Abstract
Angioinvasion remains the important prognostic feature in papillary thyroid cancer (PTC) patients. Literature data indicates several markers that may be associated with oxidative stress and/or angioinvasion. Therefore, we assessed the utility of selected parameters in angioinvasion and metastasis screening in serum of PTC patients. Serum antioxidant capacity (TAC) and sirtuin 3 (SIRT3) levels were decreased (all p < 0.05) and both DNA/RNA oxidative stress damage products (DNA/RNA OSDP) and malondialdehyde (MDA) levels were increased in PTC patients with angioinvasion and metastasis (study group) when compared with PTC patients without these features (all p < 0.01). The highest screening utility in differentiation between angioinvasion and metastasis presence and absence in PTC patients was presented for DNA/RNA OSDP (AUC = 0.71), SIRT3 (AUC = 0.70), and TAC (AUC = 0.67) (all p < 0.05). Our study suggests that peripheral concentration of oxidative stress markers could be useful as angioinvasion and metastasis indicator in PTC patients.
Similar content being viewed by others
Introduction
Papillary thyroid cancer (PTC) is one of the most commonly occurring endocrine neoplasms worldwide1. PTC originating from the follicular epithelium usually presents a good prognosis with an excellent survival rate. However, the diagnosis of aggressive types, manifested by local invasion, recurrence, and distant metastases, is still a significant clinical problem2. Angioinvasion has been identified as an important and independent prognostic factor in many cancers, including PTC. The diagnosis of thyroid cancer dedifferentiation with local angioinvasion is of great importance in further clinical management3. The molecular criteria for angioinvasion have varied both in practice as well as in studies assessing the clinical significance of these findings. This inconsistency may be attributed to application of inappropriate criteria4.
Oxidative stress is predicted to have a major impact both in the development and the progression of thyroid cancer, since, due to the process of hormonogenesis, the thyroid gland is exposed to a high level of reactive oxygen species (ROS). What should be emphasized, PTC is the most prevalent thyroid cancer associated with oxidative stress5. It has been demonstrated that ROS promote angiogenesis, either directly or via the generation of active oxidation products, including peroxidized lipids6,7.
Several markers may be associated with oxidative stress and/or angioinvasion. The total oxidative stress (TOS) and antioxidant capacity (TAC) are used to estimate the general oxidation status and antioxidant ability of the body, respectively. Malondialdehyde (MDA), the product of lipid peroxidation, is a commonly used as biological marker of this process. DNA/RNA oxidative stress damage products (DNA/RNA OSDP) show the extent of nucleic acid oxidative modifications8,9. Moreover, growing literature data links oxidative stress with DNA methylation in cancer development and progression. Nuclear factor kappa B (NF-κB) and interleukin-6 (IL-6) play a significant role in immune and inflammatory processes. In turn, sirtuin 1 and 3 (SIRT1 and SIRT3) and forkhead box protein 01 (FOXO) act as regulators of important metabolic pathways including redox homeostasis10. Protein 53 (p53), 5′-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) regulate metabolic reprogramming and antioxidant/mitochondrial function11. Their usefulness in angioinvasion screening in PTC remains to be elucidated.
It can be assumed the interplay between oxidative stress, chronic inflammation, and angiogenesis in cancer progression. Thus, our hypothesis is that circulating oxidative stress markers, inflammation and/or angiogenesis related parameters can be helpful in angioinvasion screening in order to support clinical evaluation in questionable PTC cases. Thus, the aim of this study was to assess the utility of selected parameters in angioinvasion and metastasis screening in serum of PTC patients.
Results
Biochemical characterization
To test the working hypothesis the group of PTC patients was divided into 2 subgroups: PTC patients with both angioinvasion and metastasis (study group) and PTC patients without these features (reference group, considered as very low-risk PTC group according to American Thyroid Association guidelines)12 (Table1).
