Abstract
Thiamine (vitamin B1) is required by all living organisms in multiple metabolic pathways. It is scarce in natural systems, and deficiency can lead to reproductive failure, neurological issues, and death. One major cause of thiamine deficiency is an overreliance on diet items containing the enzyme thiaminase. Thiaminase activity has been noted in many prey fishes and linked to cohort failure in salmonid predators that eat prey fish with thiaminase activity, yet it is generally unknown whether evolutionary history, fish traits, and/or environmental conditions lead to production of thiaminase. We conducted literature and GenBank BLAST sequence searches to collect thiaminase activity data and sequence homology data in expressed protein sequences for 300 freshwater and marine fishes. We then tested whether presence or absence of thiaminase could be predicted by evolutionary relationships, trophic level, omega-3 fatty acid concentrations, habitat, climate, invasive potential, and body size. There was no evolutionary relationship with thiaminase activity. It first appears in Class Actinoptergyii (bony ray-finned fishes) and is present across the entire Actinoptergyii phylogeny in both primitive and derived fish orders. Instead, ecological factors explained the most variation in thiaminase: fishes were more likely to express thiaminase if they fed closer to the base of the food web, were high in polyunsaturated fatty acids, lived in freshwater, and were from tropical climates. These data provide a foundation for understanding sources of thiaminase leading to thiamine deficiency in fisheries and other organisms, including humans that eat uncooked fish.
Similar content being viewed by others
Introduction
Thiamine (vitamin B1) is an essential cofactor in multiple enzyme complexes required for metabolism of carbohydrates and amino acids1. Yet despite being necessary for all life, animals cannot synthesize thiamine de novo, and so the majority must obtain it through diet or direct uptake in the case of fry2,3,4,5. Biological thiamine synthesis is energetically expensive and complicated6, 7. Thiamine in aquatic systems is present at extremely low (picomolar) concentrations; spatially heterogenous; degrades rapidly in the presence of UV8, alkaline conditions9, and high temperatures10; and tends to be rapidly taken up after synthesis11,12,13. Furthermore, having too little thiamine leads to a suite of cardiovascular and neurological issues in humans3, foxes14, fishes15, and other wildlife16. Although some effects of thiamine deficiency are reversible, early life deficiency can cause death3, 17, and permanent brain damage has been documented in humans18. The long-term effects of temporary or intermittent thiamine deficiency in fishes17 and the reasons thiamine deficiency complex is showing up more in wild populations19 remain poorly understood, in part because thiamine supplementation is inexpensive and easily applied in managed populations.
There are two demonstrated mechanisms for aquatic animals to become thiamine deficient: through a diet that lacks enough thiamine16 (e.g., due to poor absorption or lacking nutrients) or through eating something that destroys thiamine before it can be absorbed17, 20, 21. Previous research has hypothesized that a diet of lipid-rich prey can lead to thiamine deficiency in fish predators, but these correlative studies have not considered if lipid-rich forage fish also contain thiaminase22. Early life stage mortality and sublethal effects in salmonids is linked with low egg thiamine concentrations caused by elevated thiaminolytic enzymes (i.e., thiaminase) present in the maternal diet21, 23, 24. Fishes differ drastically in their thiaminase activity25,26,27, but the sources and reasons for thiaminase production are relatively unknown. Bacteria can use thiaminase as a salvage pathway for thiamine biosynthesis28, and originally thiaminase activity in forage fishes was thought to be linked to thiaminase-producing bacteria such as Paenibacillus thiaminolyticus that had been isolated from Alewife (Alosa pseudoharengus)29. However, later research revealed no relationship between thiaminase activity and either the amount of P. thiaminolyticus thiaminase I protein or the abundance of P. thiaminolyticus cells30. More recently, Richter et al.31 provided evidence for de novo production of thiaminase I by fish (Zebrafish, Danio rerio) and identified the genetic basis for thiaminase production in fishes.
The question remains why fishes produce thiaminase if it destroys an essential nutrient that fish cannot synthesize? It is highly unlikely that thiaminase production is a prey response to limit predators for two reasons: (1) predators develop TDC when a large portion of their diet has thiaminase, but it can take years21; and (2) this feedback loop is too slow to benefit prey producing thiaminase who might have two or three generations until predator populations begin to experience reproductive failure. One possible explanation of thiaminase production is the result of strong selective pressure as a method to partially resynthesize thiamine6. Fish that express thiaminase activity are not themselves deficient in thiamine26, suggesting that mechanisms exist to partition thiaminase activity from thiamine within the tissues of fish that express thiaminase. However, the mechanisms, efficiency, and energetic requirements of thiaminase partitioning have not been elucidated. Thiaminase activity has been found to increase with disease challenges32 and with diet quality and stress33. However, not all fishes produce thiaminase, and thiaminase activity levels are highly variable26, 33, 34. The evolutionary history of thiaminase production in fishes is also unknown. Thiaminase may be more common in primitive than derived North American freshwater fishes34, 35, but these studies did not consider marine species.
We sought to explore whether evolutionary and ecological characteristics could explain thiaminase presence in fishes. Specifically, we evaluated: (1) if phylogenetic relationships would predict thiaminase presence or activity, consistent with previous work on North American freshwater fishes34, 35; and (2) if ecological or physiological characteristics of trophic level, habitat use during foraging, salinity tolerance, or lipid content predicted thiaminase presence or activity in fishes. Associations of thiaminase with these ecological or physiological factors would aid in evaluation of risk for thiaminase-induced TDC in piscivorous fishes, wildlife, and humans.
