Abstract
To better understand the relationship among cell adhesion molecules (CAM) and investigate the clinical diagnostic and prognostic application of ICAM-1 (ICAM1), LFA-1 (ITGAL), and L-selectin (SELL) proteins and mRNA corresponding expression in thyroid cancer. Gene expression was evaluated by RT–qPCR, and protein expression was evaluated by immunohistochemistry. We evaluated 275 patients (218 women, 57 men, 48.4 ± 14.5 years old), including 102 benign and 173 malignant nodules. The 143 papillary thyroid carcinoma (PTC) and 30 follicular thyroid carcinoma (FTC) patients were managed according to current guidelines and followed-up for 78.7 ± 54.2 months. Malignant and benign nodules differed concerning mRNA (p = 0.0027) and protein (p = 0.0020 for nuclear) expression of L-selectin and ICAM-1 (mRNA: p = 0.0001 and protein: p = 0.0014) and protein expression of LFA-1 (p = 0.0168), but not mRNA expression of LFA-1 (p = 0.2131). SELL expression was more intense in malignant tumors (p = 0.0027). ICAM1 (p = 0.0064) and ITGAL (p = 0.0244) mRNA expression was higher in tumors with lymphocyte infiltrate. ICAM-1 expression correlated with younger age at diagnosis (p = 0.0312) and smaller tumor size (p = 0.0443). Also, LFA-1 expression correlated with higher age at diagnosis (p = 0.0376) and was more intense at stage III and IV (p = 0.0077). In general, the protein expression of the 3 CAM decreased as the process of cellular dedifferentiation occurred. We suggest that the SELL and ICAM1 genes and L-selectin and LFA-1 protein expression may help confirm malignancy and assist in the histological characterization of follicular patterned lesions, but we were unable to correlate these CAMs with patient outcomes.
Similar content being viewed by others
Introduction
Cell adhesion molecules (CAMs) are glycoproteins present in the cell membrane1. They play an important role in inflammatory and neoplastic diseases by recruiting immune cells to injured sites2,3. The migration of cells from the immune system consists of four main steps: capture, rolling and activation, arrest, and transmigration2,3. Initially, leukocytes are attracted to the vascular endothelium mainly by selectin molecules. Rolling begins with contact with chemoattractant and leukocyte activation. After that, integrins interact with immunoglobulins, enabling adhesion to the endothelial surface and transmigration4.
L-selectin, ICAM-1 and LFA-1 are pivotal CAMs that collaborate in the cell migration process from capture to the transmigration process but acting at different stages of the process and in different ways. The literature has been reporting their expression and potential role in aggressive cancers, but the way they interact in the progression and proliferation of thyroid cells is still unclear.
L-selectin is a transmembrane molecule encoded by the SELL gene. Its cleavage site allows a soluble form of the molecule, and the cleavage of the structure occurs after its activation5,6. L-selectin expression was initially considered exclusive to the leukocyte surface3,5, but other cell types and several types of cancer also showed this molecule7,8,9,10. In general, L-selectin favors interactions that allow both leukocytes3 and metastatic tumor cells7,8,9,10 to start the rolling process. L-selectin expression in thyroid neoplastic cells has been related to more aggressive tumor behavior since it was found to be associated with lymph node metastasis7 and BRAFV600E mutation11.
ICAM-1 is a transmembrane glycoprotein generally expressed on the surface of several cell types, such as leukocytes, endothelial cells, and fibroblasts12. This protein is involved in the regulation of leukocyte trafficking across the endothelial barrier and therefore plays a key role during the inflammatory response. ICAM-1 interacts with lymphocyte function-associated antigen 1 (LFA-1), facilitating the migration of immune cells to the injured site12. This binding promotes stable adhesion of leukocytes to endothelial cells, which is an essential step for recognition of the transmigration site. Studies have found that ICAM-1 is overexpressed in different types of cancer13, including thyroid cancer, and has associated it with aggressive features such as extrathyroidal extension, lymph node metastasis, and BRAFV600E mutation14,15,16.
LFA-1 is an integrin also known as ALB2 integrin and is encoded by the ITGAL gene. Only leukocytes express the B2 group integrins17, which are inactive under basal conditions. However, when chemoattracted to injury or tumor sites, they experience conformational changes that expose allosteric sites and allow interaction with other CAMs18. The main ligand of LFA-1 is ICAM-119, which binds with high affinity20,21 and acts in the stable adhesion step22. This molecule is expressed on the surface of all leukocytes23 and has recently been described in several types of tumor cells, including breast24, colorectal25,26, and melanoma20. LFA-1 expression has been associated with metastatic progression10, whereas the B2 integrin expression decrease in colorectal cancer tumor cells has been correlated with lower chances of liver metastasis outbreaks27.