The cholesterol (CHOL) and low-density lipoprotein (LDL) concentrations were increased, and high-density lipoprotein (HDL) was decreased in study group when compared with reference group (p < 0.05; p < 0.001; p < 0.05; respectively). Yet, other parameters did not differ among groups (Table 2).
Oxidative stress and PTC angioinvasion and metastasis
To evaluate the significance of oxidative stress in angioinvasion and metastasis features in PTC, several circulating oxidative stress-related markers were measured in PTC patients. Thus, patients with angioinvasion and metastasis were considered as the study group and patients without these characteristics constituted the reference group. Serum TAC and SIRT3 levels were decreased (all p < 0.05) and both DNA OSDP and MDA levels were increased in study group when compared with reference PTC patients (all p < 0.01). The study and reference groups did not differ in terms of TOC, SIRT1, DNA methylation, FOXO, PRKAA1, PGC1α, p53, NF-κB, and IL-6 (all p > 0.01) (Table 3).
Angioinvasion and metastasis indicators
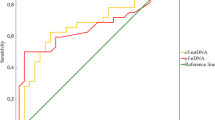
Furthermore, to assess the significance of TAC, SIRT3, DNA/RNA OSDP, and MDA as angioinvasion and metastasis indicators among PTC patients, the ROC curve analysis was performed. The highest clinical utility in angioinvasion and metastasis detection in PTC patients was observed for DNA/RNA OSDP (AUC = 0.71), SIRT3 (AUC = 0.70), TAC (AUC = 0.67) (all p < 0.05) measurements. However, the AUC for MDA (AUC = 0.66), somewhat high, did not reach significance when compared with AUC = 0.50 (p > 0.01) (Fig. 1).
Correlation
A Spearman correlation analysis was performed to study the relationship between biochemical parameters in total group. The conducted correlation analysis included both groups: the study group, consisting of PTC patients with angioinvasion and metastasis, and the reference group, without the angioinvasion and metastasis, to assess the relationship between studied parameters in PTC patients (Fig. 2).
The studied parameters correlation; IL-6 interleukin 6, FOXO Forkhead box O family member proteins, NF-κB -nuclear factor kappa B, MDA malondialdehyde, P53 protein 53, SIRT1 Sirtuin 1, SIRT3 Sirtuin 3, TAC total antioxidant capacity, TOC total oxidative capacity, CHOL cholesterol, CRP C-reactive protein, PTC papillary thyroid cancer, fT3 free triiodothyronine, fT4 free thyroxine, HDL high-density lipoprotein, LDL low-density lipoprotein, TG triglyceride, TGB thyroglobulin, TGBAb antithyroglobulin antibodies, TSH thyroid-stimulating hormone, 25-OH VITD–25-OH vitamin D, PRKAA1 5'-AMP-activated protein kinase catalytic subunit alpha-1, PGC1α peroxisome proliferator-activated receptor gamma coactivator 1-alpha, MDA malondialdehyde, DNA/RNA DNA/RNA oxidative stress damage products.
Considering all the biochemical parameters in PTC patients and their significant associations, strong positive correlation between p53 and NF-κB (r = 0.75; p < 0.001) and between p53 and FOXO (r = 0.79; p < 0.01) was shown. Moreover, medium negative correlation between TAC and MDA measurements was demonstrated (r = − 0.54; p < 0.001). Medium positive correlation between p53 and PRKAA1 (r = 0.58; p < 0.01) and between NF-κB and PGC1α (r = 0.65; p < 0.01) was also observed. Additionally, medium positive correlation between PRKAA1 and PGC1α (r = 0.69; p < 0.05) was shown.
Logistic regression analysis
Furthermore, to distinguish whether the oxidative stress markers originate from the cancer itself or are a systemic phenomenon caused by the resultant peripheral processes, the logistic regression analysis was performed. We demonstrated the association between both angioinvasion and metastasis presence and CHOL, LDL, and HDL concentrations in PTC patients (p < 0.05). Moreover, we also observed that both angioinvasion and metastasis presence influence serum TAC, DNA/RNA OSDP, SIRT3, and NF-κB concentrations in PTC patients (p < 0.05) (Table 4).