Methods
Data collection
Data on thiaminase I activity of 300 fishes were compiled from existing literature25,26,27, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 and a GenBank search for protein sequences coded by expressed transcripts with significant homology to Zebrafish (Danio rerio) thiaminase I (Richter et al. 2023; GenBank accession number NP_001314821.1). The thiaminase I enzyme of Zebrafish is homologous to a candidate thiaminase gene identified in Alewife. The empirically derived mass and isoelectric point of the thiaminase I activity extracted from Alewife tissues exactly matched that predicted for the candidate Alewife thiaminase I gene31. We limited the search to fish species with at least 10,000 predicted protein sequences in GenBank. We conducted a protein BLAST sequence search for each of the species against the Zebrafish thiaminase predicted protein sequence31. Fishes were scored thiaminase positive if they had an expressed predicted protein sequence with at least 35% sequence identity56 to Zebrafish thiaminase and contained the predicted active site cysteine (C153 in NP_001314821.1).
The BLAST search and literature agreed for 23 fishes where both genetic and literature thiaminase data were available. There were few disagreements. Sea Lamprey (Petromyzon marinus) were categorized as thiaminase positive in the literature36 but negative in BLAST. Since Boggs et al. (2019) suggested little to no thiaminase activity for another lamprey species and the BLAST data are more comprehensive, we categorized them as negative. Ninespine Stickleback (Pungitius pungitius) was listed as thiaminase positive in Riley and Evans35 based on activity of 85 ± 60 pmol/g/min26. However, the BLAST data indicated it was thiaminase negative and the high standard deviation in measurements suggested many low values. Furthermore, a more recent study showed very little thiaminase activity57. Thus, we categorized the Ninespine Stickleback as thiaminase negative. Lake Whitefish (Coregonus clupeaformis) was categorized as thiaminase positive based on Deutsch and Hasler58 but was BLAST negative. Since we have lower confidence in the sole literature value, we categorized Lake Whitefish as negative. Bowfin (Amia calva) was positive in two studies36, 46 but also listed as both positive and negative36. Thiaminase analysis in these studies was conducted on whole-body Bowfin homogenates, and it is possible that positive results may have resulted from analyzing Bowfin that contained thiaminase-rich prey in their guts, so they were eliminated from analysis. European Perch (Perca fluviatilis) was positive in one study48 but negative in BLAST. We put higher trust in the BLAST data since published work48 only reports positive or negative activity and did not report a range of measured values. All entries were checked closely by two separate people for completeness and accuracy.
We obtained family, order, and ecological data from fishbase.org59 for all species included in our final thiaminase database. Data on maximum total length (cm), trophic level estimate, and median Omega-3 concentration were treated as continuous variables. A species’ invasive ability (i.e., documented negative ecological impacts in areas where they are introduced or labeled as potential pest species on Fishbase59), climate range (i.e., polar, boreal, temperate, subtropical, tropical, deep-water), habitat (benthic, benthopelagic, or pelagic), and whether a fish spends the majority of its life in marine or freshwater environments were treated as categorical variables.
Data analysis
We analyzed all data in R v4.1.260. We tested for phylogenetic relationships among fish families and presence/absence of thiaminase using a published fish phylogeny including the Classes Sarcopterygii, Chondrichthyes, and Actinopterygii61, and among orders using a phylogeny of ray-finned fishes of Class Actinopterygii only62. We used the R package ape63 to prune the tree to fish orders/families where we had data using the ‘drop.tip’ function. We then used ‘make.simmap’ in the phytools package64 to fit continuous-time reversible Markov models to estimate the evolution of thiaminase at each node for 500 simulations. The models assumed equal (0.5/0.5) root node prior probabilities of presence or absence of thiaminase conditioned on the published fish phylogenies. We used these simulations to estimate the probability that an ancestral state/root node had thiaminase, represented as pie charts at each node.
To explore the ecological determinants of thiaminase activity we used Bayesian binomial models with a logit-link function in the rstanarm65 R package. We used weakly informative priors with a mean of zero and standard deviation of 2.5. We ran each Bayesian model for 10,000 iterations and discarded the first half as a warm-up to obtain 20,000 simulations for analysis. We confirmed convergence using Gelman–Rubin statistic (R̂ < 1.01)66 and by examining trace plots. None of the models had influential outliers as assessed by leave-one-out cross-validation (“loo”) in the rstan package67. We report the coefficients as the mean and the 95% credible interval (95% CRI), which is the range of values for posterior samples. We computed Bayesian R2 for the regression models (i.e., multiple regression, trophic level, and omega-3 models) to explore the proportion of variance explained by the models68.
Results
Our species pool included 300 fishes that were tested for thiaminase activity or were searched for thiaminase proteins in their expressed sequence libraries. Of these, less than half (n = 119) had thiaminase. These species represent a broad range of sizes, climates, and habitats (Supplementary Table S1), with representation from 124 families, 56 orders, and 3 classes of fishes. Despite having broad evolutionary representation, we found no evidence of an evolutionary pattern in thiaminase of fishes (Fig. 1, Supplementary Fig. S1). We found no evidence for thiaminase production in more primitive fishes like Coelacanth (Class Sarcopterygii), lampreys (Class Hyperoartia), or cartilaginous fishes like sharks, rays, and skates (Class Chondrichthyes; Supplementary Fig. S1). Thiaminase first appears in the most primitive ray-finned fishes (Class Actinopterygii) such as the Bichir (Order Polypteriformes), Mississippi Paddlefish (Order Acipenseriformes), and Spotted Gar (Order Lepisosteiformes). Thiaminase is distributed across the entire Actinopterygii phylogeny to more derived orders such as the live-bearing fishes (Order Cyrinidontiformes; Fig. 1). The Markov model simulations indicated equal probability of thiaminase across all nodes of the phylogeny (Fig. 1, Supplementary Fig. S1). Ecological factors explained nearly 40% of the variation in thiaminase presence or absence (Bayesian R2 = 0.36, Fig. 2). Trophic level (bTL = − 0.88, 95% CRI [− 1.72, 0.11]; Supplementary Table S2), and association with marine environments (bmarine = − 2.01, 95% CRI [− 3.07, − 1.12]) were negatively related to thiaminase presence in fishes (Fig. 2). Habitat (benthic, benthopelagic, or pelagic), invasive potential, and size (as maximum total length) were not predictors of thiaminase (Fig. 2).
