal cramping in ISM/SSM. A concurrent strong mucosal MC infiltration in UGIT and LGIT was identified in 21% of patients and in either UGIT or LGIT in further 13% of patients. A strong mucosal MC infiltration in one or both regions was independent of symptoms, markers of MC burden or subtype. In fact, 42% of those patients had ISM/SSM highlighting a discordant quantitative MC infiltration of BM and GI. These findings imply considerations at which level of mucosal MC infiltration and associated severe symptoms, e.g. life-threatening anaphylaxis, purely symptomatic treatment with antihistamines, MC stabilizers and local/systemic corticosteroids should be complemented by targeted treatment with KIT inhibitors in carefully selected patients even in the absence of a formal diagnosis of AdvSM17,18,19,20.
We also sought to assess to which extent the qualitative or quantitative measurement of the KIT D816V VAF in GI biopsies through dPCR may provide additional useful information. VAF levels were not correlated with the extent of MC infiltration or markers of disease burden. There was only a moderate correlation with low albumin levels and the KIT D816V VAF in PB with the KIT D816V VAF in GI biopsies, the latter even suggesting a potential mix of GI tissue and blood thus highlighting obvious flaws of the techniques applied. Therefore, as opposed to its undisputed diagnostic, prognostic and even predictive value in PB21,22,23,24,25, a routine implementation of a quantitative KIT D816V VAF testing in GI tract biopsies seems not to have an additional benefit in the diagnostic work up of SM-related GI involvement. However, the histological staining with tryptase, CD117 and CD25 as well as the presence of compact MC infiltrates are of utmost relevance in discriminating between reactive and neoplastic conditions, as MCs are usually also found in normal GI tract mucosa and can be markedly elevated in patients with inflammatory bowel disease. Even a positive staining for CD25 does not always allow a conclusive diagnosis of minor GI SM involvement as it also stains a subpopulation of background lymphocytes in other conditions. It may therefore be of limited value in the discrimination between low GI infiltration and reactive MC increase26. Earlier studies on the clinical correlation of GI tract infiltration and clinical symptoms also could not identify a correlation of clinical symptoms with the pattern and degree of MC infiltration indicating that the MC mediator release rather than the direct infiltration of the GI tract by MCs is responsible for the clinical symptoms9,10. Furthermore, the assessment and quantification of MC infiltration in GI samples is to some extent arbitrary and subject to a high degree of interindividual variation. MC thresholds for the different sections of the GI tract like the ones for eosinophils in hypereosinophilic conditions do not exist given the limitations of MC assessment as outlined above. In patients with unexplained GI symptoms, exclusion of an underlying SM should therefore not only be performed by endoscopy but should also include a serum tryptase screening and a BM analysis at least in patients with elevated levels. If SM diagnosis is excluded, patients with unexplained high levels of serum tryptase and GI symptoms should be evaluated for hereditary alpha-tryptasemia (HαT)27. Diagnosis of HαT and SM was concurrently seen in 2/2 patients tested within our population. Both patients had high objective disease parameters as well as a high disease burden, showing that HαT positivity is not restricted to SM negative cases but otherwise no further conclusions can be drawn given the small number of analyzed samples.
Limitations
This study focused solely on the mere presence or absence of the five symptoms outlined in the methodology, omitting consideration of additional symptoms potentially associated with SM such as constipation, bloating, flatulence, feeling of fullness, loss of appetite, dysphagia, or gastrointestinal bleeding. Consequently, the selected symptoms in this study may not comprehensively capture the spectrum of GI manifestations in SM, potentially leading to the oversight of patients experiencing more severe gastrointestinal symptoms. Individual symptoms were not quantitatively assessed, precluding a more comprehensive analysis upon the association of symptom and GI disease burden. In terms of the techniques employed, we observed a moderate correlation between the KIT D816V VAF in PB and GI biopsies, suggesting the possibility of a mixture of GI tissue and blood and methodically flaws. Additionally, the evaluation and quantification of MC infiltration in GI samples exhibit a degree of subjectivity and substantial interindividual variation as outlined above. Taken together, these findings underline the difficulties in assessment of GI tract involvement of SM. A multidisciplinary approach in SM patients with suspected GI involvement seems prudent. Whereas the assessment of GI involvement can be important to establish a diagnosis of SM, due to the rate of false positive results and possible misinterpretations by less experienced pathologists, undirected diagnostics with multiple stainings in patients without otherwise proven SM should be avoided. Histopathological assessment of the BM should therefore remain the diagnostic mainstay for detection of the disease in patients with suspected SM diagnosis.
Conclusions
Although GI manifestations in SM patients are highly prevalent and often disabling, clinical symptoms do not correlate with histologic findings of disease course or disease subtyping. MC mediator related diarrhea and malabsorption remain challenging to quantify but might be directly related to suboptimal absorption and bioavailability (pre-systemic elimination) of drug therapies in patients with SM and should therefore be taken into account.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Valent, P. et al. Updated diagnostic criteria and classification of mast cell disorders: A consensus proposal. Hemasphere 5(11), e646 (2021).