Discussion
The literature data based results assessing the predictive value of angioinvasion in distant metastasis development, especially in well-differentiated thyroid cancer, are discrepant13,14,15. This may be due to the inconsistent criteria for angioinvasion detection in endocrine tumors, especially thyroid cancer13. Assessment of angioinvasion in primary tumors is currently based upon examination of sections stained with haematoxylin and eosin. Moreover, several vascular markers have been characterized and studied to detect cancer angioinvasion, e.g., a commonly used endothelial marker CD31, platelet-associated protein CD61, and von Willebrand factor (factor VIII-related antigen)16,17,18,19.
There is clear evidence for a higher level of oxidative stress, including a compromised antioxidative defense system in cancer20. Janion et al.21 showed significantly lower serum MDA and TAS levels and higher TOS levels in the colorectal cancer (CRC) group compared to the control group. Oxidative stress has been also suggested as significant PTC risk factor and the level of ROS generation correlated with cancer aggressiveness22. Therefore, potential modulation of oxidative status may influence the cancer progression. It has been hypothesized that oxidative stress markers in thyroid tumors may have diagnostic, prognostic, and therapeutic relevance22. To date, little studies have been performed to establish the tools for angioinvasion detection in cancer. Thus, new potential circulating biomarkers indicative of angioinvasion and metastasis are needed.
Accumulating evidence suggest that ROS function as signaling molecules to mediate various growth-related responses including angiogenesis23. It has been observed that oxidative stress stimulates the secretion of angiogenic modulators in cancer cells via hypoxia-dependent and -independent pathways24. In our study, the level of serum oxidative stress markers was evaluated to assess the potential screening utility in PTC-related angioinvasion with metastasis. PTC patients with both angioinvasion and metastasis presented decreased serum TAC and SIRT3 and increased DNA OSDP and MDA levels than PTC patients without these features. The results indicate that oxidative stress is involved in angioinvasion development among PTC patients and TAC, SIRT3, DNA/RNA OSDP, and MDA could be potentially considered as PTC angioinvasion screening tools. TAC measures the peroxyl-scavenging capacity of the extracellular antioxidant system and indirectly reflects the level of oxidative stress. A low serum TAC has been reported to have a strong association with various cancers25. Reduced TAC in studied PTC patients when compared with reference PTC patients could reflect the consumption of antioxidants in free radical reactions by the cancer invasion. This suggests that TAC may have a protective role against angioinvasion and metastasis.
Based on MDA, lipid peroxidation was significantly increased in PTC patients with angioinvasion and metastasis compared with respective reference group. These results also point to increased oxidative stress in those patients. Furthermore, Maurya et al.26 demonstrated that pre chemotherapy serum MDA level was significantly higher in patients with primary ocular carcinoma having larger tumor and having lymph node metastasis than those without lymph node metastasis suggestive of its potential significance in cancer progression.
SIRT3, the major deacetylase in mitochondria, plays a crucial role in regulating glucose metabolism, modulating ROS, and limiting the oxidative damage in cellular components27. The roles of SIRT3 vary in different types of cancers and they have been thoroughly discussed. In some types of cancer SIRT3 functions as a tumor promoter by lowering ROS levels to maintain cell proliferation, other studies describe SIRT3 as a tumor suppressor, as it can promote apoptosis in cancer cells under stress conditions28. SIRT3 plays a crucial role by mediating interactions between mitochondria and intracellular signaling29. It has been observed that increased ROS production in SIRT3 null cells contributes to increased HIF1α stabilization leading to the transcriptional activation of multiple metastasis-related pathways30,31. Our study suggests that SIRT3 plays a role as angiogenic factor in PTC, however, its involvement in PTC progression is yet to be elucidated.