Presence (black) or absence (white) of thiaminase across 42 fish orders within Actinoptergyii that overlap between our data and Rabosky, et al.62. If at least one species within the order had evidence of thiaminase activity, we coded it as having thiaminase present. Branch lengths indicate time since evolution such that longer branches show orders that evolved longer ago. The pie chart at each node is the probability of the ancestral state having thiaminase based on 500 Monte Carlo simulations. Silhouettes represent common body forms within each order (downloaded from phylopic.org; all images public domain/creative commons).
Results from the Bayesian multiple regression of ecological variables vs. thiaminase presence/absence. Trophic level, omega-3, and maximum length (cm) are continuous variables, and the others are categorical (yes = 1, no = 0). The dotted line shows 0, so posterior histograms that do not overlap zero have good evidence of being related to thiaminase (e.g., if most of the posterior is positive, this suggests it is related to thiaminase presence). The 95% credible interval within each histogram is shaded and the median is represented as vertical solid line. Overall, this model explained 36% of the variation in the data.
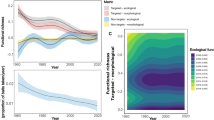
Probability of thiaminase production decreased as trophic level increased, meaning that lower trophic levels were more likely than top predators to have thiaminase, and the trophic level model alone explained 10% of the variation in the data (Bayesian R2 = 0.10, Fig. 3a). Marine species were less likely to have thiaminase (Fig. 3c); only 21.8% of marine fishes compared to 59.5% of freshwater fishes had thiaminase. Two ecological traits increased probability of thiaminase. Omega-3 concentration (bomega-3 = 0.94, 95% CRI [0.07, 1.82]; Supplementary Table S3) and tropical climate (btropical = 1.16, 95% CRI [0.03, 2.23]) were positively related to thiaminase in fishes (Fig. 2). Higher omega-3 concentrations resulted in higher probability of thiaminase production, and omega-3 concentration alone explained 5% of the variation in the data (Bayesian R2 = 0.05, Fig. 3b). Tropical species had a nearly equal proportion of thiaminase positive fishes as non-tropical species (Fig. 3d), so the increased probability of thiaminase presence in tropical fishes only appears after trophic level, omega-3 concentrations, and marine/freshwater status are included in the model.
Exploration of coefficients that had the most support for predicting thiaminase in the multiple regression (Fig. 2). Top panels show the continuous relationships between (a) trophic level and (b) omega-3 concentration and thiaminase activity. Each point represents a fish that either does not have thiaminase (y = 0) or had evidence of thiaminase activity (y = 1) in the literature or BLAST sequence, color coded by a fish’s climate region. Each regression was fit separately with freshwater only (dotted line), marine only (dashed line), or all fishes included (solid line). Bottom panels show the presence (dark bars, labeled as “yes”) or absence (light bars, labeled as “no”) of thiaminase activity separated by (c) freshwater vs. marine, and (d) non-tropical and tropical. Percentage of fishes with thiaminase within a group is on top of the dark bars.
Discussion
There are two previous studies exploring why thiaminase is present in some fishes but not others34, 35, and with adding the current study we still do not know why any fishes make thiaminase. Yet dietary thiaminase has been linked to thiamine deficiency since the 1940s in taxa as diverse as silver foxes14, reptiles69, 70, fishes17, 71, marine mammals72, and humans3. We found that ecological traits rather than evolutionary patterns explain thiaminase presence among hundreds of fishes. Fishes with lower trophic levels, high polyunsaturated fatty acids, freshwater habitats, and from tropical climates were more likely to produce thiaminase.
Evolutionary patterns of thiaminase in fishes
Thiaminase is not present in ancient fishes. Lampreys, cartilaginous fishes, and the Coelacanth (Latimeria chalumnae) all lack the genes to produce the thiaminase protein. For both family- and order-level analyses, there was no phylogenetic relationship of thiaminase presence/absence. The trait first appears in the Class Actinopterygii (ray-finned fishes) Order Polyteriformes (e.g., bichirs, reedfishes), which evolved 368 million years ago61, but the presence/absence of thiaminase has no discernable patterns within Class Actinopterygii. Previous work has reported thiaminase activity and presence were generally higher in basal teleosts (clupeids, cyprinids, and catostomids) than in more derived neoteleosts (e.g., percids and centrarchids)34, 35. However, with a tenfold larger data set we were unable to discern any phylogenetic patterns. Certain orders have more members with thiaminase (Clupeiformes and Cypriniformes), suggesting there must be some evolutionary reason for its presence. While other species can and do produce thiaminase, clupeids in particular are well known to cause thiamine deficiency if they are a large portion of predator diets17, 50, 70, 73,74,75. A better understanding of how thiaminase in Clupeiformes such as alewife differs from that in Cypriniformes such as carps and other fishes is needed.