This study focuses on L-selectin, ICAM-1 and LFA-1 to better understand their role in the migration of neoplastic cells seeking possible practical applications in the diagnosis and prognosis of thyroid cancer.
Materials and methods
This study was conducted at the Laboratory of Molecular Genetics of Cancer (GEMOCA) and approved by the Faculty of Medical Science of State University of Campinas Research Ethics Committee (IRB00002737 and CAAE#65254916.9.0000.5404) and all methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all individual participants included in the study.
Patients
We evaluated a total of 275 thyroid nodule paraffin blocks from 218 women and 57 men, 48.4 ± 14.5 years old, patients referred to the Clinical Hospital FCM/UNICAMP and the TMB Private Surgical Clinic in Campinas/São Paulo/Brazil, who underwent partial or total thyroidectomy. Of those, 102 (37%) nodules were benign (48 goiters and 54 follicular adenomas–FA), and 173 (63%) were malignant (143 papillary thyroid carcinomas-PTC and 30 follicular thyroid carcinomas-FTC). The PTC included 55 classic PTC (CVPTC). Since we aimed to better understand CAMs role in aggressive tumors, our cohort was enriched with 38 follicular variants of PTC (FVPTC), 27 oxyphilic variants of papillary thyroid carcinoma/Hürthle cell carcinoma (OVPTC), and 23 tall cell variants of PTC (TCPTC). Hurthle cell tumors were defined as those that presented more than 75% of the tumor cells with oncocytic characteristics28. Most of the 173 malignant samples (n = 123, 71.1%) were from patients younger than 55 years. Most patients in this group (n = 44, 77.2%) were TNM I and TNM II (n = 13, 22.8%).
All patients were managed according to a well-established protocol in accordance with international consensuses and followed-up for 78.7 ± 54.2 months29,30. Data were collected from medical records and included sociodemographic information, use of medications, and performed tests, including ultrasound, whole body scan, X-rays and other imaging tests, serum TSH, thyroglobulin and other eventual serum measurements, presurgical clinical data, surgery description, and anatomopathological tissue examination. Diagnostic aggressiveness was determined according to the TNM system of classification and staging of differentiated thyroid carcinoma (DTC). A disease-free status was defined as the absence of detectable residual disease (on ultrasound and WBS) and low basal (< 0.2 ng/mL) Tg serum levels. Persistent disease was defined as the presence of a detectable residual or metastatic tumor on imaging methods and/or elevated basal (> 0.2 ng/mL) Tg serum levels. Tumor recurrence was defined as structural disease diagnosed more than one year after ablation in patients without persistent disease. From the total, 57 patients evolved free of disease, 69 patients presented lymph node and/or distant metastasis, and 7 patients died due to DTC during the observation period of this study. The remaining patients could not be included with certainty in any of these groups and were excluded from further statistical evaluation concerning evolution or prognosis. All tissues were examined by two experienced pathologists with particular attention to tumor extension beyond the thyroid capsule (extrathyroidal extension), angiolymphatic, vascular and/or perineural invasion (invasion) and lymphocytic infiltration.
Unfortunately, we could not extract good quality mRNA from all paraffin blocks, but we were able to further investigate mRNA expression in 191 thyroid nodule patients (149 women and 42 men, 47.5 ± 14.4 years old) comprising 97 benign nodules (47 goiters and 50 FA) and 94 malignant nodules (74 PTC and 20 FTC). The PTC included 29 CVPTC, 21 FVPTC, 12 OVPTC and 12 TCPTC cases.
We also obtained 3 normal thyroid tissues from the necropsy of patients who had no history or signs of thyroid disease that were used as control samples.
We excluded patients with a history of accidental or medical exposure to ionizing radiation, other malignancies, autoimmune diseases, and pregnant women and children under 18 years old. FVPTCs suspected of being noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTPs) were excluded from this study.