Valent, P., Akin, C. & Metcalfe, D. D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 129(11), 1420–1427 (2017).
Valent, P. et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leukemia Res. 25(7), 603–625 (2001).
Arber, D. A. et al. International Consensus Classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood 140(11), 1200–1228 (2022).
Khoury, J. D. et al. The 5th edition of the World Health Organization Classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 36(7), 1703–1719 (2022).
Zanotti, R. et al. Refined diagnostic criteria for bone marrow mastocytosis: A proposal of the European competence network on mastocytosis. Leukemia 36(2), 516–524 (2022).
Reiter, A., George, T. I. & Gotlib, J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 135(16), 1365–1376 (2020).
Pardanani, A. Systemic mastocytosis in adults: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 96(4), 508–525 (2021).
Sokol, H. et al. Gastrointestinal manifestations in mastocytosis: A study of 83 patients. J. Allergy Clin. Immunol. 132(4), 866–873 (2013).
Doyle, L. A. et al. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am. J. Surg. Pathol. 38(6), 832–843 (2014).
Kirsch, R. et al. Systemic mastocytosis involving the gastrointestinal tract: Clinicopathologic and molecular study of five cases. Mod. Pathol. 21(12), 1508–1516 (2008).
Siegert, S. I., Diebold, J., Ludolph-Hauser, D. & Löhrs, U. Are gastrointestinal mucosal mast cells increased in patients with systemic mastocytosis? Am. J. Clin. Pathol. 122(4), 560–565 (2004).
Nanagas, V. C. & Kovalszki, A. Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: A comprehensive review. Clin. Rev. Allergy Immunol. 57(2), 194–212 (2019).
Zanelli, M. et al. Gastrointestinal mastocytosis: A potential diagnostic pitfall to be aware. Int. J. Surg. Pathol. 27(6), 643–646 (2019).
Schwaab, J. et al. Importance of adequate diagnostic work-up for correct diagnosis of advanced systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 8(9), 3121–3127 (2020).
Jawhar, M. et al. Impact of centralized evaluation of bone marrow histology in systemic mastocytosis. Eur. J. Clin. Investig. 46(5), 392–397 (2016).
Akin, C., Arock, M. & Valent, P. Tyrosine kinase inhibitors for the treatment of indolent systemic mastocytosis: Are we there yet? J. Allergy Clin. Immunol. 149(6), 1912–1918 (2022).
Valent, P. et al. Personalized management strategies in mast cell disorders: ECNM-AIM user’s guide for daily clinical practice. J. Allergy Clin. Immunol. Pract. 10(8), 1999–2012 (2022).
Lübke, J. et al. Superior efficacy of midostaurin over cladribine in advanced systemic mastocytosis: A registry-based analysis. J. Clin. Oncol. 40(16), 1783–1794 (2022).
Lübke, J. et al. Inhibitory effects of midostaurin and avapritinib on myeloid progenitors derived from patients with KIT D816V positive advanced systemic mastocytosis. Leukemia 33(5), 1195–1205 (2019).
Erben, P. et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann. Hematol. 93(1), 81–88 (2014).
Naumann, N. et al. Adverse prognostic impact of the KIT D816V transcriptional activity in advanced systemic mastocytosis. Int. J. Mol. Sci. 22(5), 2562 (2021).
Hoermann, G. et al. Standards of genetic testing in the diagnosis and prognostication of systemic mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J. Allergy Clin. Immunol. Pract. 10(8), 1953–1963 (2022).
Arock, M. et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 29(6), 1223–1232 (2015).
Kristensen, T., Vestergaard, H., Bindslev-Jensen, C., Moller, M. B. & Broesby-Olsen, S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am. J. Hematol. 89(5), 493–498 (2014).
Shivji, S., Conner, J. R. & Kirsch, R. Mast cell evaluation in gastrointestinal biopsies: Should we be counting? A critical review and practical guide for the surgical pathologist. Histopathology 82, 960 (2023).
Giannetti, M. P. et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation-related symptomatology including anaphylaxis. Ann. Allergy Asthma Immunol. 126(6), 655–660 (2021).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.L., N.N. and O.H. have assessed and verified the data. J.L., N.N., O.H., A.R. and J.S. contributed to concept and design. J.L., N.N., O.H., A.R. and J.S. were involved in acquisition of data. J.L., N.N. and O.H. contributed to statistical analysis. J.L., N.N., O.H., A.R. and J.S. were involved in interpretation of data. J.L., A.R. and J.S. were involved in manuscript writing. All authors contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. All authors are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lübke, J., Naumann, N., Hoffmann, O. et al. A clinical, morphological and molecular study of 70 patients with gastrointestinal involvement in systemic mastocytosis. Sci Rep 14, 702 (2024). https://doi.org/10.1038/s41598-023-49749-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49749-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.