Ecological patterns of thiaminase in fishes
Ecology appears to be an important determinant of thiaminase presence in fishes. Thiaminase I activity in carp increased in response to pathogenic bacterium exposure, suggesting that thiaminase may be modulated in response to disease challenges32. It may be that tropical and freshwater fishes are more likely to have thiaminase because of higher exposure to pathogens. Previous work has found higher disease prevalence for shorebirds occupying tropical freshwater than marine temperate or arctic regions76, and viral and bacterial loads are higher in freshwater systems77. Moreover, a large-scale metanalysis across taxa found biotic interactions are stronger in the tropics78. This suggests that habitat and climate and their influence on exposure to pathogens may be a reason for thiaminase presence.
Trophic level was the strongest predictor of thiaminase among the ecological variables we explored. Very few top trophic level predators (TL > 4) were thiaminase positive. The reasons for fishes producing thiaminase remain unclear. Lower trophic food items like seston and zooplankton are highly variable in thiamine concentrations79. Likewise, median thiamine concentrations can dilute with increases in trophic level80, 81. There is some evidence that thiaminase production may be related to diet composition33, but there are many remaining questions. More research is needed to understand how thiaminase-producing fishes compartmentalize thiamine and thiaminase within their tissues and in identifying the ecological advantages of producing thiaminase.
One of the more interesting results was the strong positive relationship between omega-3 concentration and thiaminase presence, independent from trophic level (omega-3 vs. trophic level: F1, 154 = 0.203, p = 0.653). Thiamine-deficiency complex in North American fisheries is thought to be the result of thiaminase in prey fishes destroying thiamine in predators gut contents as they pass through the gut15, 17, 23, 26. In contrast, thiamine deficiency (called M74) in Atlantic Salmon (Salmo salar) from the Baltic Sea has been correlated with consumption of Sprat (Sprattus sprattus) and Atlantic Herring (Clupea harengus)50. In the Baltic, thiamine deficiency is presently thought to be caused by high lipid density leading to low thiamine concentrations per unit energy22, 82, 83. However, there has been no reported evidence that oxidative stress from highly unsaturated omega-3 fatty acids results in thiamine deficiency in consumers eating diets without thiaminase. Diets containing both high concentrations of omega-3 fatty acids and thiaminase confound attempts to uncover drivers of thiamine deficiency observed in M74-affected fish. It is also possible forage fish with high lipids22 and thiaminase presence such as in Sprat27 have additive negative effects on thiamine concentrations in predators. More efforts to disentangle whether thiaminase, high lipids (such as omega-3), or both cause thiamine deficiency are needed to understand threats to fisheries.
Future directions
A critical step forward in determination of which fish species produce thiaminase will come from our understanding of biological function(s) of thiaminases in fish. Why fishes produce thiaminase remains unknown, but the discussions may have been hindered because of the tendency to focus on thiaminase’s thiamine-degrading properties (and aforementioned impact on predators) rather than its function as a benefit to organisms. In bacteria, thiaminase II hydrolyzes the thiamine break-down product of formylaminopyrimidine (N-formyl-4-amino-5-aminomethyl-2-methylpyrimidine) to 4-amino-5-hydroxymehtyl-2-mehtylpyrimidine (HMP) which is then recycled in a thiamine biosynthetic pathway7. More recently, Sannino et al.28 demonstrated that the bacterium Burkholderia thailandensis uses thiaminase I to salvage precursors from environmentally available thiamine derivatives, and then preferentially uses these precursors for thiamine synthesis. This preference of auxotrophic B. thailandensis for thiamine precursors over thiamine itself has also been observed in the abundant SAR11 clade marine bacteria84. These mechanisms for salvage of thiamine precursors have not been demonstrated in fishes but offer areas of investigation within the fish microbiome.
Thiaminase I most certainly offers a selective advantage in fishes that possess this gene. Some possible advantages offered by thiaminase production include: (1) aide in thiamine production of commensal bacteria of the microbiome; (2) ecological advantages through population control of predatory species that forage on thiaminase-producing fishes; and (3) enhanced health of thiaminase-producing species of fish through greater immune function. Thiamine is the least metabolically stable B vitamin85 due to an oxidative side-reaction that readily damages the thiazole moiety86. Thiamine is also degraded in the presence of UV, sulfites, or high pHs, exacerbating its scarcity in natural environments and offering an advantage to organisms with microbiomes containing the ability to resynthesize thiamine from its breakdown products or precursors2. It is interesting that thiaminase production is most common in lower trophic levels, meaning that forage fishes are most likely to produce it. Thiaminase I as a thiamine salvage pathway in their microbiome would offer a strong advantage to fishes in environments with low and unstable thiamine supplies. However, it is unlikely that thiaminase I in forage species serves as a selective force to control reproduction and subsequently population size in salmonine predators. Laboratory experiments demonstrated that it took at least two years to induce TDC in eggs and fry of Lake Trout fed a thiaminase-rich diet21, a timeline too long for the feedback loop to benefit prey producing thiaminase.
Last, there is evidence that thiaminase I in fishes may be associated with immune function. Thiaminase activity within fishes is found to be greatest in tissues known to have immune function, such as head, kidney, gill, and spleen44, 47. Additionally, thiaminase activity increased in carp injected with a pathogenic bacterium (Aeromonas salmonicida), suggesting a relationship between thiaminase expression in fish and immune status32. Thiaminase I in fishes may have antimicrobial activity, which would be a significant health benefit for survival. The subcellular localization of thiaminase in lysosomes87 is consistent with such an antimicrobial activity.