Immunohistochemistry analysis
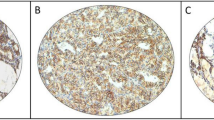
A tissue microarray (TMA) was assembled from formalin-fixed paraffin-embedded tissues representative of tumor regions, previously selected by two experienced pathologists. Each case was added to the TMA in duplicate. Five micrometer sections were cut with the microtome CUT5062 (SLEE Medical, Mainz, DE). The immunohistochemistry reaction was carried out with deparaffinization and rehydration of the tissues. Endogenous peroxidase was blocked with 10 volumes of hydrogen peroxide. Tissues were incubated in Trizma-Ethylenediaminetetra acetic Acid (Tris–EDTA) buffer antigen recovery buffer for 40 min at 97 °C in a steamer (Hamilton Beach Brands, Virginia, USA) and left to rest in 3% milk for 30 min. We added 150 µL of antibody to each slide using specific antibodies for each protein (L-selectin—Anti-CD62L, polyclonal antibody, dilution 1:50; ICAM-1—Anti-ICAM1, monoclonal antibody, dilution 1:1000 and LFA-1—Anti-CD11a, monoclonal antibody, dilution 1:750). The antibody dilution tests were previously performed individually with their respective positive controls. The slides were then incubated in a humid chamber (60 min at 37 °C) and stored in a refrigerator (4 °C) for 16–18 h. Slides were washed in Phosphate-buffered Saline (PBS) 1X, 150 µL of secondary antibody was added and they were incubated (30 min at 37ºC). After that, 3.3-diaminobenzidine-tetrahydrochloride (DAB—150 µL) was added onto the slides before they were packed in PBS 1X until counterstaining with Mayer’s hematoxylin. Positive and negative controls were run in the same batch of reaction. Different concentrations of alcohol and xylol were used to dehydrate the tissue The final score was assigned based on slide analysis performed independently by two different pathologists with extensive thyroid experience, both blinded to tumor characteristics. The analysis included an evaluation of the intensity (weak, moderate, and strong) and the proportion (focal or diffuse) of expression of the studied proteins.
RNA extraction and reverse transcription
Total RNA from 191 formalin-fixed paraffin-embedded (FFPE) thyroid tumor samples was extracted using the Recover All* Total Nucleic Acid Isolation Kit FFPE (Life Technologies, California, USA) according to the manufacturer’s instructions. All samples were treated with DNaseI (Thermo Fisher Scientific, Waltham, EUA). RNA purity was confirmed by spectrophotometry using the Epoch™ Spectrophotometer System (BioTek Instruments Inc., Vermont, USA) with the Take3™ Multivolume Plate.
First-strand complementary DNA (cDNA) synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, California, USA) according to the manufacturer’s instructions. The initial RNA template used for the reaction was 1000 ng. All samples were amplified in a thermocycler (Biosystems, Barcelona, ES) according to the manufacturer’s instructions. After the reaction, the cDNA was diluted to a final concentration of 5 ng/µL.
Primer optimization and reference gene testing
Primers were designed and verified using the free online software Primer3Plus31, OligoAnalyzer32, and insilicoPCR33 and were tested at concentrations of 100 nM, 200 nM, 400 nM, and 800 nM, evaluating the best concentration without losses in the amplification cycle due to the absence of primers in the reaction. We used the following primers and concentrations for the respective genes: SELL—5’TACTGCATTCTCTGGGTTGG3’ and 5’CACCAAGGGCGATTTAATATG3’ (200 nM); ICAM1—5’AGCTTCTCCTGCTCTGCAAC3’ and 5’CATTGGAGTCTGCTGGGAAT3’ (100 nM) and ITGAL—5’GTTGACGTGGTGTATGAGAAGC3’ and 5’GTTGACGTGGTGTATGAGAAGC3’ (200 nM). The efficiency curve was performed with a cDNA1:10 dilution at an initial concentration of 50 ng/µL. We also used four reference genes (ACTB—5’GTCTTCCCCTCCATCGTG3’ and 5’CCACATAGGAATCCTTCTGACC3’; HPRT1—5’CTTTGCTTTCCTTGGTCAGG3’ and 5’TTCGTGGGGTCCTTTTCAC3’; RPL19—5’AGACCCCAATGAGACCAATG3’ and 5’GGATGATCAGCCCATCTTTG3’ and; GAPDH—5’CAACGACCACTTTGTCAAGC3’ and 5’TTCCTCTTGTGCTCTTGCTG3’, all with 200 nM concentration) as normalizers for our samples. The analysis was performed using two software programs: BestKeeper34 and GeNorm35. Both software programs indicated that the GAPDH gene would be the gene with the least stability among the reference genes tested for our samples. Thus, we chose the ACTB, HPRT1, and RPL19 genes as references for this research.
Quantitative real-time PCR (qPCR)
Reactions were prepared with 5 µL of ItaQ Universal SYBR Green Supermix (Bio–Rad, California, USA) 2 × , 2.5 µL of cDNA (concentration = 5 ng/µL) and varying volumes of Milli-Q® water and Primers, according to the concentration established in the concentration tests, to reach the final reaction volume of 10 µL. Analysis was performed with a 7500RT-PCR system (Applied Biosystems, California, USA) according to the manufacturer’s instructions. Each sample was assayed in triplicate. The mRNA expression was calculated using the Pfaffl method36, and the expression levels were given in arbitrary units (AU).