Interestingly, oxidative damage to nucleic acid has been associated with the development of various diseases including cancer32,33,34,35. 8-Oxo-7,8-dihydro-2’-deoxyguanosine, 8-oxo-7,8-dihydroguanosine, and 8-hydroxy-2′-deoxyguanosine (8-OHdG) are generated by the oxidative damage to DNA. Increased serum 8-oxodG concentration compared to the control group has been observed in CRC patients21. Moreover, it has been demonstrated increased levels of urinary 8-OHdG in CRC patients and patients with tumor metastasis, compared to healthy controls and patients without tumor metastasis, respectively36. This line of evidence suggest that peripheral concentration of DNA/RNA OSDP may be useful in noninvasive screening of angioinvasion and metastasis in cancer patients.
Oxidative stress can also activate a variety of transcription factors including NF-κB, FOXO, and p53. P53 is the most extensively studied tumor suppressor deregulated in most human cancers. In turn, NF-κB transcription factors play essential regulatory function in inflammation, cell proliferation, and apoptosis37. FOXO proteins are growth factor and stress regulated transcription factors known to cooperate with p5338. The reciprocal regulation of these transcription factors can influence several important cancer pathways: tumor cell metabolism, DNA damage, mitochondrial function, and ATP production39. Depending on the cellular context, the activation of NF-κB can have different related output to the p53 function. Our study confirmed strong positive correlation between p53 and NF-κB (r = 0.75; p < 0.001) and between p53 and FOXO (r = 0.79; p < 0.01) in PTC patients. FOXO mediates cellular oxidative stress to maintain metabolic stability by promoting transcription of several genes coding for antioxidant proteins40.
Moreover, medium negative correlation between TAC and MDA measurements was demonstrated (r = − 0.54; p < 0.001). As MDA is a marker for lipid peroxidation, the results indicate the imbalance between oxidant and antioxidant capacity in PTC, and, therefore, oxidative damage in cells and tissues. Positive correlation between p53 and PRKAA1 (r = 0.58; p < 0.01) and between NF-κB and PGC1α (r = 0.65; p < 0.01) was also observed in all PTC patients. Additionally, medium positive correlation between PRKAA1 and PGC1α (r = 0.69; p < 0.05) was shown. PRKAA1 is a major cellular energy sensor implicated in angiogenesis in hypoxia-induced diseases41. In turn, PGC1α plays a pivotal role in mitochondrial biogenesis and multiple other pathways (inflammation, endothelial dysfunction, and oxidative stress)42. As no difference in serum PRKAA1 and PGC1α concentration was observed between groups, it can be concluded that their peripheral level is not influenced by angioinvasion development as they are produced and exert effects locally.
Bauerle et al. demonstrated the key role of NF-κB in angiogenesis and growth of primary and metastatic thyroid cancer43. Since, considering these markers, no difference was observed between PTC patients with both angioinvasion and metastasis and PTC patients without these features, the associations need further study to evaluate their role in PTC invasion44. Targeting oxidative stress-sensitive pathways and transcription factors offers great promise for cancer prevention and therapy45. Nevertheless, the direct roles of ROS and distinct oxidative stress markers in angiogenesis in PTC patients remain to be elucidated. However, serum oxidative status marker levels are good indicators for the systemic oxidant/antioxidant status.