The physiological function of thiaminase I in fishes remains in question at this point. Better understanding of these functions will ultimately help our predictions of ecological determinants for thiaminase production in fishes, as well as evolutionary significance of this fascinating enzyme.
Conclusions
The present work shows that de novo thiaminase production in fishes is widespread. We found no evolutionary relationship with thiaminase activity. Thiaminase appears in Class Actinoptergyii and is present across the entire phylogeny in both primitive and derived fish orders within this Class. Computer simulation resulted in the probability of all families in the Actinoptergyii Class having thiaminase, suggesting the genes have been widely retained. Ecological factors explained the most (40%) variation in thiaminase; fishes were more likely to express thiaminase if they feed closer to the base of the food web, were high in polyunsaturated fatty acids, lived in freshwater, and were from tropical climates.
Determining sources of thiaminase can help predict spatial and temporal patterns of the risks of thiamine deficiency globally. Thiamine deficiency is considered one of the top emerging issues for wildlife19. As the climate changes, certain fish communities are shifting their ranges. Species like Northern Anchovy (Engraulis mordax, a thiaminase positive fish) have reached record abundances in the Pacific Ocean along the southern portion of the United States88, causing thiamine deficiency in Pacific Salmon74. Understanding which prey species produce thiaminase, why they produce it, and how prey range and population sizes may change with climate is a necessary foundation for predicting and managing thiamine deficiency in fisheries.
Data availability
All data and R code are publicly available on Zenodo (https://doi.org/10.5281/zenodo.8263918).
References
Thurnham, D. I. In Encyclopedia of Human Nutrition (ed. Caballero, B.) 274–279 (Elsevier, 2013).
Kraft, C. E. & Angert, E. R. Competition for vitamin B1 (thiamin) structures numerous ecological interactions. Q. Rev. Biol. 92, 151–168. https://doi.org/10.1086/692168 (2017).
Whitfield, K. C. et al. Thiamine deficiency disorders: Diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 1430, 3–43. https://doi.org/10.1111/nyas.13919 (2018).
Tylicki, A., Łotowski, Z., Siemieniuk, M. & Ratkiewicz, A. Thiamine and selected thiamine antivitamins—biological activity and methods of synthesis. Biosci. Rep. https://doi.org/10.1042/BSR20171148 (2018).
Amcoff, P., Börjeson, H., Eriksson, R. & Norrgren, L. in Early life stage mortality syndrome in fishes of the Great Lakes and Baltic Sea. American Fisheries Society, Symposium. 31–40.
Bettendorff, L. At the crossroad of thiamine degradation and biosynthesis. Nat. Chem. Biol. 3, 454–455. https://doi.org/10.1038/nchembio0807-454 (2007).
Jenkins, A. H., Schyns, G., Potot, S., Sun, G. & Begley, T. P. A new thiamin salvage pathway. Nat. Chem. Biol. 3, 492–497. https://doi.org/10.1038/nchembio.2007.13 (2007).
Carlucci, A., Silbernagel, S. & McNally, P. Influence of temperature and solar radiation on persistence of vitamin B12, thiamine, and biotin in seawater. J. Phycol. 5, 302–305. https://doi.org/10.1111/j.1529-8817.1969.tb02618.x (1969).
Maier, G. D. & Metzler, D. E. Structures of thiamine in basic solution. J. Am. Chem. Soc. 79, 4386–4391. https://doi.org/10.1021/ja01573a040 (1957).
Gold, K., Roels, O. A. & Bank, H. Temperature dependent destruction of thiamine in seawater. Limnol. Oceanograp. 11, 410–413. https://doi.org/10.4319/lo.1966.11.3.0410 (1966).
Suffridge, C. P. et al. Exploring vitamin B1 cycling and its connections to the microbial community in the north Atlantic Ocean. Front. Marine Sci. https://doi.org/10.3389/fmars.2020.606342 (2020).
Sañudo-Wilhelmy, S. A. et al. Multiple B-vitamin depletion in large areas of the coastal ocean. Proc. Nat. Acad Sci. 109, 14041–14045. https://doi.org/10.1073/pnas.1208755109 (2012).
Sañudo-Wilhelmy, S. A., Gómez-Consarnau, L., Suffridge, C. & Webb, E. A. The role of B vitamins in marine biogeochemistry. Ann. Rev.Marine Sci. 6, 339–367. https://doi.org/10.1146/annurev-marine-120710-100912 (2014).
Lee, C. F. Thiaminase in fishery products: A review. Commer. Fish. Rev. 10, 7–17 (1948).
Fitzsimons, J. D., Brown, S. B., Honeyfield, D. C. & Hnath, J. G. A review of early mortality syndrome (EMS) in great lakes salmonids: Relationship with thiamine deficiency. Ambio (Sweden) 28, 9–15 (1999).
Balk, L. et al. Widespread episodic thiamine deficiency in Northern Hemisphere wildlife. Sci. Rep. 6, 1–13. https://doi.org/10.1038/srep38821 (2016).
Harder, A. M. et al. Thiamine deficiency in fishes: Causes, consequences, and potential solutions. Rev. Fish Biol. Fish. 28, 865–886. https://doi.org/10.1007/s11160-018-9538-x (2018).
Ogershok, P. R., Rahman, A., Brick, J. & Nestor, S. Wernicke encephalopathy in nonalcoholic patients. Am. J. Med. Sci. 323, 107–111. https://doi.org/10.1097/00000441-200202000-00010 (2002).