Statistical analysis
The statistical analysis was performed using GraphPad Prism software (version 7.00 for Windows, GraphPad Software, California, USA). The Mann–Whitney or Wilcoxon tests were used to compare continuous random variables between two groups, and the Kruskal–Wallis test was used to compare three or more groups whose variables did not have a normal distribution. Spearman’s coefficient was used to verify linear associations between variables. All tests were conducted at the 5% significance level.
Results
SELL
SELL mRNA expression (n = 157 cases, of which 77 were benign and 80 were malignant) was higher in malignant (0.85 ± 1.54 AU) than in benign thyroid nodules (0.54 ± 0.71 AU, p = 0.0027). SELL mRNA expression could differentiate PTC from FA (p = 0.0018) and FTC from FA (p = 0.0078). Also, SELL mRNA expression was significantly lower in Hürthle cell tumors (0.45 ± 0.63 AU vs 0.95 ± 1.46 AU, p < 0.0001), but we could not demonstrate any other association with clinical or pathological features (Table 1). SELL expression was not associated with any characteristic of tumor aggressiveness or patient outcome.
Regarding protein expression (n = 229 cases, of which 98 benign and 131 malignant), nuclear expression (Fig. 1A) showed weaker intensity in malignant tumors (p = 0.0020) but not cytoplasm (p = 0.3344, Fig. 1B). However, both nuclear and cytoplasmic staining could differentiate follicular patterned lesions (Nuclear: PTC vs goiter, p < 0.0001; PTC vs FA, p = 0.0020; PTC vs FTC, p = 0.0133. FTC vs goiter, p < 0.0001; FTC vs FA, p < 0.0001, and Cytoplasmic: FA vs PTC, p = 0.0026; FA vs FTC, p = 0.0005 and Goiter vs FTC, p = 0.0062).
The group of patients younger than 55 years that were classified as TNM I + II (n = 32) did not differ from the remaining patients, both concerning mRNA gene expression (0.790 ± 1.710 vs III + IV, n = 7, 0.910 ± 1.880; p = 0.8397) and protein expression (nuclear, p = 0.4304 and cytoplasmic, p = 0.8040).
The nuclear and cytoplasmic protein expression of L-selectin were not correlated with any other clinical feature or to patient outcome (Table 2).
ICAM1
ICAM1 mRNA expression (n = 147 cases, of which 70 were benign and 77 were malignant) was higher in malignant (0.99 ± 1.413 AU) than in benign thyroid nodules (0.46 ± 0.85 AU, p = 0.0001), and it differentiated PTC from goiter (p = 0.0030). Likewise SELL, ICAM1 expression was lower in Hürthle cell tumors (0.44 ± 0.82 AU vs 0.95 ± 1.60 AU, p < 0.0001) and was higher in the presence of lymphocytic infiltration (1.16 ± 3.04 AU vs 0.52 ± 0.96 AU, p = 0.0064), but we could not associate it with any other clinical or pathological features (Table 1). ICAM1 expression was also not associated with any characteristic of tumor aggressiveness or patient outcome.
Apical protein expression of ICAM-1 (Fig. 1C) was positive in 52.5% (n = 21) of our goiter samples, 51.2% (n = 22) of FA samples, and 31.8% (n = 7) of FTC samples. A moderate intensity of ICAM-1 distinguished malignant (25%) from benign nodules (6.0%; p = 0.0014). The group of patients younger than 55 years that were classified as TNM I + II (n = 34) did not differ from the remaining patients, both concerning mRNA gene expression (1.130 ± 3.461 vs III + IV, n = 7, 1.640 ± 3.540; p = 0.8722) and protein expression (p = 0.6766).
ICAM-1 expression correlated with smaller tumor size (p = 0.0443) but not with any other clinical feature or to the patients’ outcome (Table 2).
ITGAL
ITGAL mRNA expression (n = 161 cases, of which 81 were benign and 80 were malignant) did not differ between malignant (1.04 ± 1.63 AU) and benign thyroid nodules (0.76 ± 1.21 AU, p = 0.2131) or among the histological types evaluated (PTC vs goiter, p = 0.1652; PTC vs FA, p = 0.7297; PTC vs FTC, p = 0.6662; FTC vs goiter, p = 0.2335; FTC vs FA, p = 0.4256 and FA vs goiter, p = 0.3029). However, ITGAL expression was correlated with several clinical pathological features associated with good prognosis: mRNA expression was higher in the presence of lymphocytic infiltration (1.17 ± 1.54 AU vs 0.49 ± 1.39 AU, p = 0.0244, Fig. 2A); in the absence of lymphovascular infiltration (1.26 ± 1.96 AU vs 0.74 ± 1.01 AU, p = 0.0427, Fig. 2B); and in the absence of extrathyroidal extension (1.25 ± 1.86 AU vs 0.72 ± 0.95 AU, p = 0.0111, Fig. 2C). Conversely, ITGAL was higher in cases with distant metastasis at diagnosis (1.53 ± 2.18 AU vs 0.57 ± 1.10 AU, p = 0.0217, Fig. 2D). We could not associate ITGAL expression with patient outcome (Table 1).