To assess the potential screening utility of the parameters, ROC curves were constructed. DNA/RNA OSDP (AUC = 0.71), SIRT3 (AUC = 0.70), and TAC (AUC = 0.67) have been demonstrated to differentiate between PTC patients with both angioinvasion and metastasis and PTC patients without these features (all p < 0.05). In our regression analysis results, a significant influence of angioinvasion and metastasis on various peripheral parameters, which showed statistical significance (p < 0.05), was observed. Serum parameters such as CHOL, LDL, HDL, TAC, OSDP, SIRT3, and NFκB showed significant association with the presence of both angioinvasion and metastasis in PTC patients. Thus, it can be concluded that the concentration change of these parameters originates from the cancer itselft. In previously conducted studies, we also observed a positive correlation between the concentration of MDA measured among advanced PTC patients and their lipid profile. This finding suggests that individuals with advanced PTC may be at risk of having deregulated lipid levels, which, in turn, is associated with the metastasis process46,47. On the other hand, in our analysis, it has been shown the difference in the MDA concentration between groups, however, our regression analysis did not demonstrate the association between angioinvasion and metastasis status and serum MDA level. Therefore, it can be assumed that the change in MDA concentration results from the peripheral oxidative stress rather than the cancer. It can be hypothesized that additional mechanisms involving lipids and MDA are associated with PTC progression, which need further detailed evaluation. Additional investigations on these peripheral metabolic processes will enable to better understand their role and potential clinical significance. Larger studies are also needed to confirm the results and assess the possibility to support of serum oxidative stress markers in challenging PTC screening.
To our knowledge, this is the first study assessing circulating oxidative stress markers as angioinvasion and metastasis indicators in PTC patients. Considering that the parameters were measured in serum, their peripheral concentration results not only from thyroid cancer tissue but also from the imbalance of homeostatic processes. Although the involvement of oxidative stress in PTC angioinvasion and metastasis seems to be important, our hypotheses need to be verified in further molecular studies.
Investigation of thyroid cancer progression from the overview of oxidative stress may be helpful in the understanding of the cancer etiology and may contribute to the development of new approaches to cancer therapy.
Materials and methods
Study design
This research was conducted at the Department of Endocrinology, Diabetology, and Internal Diseases, Medical University of Bialystok, Poland. All patients were diagnosed as having PTC based on clinical laboratory tests and ultrasound imaging, then confirmed by fine needle aspiration biopsy (FNAB), followed by histopathological examination. For this study 80 patients diagnosed with various stages of PTC after total thyroidectomy were enrolled (Table 1). To assess the clinical utility of the selected parameters as indicators of angioinvasion and metastasis, the study group comprised patients with PTC presenting angioinvasion and metastasis (study group), while the reference group consisted of PTC patients without these specific features (reference group). The angioinvasion was identified through post-thyroidectomy histopathological evaluation. To conduct this study, patient recruitment has been ongoing since 2018.
Material and methods
Venous blood (5.5 mL) was obtained and centrifuged, with serum subsequent separation and then frozen at − 80 °C. The procedures were approved by the Local Ethics Committee of the Medical University of Bialystok, Poland, and written informed consent was obtained from each participant (R-I-002/491/2019).
The triglyceride (TG), LDL, HDL, CHOL, and C-reactive protein (CRP) concentrations were assayed using the enzymatic colorimetric method on a Roche C111 device (Roche Diagnostics, Basel, Switzerland). The thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), and antithyroglobulin antibody (TGBAb) concentrations were measured by a Roche E411 device (Roche Diagnostics, Sussex, UK) using the electrochemiluminescence (ECLIA) method. The TOS status was assessed using photometric immunodiagnostic assay (PerOx (TOS/TOC) kit, KC5100, 64625 Bensheim, Germany) and the TAC status was determined by photometric assay (ImAnOx (TAS/TAC) Kit, KC5200, 64625 Bensheim, Germany). P53, NF-κB, FOXO, SIRT1, PRKAA1, and PGC1α concentrations were determined using an enzyme-linked immunosorbent assay (ELISA) (Enzyme-linked Immunosorbent Assay Kit; Cloud-Clone Corp., Wuhan, China; SEA928Hu; SEB824Hu; SEA764Hu; SEE912Hu; SEA679Hu; SEH337Hu; respectively) according to the manufacturer’s instructions. SIRT3 and IL-6 concentrations were determined using ELISA method (Human Sirtuin 3, Thermofisher, CA 92008, Carlsband, USA; and Human IL-6; R&D Systems, D6050, Inc., Minneapolis, MN 55413, Canada. MDA was evaluated accordingly the ELISA method (Malondialdehyde ELISA kit, E-EL-0060, Elabscience, Wuhan, China). Moreover, DNA/RNA OSDP and DNA methylation were assessed using an immunoassay kit (DNA/RNA Oxidative Damage (High Sensitivity) ELISA Kit, Cayman Chemicals, 589320, Ann Arbor, Michigan, MI, USA; DNA Methylation ELISA Kit, Cayman Chemicals, 589324, Ann Arbor, Michigan, MI, USA).