Sutherland, W. J. et al. A 2018 horizon scan of emerging issues for global conservation and biological diversity. Trends Ecol. Evolut. 33, 47–58. https://doi.org/10.1016/j.tree.2017.11.006 (2018).
Sealock, R. R. & Goodland, R. L. Thiamine inactivation by the Chastek-Paralysis factor: Inhibition of thiamine inactivation. J. Am. Chem. Soc. 66, 507–510. https://doi.org/10.1021/ja01232a001 (1944).
Honeyfield, D. C. et al. Development of thiamine deficiencies and early mortality syndrome in lake trout by feeding experimental and feral fish diets containing thiaminase. J. Aquat. Anim. Health 17, 4–12. https://doi.org/10.1577/H03-078.1 (2005).
Vuorinen, P. J. et al. Model for estimating thiamine deficiency-related mortality of Atlantic salmon (Salmo salar) offspring and variation in the Baltic salmon M74 syndrome. Marine Freshwater Behav. Physiol. 54, 97–131. https://doi.org/10.1080/10236244.2021.1941942 (2021).
Brown, S. B., Fitzsimons, J. D., Honeyfield, D. C. & Tillitt, D. E. Implications of thiamine deficiency in great lakes Salmonines. J. Aquat. Anim. Health 17, 113–124. https://doi.org/10.1577/H04-015.1 (2005).
Fisher, J. P., Connelly, S., Chiotti, T. & Krueger, C. C. Interspecies comparisons of blood thiamine in salmonids from the Finger Lakes, and effect of maternal size on blood and egg thiamine in Atlantic Salmon with and without Cayuga Syndrome. Am. Fish. Soc. Symp. 21, 112–123 (1998).
Suomalainen, P. & Pihlgren, A.-M. On the thiaminase activity of fish and some other animals and on the preservation of thiaminase in silage made from fish. Acta Agralia Fennica 83, 221–229 (1955).
Tillitt, D. E. et al. Thiamine and thiaminase status in forage fish of salmonines from Lake Michigan. J. Aquat. Anim. Health 17, 13–25. https://doi.org/10.1577/H03-081.1 (2005).
Wistbacka, S. & Bylund, G. Thiaminase activity of Baltic salmon prey species: A comparision of net-and predator-caught samples. J. Fish Biol. 72, 787–802. https://doi.org/10.1111/j.1095-8649.2007.01722.x (2008).
Sannino, D. R., Kraft, C. E., Edwards, K. A. & Angert, E. R. Thiaminase I provides a growth advantage by salvaging precursors from environmental thiamine and its analogs in Burkholderia thailandensis. Appl. Environ. Microbiol. 84, e01268-e1218 (2018).
Honeyfield, D. C., Hinterkopf, J. P. & Brown, S. B. Isolation of thiaminase-positive bacteria from alewife. Trans. Am. Fish. Soc. 131, 171–175. https://doi.org/10.1577/1548-8659(2002)131%3c0171:IOTPBF%3e2.0.CO;2 (2002).
Richter, C. A. et al. Paenibacillus thiaminolyticus is not the cause of thiamine deficiency impeding lake trout (Salvelinus namaycush) recruitment in the Great Lakes. Can. J. Fish. Aquat. Sci. 69, 1056–1064. https://doi.org/10.1139/f2012-043 (2012).
Richter, C. A., Evans, A. N., Heppell, S. A., Zajicek, J. L. & Tillitt, D. E. Genetic basis of thiaminase I activity in a vertebrate, zebrafish Danio rerio. Sci. Rep. 13, 698. https://doi.org/10.1038/s41598-023-27612-5 (2023).
Wistbacka, S., Lönnström, L.-G., Bonsdorff, E. & Bylund, G. Thiaminase activity of crucian carp Carassius carassius injected with a bacterial fish pathogen, Aeromonas salmonicida subsp. salmonicida. J. Aquat. Anim. Health 21, 217–228. https://doi.org/10.1577/H08-010.1 (2009).
Lepak, J., Kraft, C. & Vanni, M. Clupeid response to stressors: The influence of environmental factors on thiaminase expression. J. Aquat. Anim. Health 25, 90–97. https://doi.org/10.1080/08997659.2013.768560 (2013).
Boggs, K. et al. Phylogeny and foraging mode correspond with thiaminase activity in freshwater fishes: Potential links to environmental factors. Freshwater Sci. 38, 605–615. https://doi.org/10.1086/704927 (2019).
Riley, S. C. & Evans, A. N. Phylogenetic and ecological characteristics associated with thiaminase activity in Laurentian Great Lakes fishes. Trans. Am. Fish. Soc. 137, 147–157. https://doi.org/10.1577/T06-210.1 (2008).
Greig, R. A. & Gnaedinger, R. H. Occurance of thiaminase in some common aquatic animals of the United States and Canada. (Special Scientific Report - Fisheries, 1971).
Sidwell, V. D., Loomis, A. L., Foncannon, P. R. & Buzzell, D. H. Composition of the edible portion of raw (fresh or frozen) crustaceans, finfish, and mollusks. Marine Fish. Rev. 40, 1–16 (1978).
Honeyfield, D. C., Hanes, J. W., Brown, L., Kraft, C. E. & Begley, T. P. Comparison of thiaminase activity in fish using the radiometric and 4-Nitrothiophenol colorimetric methods. J. Great Lakes Res. 36, 641–645. https://doi.org/10.1016/j.jglr.2010.07.005 (2010).
Neilands, J. Thiaminase in aquatic animals of Nova Scotia. J. Fish. Board Canada 7, 94–99. https://doi.org/10.1139/f47-011 (1947).