LFA-1 protein expression (Fig. 1D, n = 221 cases, of which 99 were benign and 122 were malignant) was more frequently negative in malignant tumors (p = 0.0168). We observed that this negativity was increasingly present in dedifferentiated samples (15,6% (n = 7) goiter; 14,8% (n = 8) FA; 26,2% (n = 26) PTC and 42,1% (n = 8) FTC were negative, p = 0.0489). Also, LFA-1 expression was more intense at stage III and IV(p = 0.0077). However, a comparison of patients younger than 55 years that were classified as TNM I + II (n = 31) with the remaining patients did not show any difference, both concerning mRNA gene expression (0.720 ± 1.550 vs III + IV, n = 7, 0.830 ± 2.230; p = 0.6112) and protein expression (p = 0.5519). Moderate expression of LFA-1 correlated with older age at diagnosis (p = 0.0376) but not with any other clinical feature or to the patients’ outcome (Table 2).
Discussion
The importance of the immune response in tumor progression and patient prognosis is becoming increasingly clear, and our group is one of those who has been dedicating intense attention to the clinical application of the several cells and immune system mediators involved in this response37,38,39,40,41,42,43,44. However, interpreting data is difficult since tumor microenvironment elements can play a dual role, either suppressing malignancy or cooperating with tumor growth45,46. CAMs are essential for the migration of leukocytes to sites of inflammation and play an important role in stimulating intracytoplasmic signals that regulate cell differentiation, survival, and proliferation47. In fact, our data indicate that both ICAM1 and ITGAL are associated with lymphocytic chronic infiltration. Due to their multiple interactions in different signaling pathways, CAMs correlate with a good prognosis in some tumor types, whereas in other neoplasm types, their expression is correlated with a worse prognosis and aggressive characteristics5,7,8,9,13,14,15,16,20,21,26,27,48,49,50,51,52. Taking advantage of a very well-characterized group of DTC patients carefully followed-up for more than 6 years by the same group in a single institution, we investigated the role of SELL, ITGAL, and ICAM1 mRNA and protein expression in the characterization of thyroid nodules and their possible clinical utility in identifying aggressive tumors.
L-selectin is a molecule that acts mainly in the capture and rolling of migratory cells, both leukocytes and metastatic cells10,49. Its expression has been studied in several types of cancer, such as ovarian50, bladder48, and thyroid cancer7,9,11, and is usually related to tumor aggressiveness. Muzza et al.9 found higher expression of L-selectin in 15 PTC than in 4 normal (contralateral) thyroid tissues. We also observed higher SELL mRNA expression in malignant tumors than in benign thyroid nodules (p = 0.0027). We also showed that SELL mRNA expression could differentiate some of the histopathology follicular patterned cases, especially FA from FTC (p = 0.0078). Despite the trend toward higher SELL expression in tumors with lymphocytic infiltrate (1.40 ± 2.16 AU) when compared with tumors without this characteristic (0.88 ± 1.44 AU, p = 0.1883), we could not confirm the findings of Muzza et al.9 on this association9.
We observed decreased expression of both nuclear and cytoplasmic L-selectin in more aggressive histological types, suggesting that nuclear expression of this molecule decreases as neoplastic cells become less differentiated. On the other hand, Choudhary et al. observed that membrane L-selectin expression was higher in PTC than in benign nodules 5. This finding may be explained by L-selectin being cleaved after its activation, releasing its extracellular portion in soluble form5,48. Hence, the activation of this molecule may contribute to the cellular metastatic process. Indeed, the literature has reported a higher serum concentration of L-selectin in several types of malignant tumors8,50,53,54. The increase in soluble L-selectin and the possible relocation of its cytoplasmic portion may stimulate the increase in mRNA levels, explaining the higher SELL mRNA expression observed in malignant tumors alongside the lower expression of both nuclear and cytoplasmic proteins. However, note that L-selectin is a molecule participating in a process that involves a tangle of other molecules, cytokines, chemokines, transcription factors, signaling pathways, and other components that act in a systemic and integrated way. Thus, other yet undescribed factors may modulate L-selectin function.