Samples and controls were measured in duplicate using the blind analysis method in the same run.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v. 9.0 (GraphPad Software, Inc., San Diego, CA, USA). The lack of normality in data distribution using the Shapiro–Wilk test was demonstrated. Thus, the groups were compared using the nonparametric tests were used and p < 0.05 was considered statistically significant. To assess the screening utility, the receiver operating characteristic (ROC) curves were determined and the area under the ROC curves (AUC) was analyzed. Moreover, Spearman correlations and logistic regression analyses were performed.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Bialystok, Poland (R-I-002/491/2019).
Informed consent
Written informed consent has been obtained from the patients to publish this paper.
Conclusions
Reporting angioinvasion as prognostic factor in PTC patients should be performed to ensure proper management and, therefore, screening tools to for objective evaluation of the process are of great importance. Our study confirmed the screening utility of serum oxidative stress markers as angioinvasion and metastasis indicators in PTC patients. The mechanisms that may connect to the angioinvasion process and oxidative stress in PTC need to be studied in depth.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Miranda-Filho, A. et al. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 9, 225–234. https://doi.org/10.1016/S2213-8587(21)00027-9 (2021).
Schmidbauer, B., Menhart, K., Hellwig, D. & Grosse, J. Differentiated thyroid cancer—treatment: State of the art. Int. J. Mol. Sci. 18, 1292 (2017).
Matrone, A. et al. Differentiated thyroid cancer, from active surveillance to advanced therapy: Toward a personalized medicine. Front. Endocrinol. (Lausanne) 10, 884. https://doi.org/10.3389/FENDO.2019.00884/BIBTEX (2020).
Nishino, M. & Jacob, J. Invasion in thyroid cancer: Controversies and best practices. Semin. Diagn. Pathol. 37, 219–227. https://doi.org/10.1053/J.SEMDP.2020.02.003 (2020).
Ameziane El Hassani, R., Buffet, C., Leboulleux, S. & Dupuy, C. Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocrine-Relat. Cancer 26, 131–143 (2019).
Kim, Y. W. & Byzova, T. V. Oxidative stress in angiogenesis and vascular disease. Blood 123, 625–631 (2014).
Xian, D. et al. Emerging roles of redox-mediated angiogenesis and oxidative stress in dermatoses. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2019/2304018 (2019).
Tanaka, M. & Chock, P. B. Oxidative modifications of RNA and its potential roles in biosystem. Front. Mol. Biosci. 8, 407. https://doi.org/10.3389/FMOLB.2021.685331/BIBTEX (2021).
Dhama, K. et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Media S.A. 6, 91 (2019).
Singh, C. K. et al. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 28, 23. https://doi.org/10.1089/ARS.2017.7290 (2018).
Deng, X. et al. p53 affects PGC1α stability through AKT/GSK-3β to enhance cisplatin sensitivity in non-small cell lung cancer. Front. Oncol. https://doi.org/10.3389/fonc.2020.01252 (2020).
Haugen, B. R. et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1. https://doi.org/10.1089/THY.2015.0020 (2016).
Mete, O. & Asa, S. L. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod. Pathol. 24, 1545–1552. https://doi.org/10.1038/MODPATHOL.2011.119 (2011).
Wreesmann, V. B. et al. Prognostic value of vascular invasion in well-differentiated papillary thyroid carcinoma. Thyroid 25, 503. https://doi.org/10.1089/THY.2015.0052 (2015).