Hirn, J. & Pekkanen, T. J. Quantitative analysis of thiaminase activity in certain fish species. Nord. Vet. Med. 27, 646–648 (1975).
Okonji, R. E., Olusola, K. O., Ehigie, L. O., Olaoluwa, P. M. & Bamitale, K. S. Thiaminase properties in the fillet and liver of Tilapia zillii from Osinmo Reservoir: A comparative study. Afr. J. Food Sci. 7, 143–149. https://doi.org/10.5897/AJFS12.176 (2013).
Chaet, A. B. & Bishop, D. W. The occurrence of thiaminase in certain fresh-water fishes. Physiol. Zool. 25, 131–134. https://doi.org/10.1086/physzool.25.2.30158349 (1952).
Kraft, C. E., Gordon, E. R. & Angert, E. R. A rapid method for assaying thiaminase I activity in diverse biological samples. PLoS One 9, e92688. https://doi.org/10.1371/journal.pone.0092688 (2014).
Zajicek, J. L., Tillitt, D. E., Honeyfield, D. C., Brown, S. B. & Fitzsimons, J. D. A method for measuring total thiaminase activity in fish tissues. J. Aquat. Anim. Health 17, 82–94. https://doi.org/10.1577/H03-083.1 (2005).
Nishimune, T., Watanabe, Y. & Okazaki, H. Studies on the polymorphism of thiaminase I in seawater fish. J. Nutr. Sci. Vitaminol. 54, 339–346. https://doi.org/10.3177/jnsv.54.339 (2008).
Gnaedinger, R. & Krzeczkowski, R. Heat inactivation of thiaminase in whole fish. Commer. Fish. Rev. 28, 11–14 (1966).
Fujita, A. Advances in enzymology and related subjects of biochemistry. Science 14, 389–421 (1954).
Arsan, O. Thiaminase activity in the liver and intestine of some freshwater fishes. Hydrobiol. J. 6, 75–77 (1970).
Anglesea, J. & Jackson, A. Thiaminase activity in fish silage and moist fish feed. Anim. Feed Sci. Technol. 13, 39–46. https://doi.org/10.1016/0377-8401(85)90040-9 (1985).
Karlsson, L., Ikonen, E., Mitans, A. & Hansson, S. The diet of salmon (Salmo salar) in the Baltic sea and connections with the M74 syndrome. Ambio 28, 37–42 (1999).
Deolalkar, S. & Sohonie, K. Thiaminase from fresh-water, brackish-water and salt-water fish. Nature 173, 489–490. https://doi.org/10.1038/173489a0 (1954).
Csepp, D., Honeyfield, D. C., Vollenweider, J. J. & Womble, J. N. Estuarine distribution, nutritional and thiaminase content of eulachon (Thaleichthys pacificus) in southeast Alaska, with implications for Steller sea lions. NOAA Technical Memorandum NMFS-AFSC 356, doi:https://doi.org/10.7289/V5/TM-AFSC-356 (2017).
Wetzel, L. A., Parsley, M. J., van der Leeuw, B. K. & Larsen, K. A. in Impact of American Shad in the Columbia River 89–105 (2011).
Honeyfield, D. C. et al. Survey of four essential nutrients and thiaminase activity in five Lake Ontario prey fish species. J. Great Lakes Res. 38, 11–17. https://doi.org/10.1016/j.jglr.2011.11.008 (2012).
Arsan, O. & Malyarevskaya, A. The effect of food on thiaminase activity and the content of thiamine in the liver and intestine of silver carp. Gidrobiologicheskii Zhurnal 5, 79–81 (1969).
Tian, W. & Skolnick, J. How well is enzyme function conserved as a function of pairwise sequence identity?. J. Mole. Biol. 333, 863–882. https://doi.org/10.1016/j.jmb.2003.08.057 (2003).
Tillitt, D. E. et al. Dreissenid mussels from the Great Lakes contain elevated thiaminase activity. J. Great Lakes Res. 35, 309–312. https://doi.org/10.1016/j.jglr.2009.01.007 (2009).
Deutsch, H. F. & Hasler, A. D. Distribution of a vitamin B1 destructive enzyme in Fish. Proc. Soc. Exp. Biol. Med. 53, 63–65. https://doi.org/10.3181/00379727-53-14186 (1943).
Froese, R. & Pauly, D. in World Wide Web electronic publication (www.fishbase.org, 2021).
R: A language and environment for statistical computing v. 4.2.2 (2022).
Betancur-R, R. et al. Phylogenetic classification of bony fishes. BMC Evolut. Biol. 17, 162. https://doi.org/10.1186/s12862-017-0958-3 (2017).
Rabosky, D. L. et al. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395. https://doi.org/10.1038/s41586-018-0273-1 (2018).
Paradis, E. & Schliep, K. ape 50: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evolut. 3, 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x (2012).
rstanarm: Bayesian applied regression modeling via Stan v. 2.21.1 (https://mc-stan.org/rstanarm., 2020).
Gelman, A. & Hill, J. Data analysis using regression and multilevel/hierarchical models. (Cambridge University Press, 2006).
Stan Developent Team. RStan: the R interface to Stan. R package version 2, 522 (2021).
Gelman, A., Goodrich, B., Gabry, J. & Vehtari, A. R-squared for Bayesian regression models. Am. Stat. 73, 307–309. https://doi.org/10.1080/00031305.2018.1549100 (2019).
Marshall, C. Nutrition of snakes, lizards and tortoises. Vet. Nurs. J. 8, 72–78 (1993).