ICAM-1 is a glycoprotein involved in both innate and adaptive immune responses55. It has been suggested that ICAM-1 acts as a facilitator of cancer cell spread through the recruitment of inflammatory cells that release stimulating factors for cell proliferation, angiogenesis, and invasion. This molecule also plays a role in the mechanisms of tumor cell progression, and studies have related it to the prognosis of some types of cancer52,56. Hayes and Seigel15 analyzed approximately 300 samples of normal, malignant, and metastatic tissues from various tumors, including thyroid cancer, and found overexpression of both the ICAM1 gene and ICAM-1 protein in malignant tissues. More recently, Buitrago et al.14 confirmed higher expression of ICAM1 in malignant tumors, corroborating our findings. However, despite also observing higher mRNA expression in the presence of lymphocytic infiltration, we could not associate ICAM1 with any characteristic of tumor aggressiveness or patient outcome.
LFA-1 is encoded by the ITGAL gene and is expressed in the leukocyte membrane23 but has also been described in several types of tumor cells20,24,25,26. LFA-1 has been associated with the progression of lymphomas57 and colorectal cancer58. Papas et al.26 evaluated LFA-1 expression in primary tumors and in metastatic tissues. They found a higher expression in primary tumors from patients without distant metastasis when compared to tumor tissue from patients who had no metastasis at diagnosis, proposing a negative correlation between the expression of LFA-1 and the metastatic process. The authors suggested a tumor suppressor role for LFA-1 in colorectal adenocarcinomas since its expression was higher in primary tumors from patients without distant metastasis. However, they also observed a higher expression of LFA-1 in the metastatic tissues when compared to the primary tumor, which may suggest a different role of LFA-1 after the initiation of the metastatic process since this molecule participates intensely in the migratory process. Our findings of lower ITGAL mRNA expression in cases with lymphovascular invasion (p = 0.0427) and extrathyroidal extension (p = 0.0111) and high mRNA expression in cases with lymphocytic infiltrate (p = 0.0244), reinforce a tumor suppressive role for LFA-1 in thyroid tumorigenesis. We know that the conformational structure of LFA-1 changes to interact with other CAMs, especially ICAM-118 making us speculate that LFA-1 role depends on its conformational structure. Alternatively, it may be regulated by other signaling pathway molecules.
In conclusion, our data confirm an important role of CAMs in thyroid cell proliferation and tumor progression. In general, the protein expression of these molecules decreases as the cell dedifferentiation process occurs. However, the relatively long and positive course of most thyroid cancer patients, and the complex role of CAMs at the different stages of tumor evolution hinders the correlation of these molecules with tumor aggressiveness or patient outcomes in a clinical setting. On the other hand, we demonstrated that mRNA expression of SELL and ICAM1 and protein expression of L-selectin and LFA-1 can help in the histological characterization of thyroid nodules with a follicular pattern.
Data availability
All data generated or analysed during this study are included in this published article.
References
Elangbam, C. S., Qualls, C. W. Jr. & Dahlgren, R. R. Cell adhesion molecules–update. Vet. Pathol. 34(1), 61–73 (1997).
Luster, A. D., Alon, R. & von Andrian, U. H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 6(12), 1182–1190 (2005).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7(9), 678–689 (2007).
Gupta, G. S. L-Selectin (CD62L) and Its Ligands. Animal Lectins: Form, Function and Clinical Applications. Vienna: Springer Vienna; 2012. p. 553–74.
Ivetic, A., Hoskins Green, H. L. & Hart, S. J. L-selectin: A major regulator of leukocyte adhesion migration and signaling. Front. Immunol. 10, 1068 (2019).
Kheradmand, F. & Werb, Z. Shedding light on sheddases: Role in growth and development. BioEssays 24(1), 8–12 (2002).
Kobawala, T. P. et al. Significance of TNF-alpha and the adhesion molecules: L-Selectin and VCAM-1 in papillary thyroid carcinoma. J. Thyroid Res. 2016, 8143695 (2016).
Korniluk, A., Kaminska, J., Kiszlo, P., Kemona, H. & Dymicka-Piekarska, V. Lectin adhesion proteins (P-, L- and E-selectins) as biomarkers in colorectal cancer. Biomarkers 22(7), 629–634 (2017).
Muzza, M. et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin. Endocrinol. (Oxf). 72(5), 702–708 (2010).
Reymond, N., d’Agua, B. B. & Ridley, A. J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 13(12), 858–870 (2013).