Ortiz, S. et al. Extrathyroid spread in papillary carcinoma of the thyroid: Clinicopathological and prognostic study. Otolaryngol. Head Neck Surg. 124, 261–265. https://doi.org/10.1067/MHN.2001.113141 (2001).
Lin, X., Zhu, B., Liu, Y. & Silverman, J. F. Follicular thyroid carcinoma invades venous rather than lymphatic vessels. Diagn. Pathol. 5, 8. https://doi.org/10.1186/1746-1596-5-8 (2010).
Patmore, S., Dhami, S. P. S. & O’Sullivan, J. M. V. Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemost. 18, 2444–2456. https://doi.org/10.1111/JTH.14976 (2020).
Cracolici, V., Parilla, M., Henriksen, K. J. & Cipriani, N. A. An evaluation of CD61 immunohistochemistry in identification of vascular invasion in follicular thyroid neoplasms. Head Neck Pathol. 14, 399–405. https://doi.org/10.1007/S12105-019-01048-8 (2020).
Mohammed, R. A. A., Ellis, I. O., Lee, A. S. H. & Martin, S. G. Vascular invasion in breast cancer; an overview of recent prognostic developments and molecular pathophysiological mechanisms. Histopathology 55, 1–9. https://doi.org/10.1111/J.1365-2559.2008.03169.X (2009).
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell 38, 167–197 (2020).
Janion, K. et al. Evaluation of malondialdehyde level, total oxidant/antioxidant status and oxidative stress index in colorectal cancer patients. Metabolites 12, 1118. https://doi.org/10.3390/METABO12111118 (2022).
Muzza, M. et al. Oxidative stress correlates with more aggressive features in thyroid cancer. Cancers (Basel) https://doi.org/10.3390/CANCERS14235857 (2022).
Ushio-Fukai, M. & Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 266, 37–52. https://doi.org/10.1016/J.CANLET.2008.02.044 (2008).
Qutub, A. A. & Popel, A. S. Reactive oxygen species regulate hypoxia-inducible factor 1α differentially in cancer and ischemia. Mol. Cell. Biol. 28, 5106. https://doi.org/10.1128/MCB.00060-08 (2008).
AbbasalizadFarhangi, M. & Vajdi, M. Dietary total antioxidant capacity (TAC) significantly reduces the risk of site-specific cancers: An updated systematic review and meta-analysis. Nutr. Cancer 73, 721–739. https://doi.org/10.1080/01635581.2020.1771385 (2021).
Maurya, R. P. et al. Serum malondialdehyde as a biomarker of oxidative stress in patients with primary ocular carcinoma: Impact on response to chemotherapy. Clin. Ophthalmol. 15, 871–879. https://doi.org/10.2147/OPTH.S287747 (2021).
Zhao, Q. Y. et al. The role and therapeutic perspectives of Sirtuin 3 in cancer metabolism reprogramming, metastasis, and chemoresistance. Front. Oncol. 12, 2497. https://doi.org/10.3389/FONC.2022.910963/BIBTEX (2022).
Bell, E. L., Emerling, B. M., Ricoult, S. J. H. & Guarente, L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30, 2986–2996 (2011).
Ouyang, S. et al. The double-edged sword of SIRT3 in cancer and its therapeutic applications. Front. Pharmacol. 13, 1376. https://doi.org/10.3389/FPHAR.2022.871560/BIBTEX (2022).
Finley, L. W. S. et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 19, 416–428. https://doi.org/10.1016/J.CCR.2011.02.014 (2011).
Chen, H., Chen, J., Yuan, H., Li, X. & Li, W. Hypoxia-inducible factor-1α: A critical target for inhibiting the metastasis of hepatocellular carcinoma. Oncol. Lett. https://doi.org/10.3892/OL.2022.13404 (2022).
Guo, C. et al. Potential application of the oxidative nucleic acid damage biomarkers in detection of diseases. Oncotarget 8, 75767. https://doi.org/10.18632/ONCOTARGET.20801 (2017).