Honeyfield, D. C. et al. Pathology, physiologic parameters, tissue contaminants, and tissue thiamine in morbid and healthy central Florida adult American alligators (Alligator mississippiensis). J. Wildlife Dis. 44, 280–294 (2008).
Wolf, L. E. Fish-diet disease of trout: a vitamin deficiency produced by diets containing raw fish. (New York State Conservation Department, Bureau of Fish Culture, 1942).
Aulerich, R., Davis, H., Bursian, S., Sikarskie, J. & Stuht, J. Suspected thiamine deficiency (Chastek’s paralysis) in northern river otter (Lutra canadensis). Scientifur 19, 297–304 (1995).
Riley, S. C., Rinchard, J., Honeyfield, D. C., Evans, A. N. & Begnoche, L. Increasing thiamine concentrations in lake trout eggs from Lakes Huron and Michigan coincide with low alewife abundance. North Am. J. Fish. Manag. 31, 1052–1064. https://doi.org/10.1080/02755947.2011.641066 (2011).
Mantua, N. J. et al. Mechanisms, Impacts, and Mitigation for Thiamine Deficiency and Early Life Stage Mortality in California’s Central Valley Chinook Salmon. 92–93 (2021).
Ross, J. P. et al. Gizzard shad thiaminase activity and its effect on the thiamine status of captive American alligators Alligator mississippiensis. J. Aquat. Anim. Health 21, 239–248. https://doi.org/10.1577/H08-002.1 (2009).
Mendes, L. et al. Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 109, 396–404. https://doi.org/10.1111/j.0030-1299.2005.13509.x (2005).
Maranger, R. & Bird, D. F. Viral abundance in aquatic systems: a comparison between marine and fresh waters. Marine Ecol. Progress Series 121, 217–226 (1995).
Schemske, D. W., Mittelbach, G. G., Cornell, H. V., Sobel, J. M. & Roy, K. Is there a latitudinal gradient in the importance of biotic interactions?. Ann. Rev. Ecol. Evolut. Syst. 40, 245–269. https://doi.org/10.1146/annurev.ecolsys.39.110707.173430 (2009).
Fridolfsson, E. et al. Seasonal variation and species-specific concentrations of the essential vitamin B1 (thiamin) in zooplankton and seston. Marine Biol. 166, 1–13. https://doi.org/10.1007/s00227-019-3520-6 (2019).
Majaneva, S. et al. Deficiency syndromes in top predators associated with large-scale changes in the Baltic Sea ecosystem. Plos One 15, e0227714. https://doi.org/10.1371/journal.pone.0227714 (2020).
Sylvander, P. & Snoeijs, P. Thiamine concentrations in the Baltic Sea pelagic food web decrease with increasing trophic level. (2013).
Hazell, A. S., Faim, S., Wertheimer, G., Silva, V. R. & Marques, C. S. The impact of oxidative stress in thiamine deficiency: A multifactorial targeting issue. Neurochem. Int. 62, 796–802. https://doi.org/10.1016/j.neuint.2013.01.009 (2013).
Vuori, K. A., Kanerva, M., Ikonen, E. & Nikinmaa, M. Oxidative stress during Baltic salmon feeding migration may be associated with yolk-sac fry mortality. Environ. Sci. Technol. 42, 2668–2673. https://doi.org/10.1021/es702632c (2008).
Carini, P. et al. Discovery of a SAR11 growth requirement for thiamin’s pyrimidine precursor and its distribution in the Sargasso Sea. ISME J. 8, 1727–1738. https://doi.org/10.1038/ismej.2014.61 (2014).
McCourt, J. A., Nixon, P. F. & Duggleby, R. G. Thiamin nutrition and catalysis-induced instability of thiamin diphosphate. British J. Nutr. 96, 636–638. https://doi.org/10.1079/BJN20061842 (2006).
Hanson, A. D. et al. Redesigning thiamin synthesis: Prospects and potential payoffs. Plant Sci. 273, 92–99. https://doi.org/10.1016/j.plantsci.2018.01.019 (2018).
Sato, M., Hayashi, S. & Nishino, K. Subcellular localization of thiaminase I in the kidney and spleen of carp, Cyprinus carpio. Comp. Biochem. Physiol. Part A: Physiol. 108, 31–38. https://doi.org/10.1016/0300-9629(94)90050-7 (1994).
Thompson, A. R. State of the California current 2018–2019: A novel anchovy regime and a new marine heatwave?. Calif. Coop. Ocean. Fish. Investig. 60, 1–65 (2019).
Acknowledgements
We thank K. Klymus and R. Betancur for help with phylogenies, and the Chinook Salmon thiamine working group for feedback on the analysis. D. Honeyfield provided excellent insight on thiaminase status of fishes in our database and on the manuscript, and C. Kraft helped us reframe the paper. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
CRediT (Contributor Roles Taxonomy) roles in the study were as follows: F.E.R., Conceptualization; F.E.R. and C.A.R., Data curation; F.E.R., Formal analysis; F.E.R., C.A.R., D.M.W., and D.E.T., Methodology; F.E.R., Writing – original draft; F.E.R., C.A.R., D.M.W., and D.E.T., Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rowland, F.E., Richter, C.A., Tillitt, D.E. et al. Evolutionary and ecological correlates of thiaminase in fishes. Sci Rep 13, 18147 (2023). https://doi.org/10.1038/s41598-023-44654-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44654-x
This article is cited by
-
Pre-analytical challenges from adsorptive losses associated with thiamine analysis
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.