Borrelli, N. et al. Role of gene expression profiling in defining indeterminate thyroid nodules in addition to BRAF analysis. Cancer Cytopathol. 124(5), 340–349 (2016).
Yang, L. et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106(2), 584–592 (2005).
Lin, Y. C., Shun, C. T., Wu, M. S. & Chen, C. C. A novel anticancer effect of thalidomide: inhibition of intercellular adhesion molecule-1-mediated cell invasion and metastasis through suppression of nuclear factor-kappaB. Clin. Cancer Res. 12(23), 7165–7173 (2006).
Buitrago, D. et al. Intercellular adhesion molecule-1 (ICAM-1) is upregulated in aggressive papillary thyroid carcinoma. Ann. Surg. Oncol. 19(3), 973–980 (2012).
Hayes, S. H. & Seigel, G. M. Immunoreactivity of ICAM-1 in human tumors, metastases and normal tissues. Int. J. Clin. Exp. Pathol. 2(6), 553–560 (2009).
Tanda, F. et al. Intercellular adhesion molecule-1 (ICAM-1) immunoreactivity in well-differentiated thyroid papillary carcinomas. Mod. Pathol. 9(1), 53–56 (1996).
Laudanna, C., Kim, J. Y., Constantin, G. & Butcher, E. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186, 37–46 (2002).
Carman, C. V. & Springer, T. A. Integrin avidity regulation: Are changes in affinity and conformation underemphasized?. Curr. Opin. Cell Biol. 15(5), 547–556 (2003).
de Fougerolles, A. R., Qin, X. & Springer, T. A. Characterization of the function of intercellular adhesion molecule (ICAM)-3 and comparison with ICAM-1 and ICAM-2 in immune responses. J. Exp .Med. 179(2), 619–629 (1994).
Ghislin, S. et al. LFA-1 and ICAM-1 expression induced during melanoma-endothelial cell co-culture favors the transendothelial migration of melanoma cell lines in vitro. BMC Cancer 12, 455 (2012).
Reina, M. & Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers (Basel). 9(11) (2017).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Manikwar, P. et al. Utilization of I-domain of LFA-1 to target drug and marker molecules to leukocytes. Theranostics. 1, 277–289 (2011).
Wang, H. S. et al. CD44 cross-linking induces integrin-mediated adhesion and transendothelial migration in breast cancer cell line by up-regulation of LFA-1 (alpha L beta2) and VLA-4 (alpha4beta1). Exp. Cell Res. 304(1), 116–126 (2005).
Fujisaki, T. et al. CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 59(17), 4427–4434 (1999).
Papas, M. G. et al. LFA-1 expression in a series of colorectal adenocarcinomas. J. Gastrointest. Cancer 43(3), 462–466 (2012).
Benedicto, A., Marquez, J., Herrero, A., Olaso, E., Kolaczkowska, E. & Arteta, B. Decreased expression of the beta2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. 17(1), 827 (2017).
Maximo, V., Lima, J., Prazeres, H., Soares, P. & Sobrinho-Simoes, M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr. Relat. Cancer 23(12), X2 (2016).
Haugen, B. R. et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1), 1–133 (2016).
Nabhan, F. & Ringel, M. D. Thyroid nodules and cancer management guidelines: comparisons and controversies. Endocr. Relat. Cancer. 24(2), R13-r26 (2017).
Untergasser, A., Nijveen, H., Rao, X., Bisseling, T., Geurts, R. & Leunissen, J. A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35(Web Server issue), W71–4 (2007).
Technolgies ID. OligoAnalyzer. 2019.
Haeussler, M. et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 47(D1), D853–D858 (2019).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26(6), 509–515 (2004).
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A. & Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3(7), Research0034 (2002).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29(9), e45 (2001).
Cunha, L. L., Marcello, M. A. & Ward, L. S. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr. Relat. Cancer 21(3), R85-r103 (2014).
Cunha, L. L. et al. Infiltration of a mixture of different immune cells may be related to molecular profile of differentiated thyroid cancer. Endocr. Relat. Cancer 19(3), L31–L36 (2012).
Cunha, L. L. & Ward, L. S. Concurrent lymphocytic thyroiditis is associated to less aggressive papillary thyroid carcinomas. Eur. Arch. Otorhinolaryngol. 269(2), 699–700 (2012).
Cunha, L. L. & Ward, L. S. Comments on “well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence”. Endocr. Pathol. 22(3), 172–173 (2011).
Cunha, L. L., Soares, F. A., Vassallo, J. & Ward, L. S. The role of tumor-infiltrating lymphocytes in papillary thyroid carcinomas. J. Endocrinol. Investig. 34(9), 733 (2011).