Kościuszko, M., Buczyńska, A., Krętowski, A. J. & Popławska-Kita, A. Could oxidative stress play a role in the development and clinical management of differentiated thyroid cancer?. Cancers (Basel) 15, 3182. https://doi.org/10.3390/CANCERS15123182 (2023).
Rogucki, M., Buczyńska, A., Krętowski, A. J. & Popławska-Kita, A. The importance of miRNA in the diagnosis and prognosis of papillary thyroid cancer. J. Clin. Med. https://doi.org/10.3390/jcm10204738 (2021).
Rogucki, M. et al. Expression profile and diagnostic significance of MicroRNAs in papillary thyroid cancer. Cancers (Basel) https://doi.org/10.3390/CANCERS14112679 (2022).
Guo, C. et al. Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-hydroxy-2′-deoxyguanosine by UPLC-MS/MS analysis. Sci. Rep. 6(1), 1–9. https://doi.org/10.1038/srep32581 (2016).
Dey, A., Tergaonkar, V. & Lane, D. P. Double-edged swords as cancer therapeutics: Simultaneously targeting p53 and NF-κB pathways. Nat. Rev. Drug Discov. 7(12), 1031–1040. https://doi.org/10.1038/nrd2759 (2008).
Farhan, M. et al. FOXO signaling pathways as therapeutic targets in Cancer. Int. J. Biol. Sci. 13, 815–827. https://doi.org/10.7150/IJBS.20052 (2017).
Carrà, G., Lingua, M. F., Maffeo, B., Taulli, R. & Morotti, A. P53 vs NF-κB: The role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell. Mol. Life Sci. 77, 4449–4458. https://doi.org/10.1007/S00018-020-03524-9 (2020).
Zhang, X., Tang, N., Hadden, T. J. & Rishi, A. K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 1813, 1978–1986. https://doi.org/10.1016/J.BBAMCR.2011.03.010 (2011).
Yang, Q. et al. PRKAA1/AMPKα1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat. Commun. 9(1), 1–17. https://doi.org/10.1038/s41467-018-07132-x (2018).
Liu, C. L. et al. PGC1α downregulation and glycolytic phenotype in thyroid cancer. J. Cancer 10, 3819. https://doi.org/10.7150/JCA.30018 (2019).
Bauerle, K. T. et al. Nuclear factor κB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/JC.2013-3636 (2014).
Rajabi, S. et al. The roles and role-players in thyroid cancer angiogenesis. Endocr. J. 66, 277–293. https://doi.org/10.1507/ENDOCRJ.EJ18-0537 (2019).
Reuter, S., Gupta, S. C., Chaturvedi, M. M. & Aggarwal, B. B. Oxidative stress, inflammation, and cancer: How are they linked?. Free Radic. Biol. Med. 49, 1603–1616 (2010).
Buczyńska, A. et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 11, 17126 (2021).
Buczyńska, A. et al. The relationship between oxidative status and radioiodine treatment qualification among papillary thyroid cancer patients. Cancers 15, 2436. https://doi.org/10.3390/CANCERS15092436 (2023).
Funding
This research was funded by internal financing of Medical University of Bialystok, grant number (SUB/1/DN/22/002/1150).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.B. and I.S.; methodology, A.B. and M.S.z.; software, M.K. and A.A; validation, A.B., P.S.Z. and J.D.Z.; formal analysis, I.S.; investigation, J.M.; resources, A.P.K.; data curation, K.S.; writing—original draft preparation, A.B. and I.S; writing—review and editing, I.S and A.J.K.; visualization, A.B.; supervision, A.P.K.; project administration, A.P.K.; funding acquisition, A.P.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buczyńska, A., Sidorkiewicz, I., Kościuszko, M. et al. Clinical significance of oxidative stress markers as angioinvasion and metastasis indicators in papillary thyroid cancer. Sci Rep 13, 13711 (2023). https://doi.org/10.1038/s41598-023-40898-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40898-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.