Marcello, M. A. et al. P53 and expression of immunological markers may identify early stage thyroid tumors. Clin. Dev. Immunol. 2013, 846584 (2013).
Cunha, L. L., Ferreira Rde, C., de Matos, P. S., da Assumpcao, L. V. & Ward, L. S. Both gender and concurrent chronic lymphocytic thyroiditis may influence the nuclear texture of papillary thyroid carcinomas cells. Endocr. Res. 39(3), 126–129 (2014).
Cunha, L. L. et al. Interleukin 10 expression is related to aggressiveness and poor prognosis of patients with thyroid cancer. Cancer Immunol. Immunother. 66(2), 141–148 (2017).
Bissell, M. J. & Hines, W. C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17(3), 320–329 (2011).
Coussens, L. M., Zitvogel, L. & Palucka, A. K. Neutralizing tumor-promoting chronic inflammation: A magic bullet?. Science 339(6117), 286–291 (2013).
Huang, S. & Ingber, D. E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1(5), E131–E138 (1999).
Choudhary, D. et al. Increased expression of L-selectin (CD62L) in high-grade urothelial carcinoma: A potential marker for metastatic disease. Urol. Oncol. 33(9), 387.e17–27 (2015).
Laubli, H. & Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 20(3), 169–177 (2010).
Majchrzak-Baczmanska, D. B., Glowacka, E., Wilczynski, M. & Malinowski, A. Serum concentrations of soluble (s)L- and (s)P-selectins in women with ovarian cancer. Prz Menopauzalny. 17(1), 11–17 (2018).
Raffler, N. A., Rivera-Nieves, J. & Ley, K. L-selectin in inflammation, infection and immunity. Drug Discov. Today Therap. Strateg. 2(3), 213–220 (2005).
Dymicka-Piekarska, V. & Kemona, H. Does colorectal cancer clinical advancement affect adhesion molecules (sP-selectin, sE-selectin and ICAM-1) concentration?. Thromb. Res. 124(1), 80–83 (2009).
Eichbaum, M. H., de Rossi, T. M., Kaul, S. & Bastert, G. Serum levels of soluble E-selectin are associated with the clinical course of metastatic disease in patients with liver metastases from breast cancer. Oncol. Res. 14(11–12), 603–610 (2004).
Haznedaroglu, I. C. et al. Serum L-selectin and P-selectin levels in lymphomas. Haematologia (Budap). 30(1), 27–30 (2000).
Lawson, C. & Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 61(1), 22–32 (2009).
Dowlati, A., Gray, R., Sandler, A. B., Schiller, J. H. & Johnson, D. H. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—An Eastern Cooperative Oncology Group Study. Clin. Cancer Res. 14(5), 1407–1412 (2008).
Roossien, F. F., de Rijk, D., Bikker, A. & Roos, E. Involvement of LFA-1 in lymphoma invasion and metastasis demonstrated with LFA-1-deficient mutants. J. Cell Biol. 108(5), 1979–1985 (1989).
Valcarcel, M. et al. Three-dimensional growth as multicellular spheroid activates the proangiogenic phenotype of colorectal carcinoma cells via LFA-1-dependent VEGF: implications on hepatic micrometastasis. J. Transl. Med. 6, 57 (2008).
Acknowledgements
The authors thank the statisticians of the School of Medical Sciences of University of Campinas (UNICAMP) and Espaço da Escrita—Pró-Reitoria de Pesquisa—UNICAMP—for the language services provided. We would also like to thank Dr. José Barreto Carvalheira and Sandra Brambilla from the Molecular Oncology Laboratory (UNICAMP) for allowing us to use laboratory facilities.
Funding
This study received financial support from the São Paulo Research Foundation (FAPESP-grant#2016/20458–0) and Coordination of Superior Level Staff Improvement (CAPES).
Author information
Authors and Affiliations
Contributions
Conception and design: LTR, KCP, NEB, VM and LSW Acquisition of data: LTR, KCP, MN, EST, AJT, ISB, LVMA and NEB Literature search and data analysis: LTR, KCP, NEB and LSW Design and optimization of RT-qPCR: LTR, KCP, MVG and NEB Design, optimization and evaluation of immunoistochemistry analysis: LTR, KCP, LLLF, ISB and NEB Statistical analysis, figures and tables: LTR, KCP and NEB Writing, review and/or revision of the manuscript: LTR, NEB and LSW Study supervision: LSW All authors approved the final manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rabi, L.T., Peres, K.C., Nascimento, M. et al. Investigation of the clinical utility of adhesion molecules in the management of thyroid nodules. Sci Rep 13, 4069 (2023). https://doi.org/10.1038/s41598-023-31302-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31302-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.