Abstract
Hypotension after cardiac arrest could aggravate prolonged hypoxic ischemic encephalopathy. The association of circulatory shock at hospital admission with outcome after cardiac arrest has not been well studied. The objective of this study was to investigate the independent association of circulatory shock at hospital admission with neurologic outcome, and to evaluate whether cardiovascular comorbidities interact with circulatory shock. 4004 adult patients with out-of-hospital cardiac arrest enrolled in the International Cardiac Arrest Registry 2006–2017 were included in analysis. Circulatory shock was defined as a systolic blood pressure below 90 mmHg and/or medical or mechanical supportive measures to maintain adequate perfusion during hospital admission. Primary outcome was cerebral performance category (CPC) dichotomized as good, (CPC 1–2) versus poor (CPC 3–5) outcome at hospital discharge. 38% of included patients were in circulatory shock at hospital admission, 32% had good neurologic outcome at hospital discharge. The adjusted odds ratio for good neurologic outcome in patients without preexisting cardiovascular disease with circulatory shock at hospital admission was 0.60 [0.46–0.79]. No significant interaction was detected with preexisting comorbidities in the main analysis. We conclude that circulatory shock at hospital admission after out-of-hospital cardiac arrest is independently associated with poor neurologic outcome.
Similar content being viewed by others
Introduction
The main driver of poor long-term outcome after out-of-hospital cardiac arrest (OHCA) is hypoxic ischemic encephalopathy (HIE)1. The early phase after cardiac arrest can be complicated by circulatory shock, due to low systemic vascular resistance and/or myocardial dysfunction2, potentially aggravating HIE through prolonged cerebral hypoperfusion. The reported incidence of circulatory shock at presentation after OHCA is 15–68%3,4,5,6. In studies circulatory shock is defined as hypotension, clinical signs of hypoperfusion, or the need for supportive measures to maintain an acceptable perfusion pressure. The lack of a universally accepted definition of circulatory shock and differences in patient selection could explain the large variation in the incidence reported. Outcomes after cardiac arrest include survival and/or neurologic recovery, dichotomized as favorable or poor, according to the International Liaison Committee of Resucitation (ILCOR)7. Observational studies have failed to prove an association between increased cardiac output post-cardiac arrest and improved long-term outcomes8,9,10,11. Current guidelines based on observational studies recommend a mean arterial pressure (MAP) above 65 mmHg during the initial phase of critical care12,13. Impaired autoregulation of cerebral perfusion has been reported in 35% of post resuscitation patients and is overrepresented in patients with preexisting arterial hypertension14. This may explain lack of beneficial effects in interventional trials targeting increased blood pressure for patients with hypotension post OHCA15,16. Despite the many studies on the association between circulatory shock on admission and outcome after OHCA the data are conflicting17,18,19. In this observational study of a large international cardiac arrest database, our hypothesis was that circulatory shock at hospital admission after OHCA is independently associated with neurologic outcome. As a secondary analysis, we explored the relative contribution of circulatory shock in patients with cardiovascular comorbidities, on neurologic outcome.
Methods
Study design and setting
This is a retrospective registry study of patients enrolled from 2006 to 2017 in The International Cardiac Arrest Registry (INTCAR), a large North American/European post cardiac arrest registry containing a defined core dataset. Ethical Review Boards (ERB) in each country approved data collection and participation. Informed consent was waived from all participants in line with the Helsinki declaration, by approval of the Ethical Review Board in Lund, reference number: REPN Lund Dnr 2007/272.
Study population
All patients in the INTCAR registry were screened for eligibility. Exclusion criteria were the following: in-hospital cardiac arrest (IHCA); Patients awake on admission defined as Glasgow Coma Score motor component equal to 6; Age below 18 years; Missing records on the presence or absence of circulatory shock on admission or Cerebral Performance Category (CPC) at hospital discharge. Subgroup analysis was performed in patients with continuous explanatory variables linearly associated with outcome.
Outcomes and definitions
According to the International Liaison Committee of Resucitation, core outcome recommendations20, the primary outcome was dichotomized as good (CPC 1–2) or poor (CPC 3–5) at hospital discharge. The CPC scale ranges from 1 to 5, with 1 representing good cerebral performance or minor disability, 2 moderate disability, 3 severe disability, 4 coma or vegetative state, and 5 brain death21. By default, poor neurologic recovery according to this definition, is correlated with mortality. Circulatory shock was defined as systolic blood pressure < 90 mm Hg and/or the need for supportive measures, such as inotropes, vasoactive drugs, mechanical circulatory support devices to maintain a systolic blood pressure ≥ 90 mmHg or end-organ hypoperfusion. Hospital admission was defined as the first unit the patient presented at after the OHCA, emergency department (ED), the intensive Care Unit (ICU) or coronary angiography lab, depending on the routines of the including hospital. Time to start of advanced life support (ALS) was defined as time from witnessed cardiac arrest or, in the event of unwitnessed arrest, time from emergency call to start of advanced life support by medical personnel. Return of spontaneous circulation (ROSC) was defined to have occurred when chest compressions were not required for 20 consecutive minutes and signs of circulation persisted. Predefined co-morbidities were registered if they involved current pharmacological or prior surgical treatment or were under active medical supervision at the time of arrest. Obesity was defined as body mass index > 35.
Statistical analysis
Continuous descriptive data are presented as medians with interquartile (IQR) range. Differences in baseline variables were tested using Chi-square test for categorical data, while continuous data were tested using Student’s t-Test or Mann–Whitney test as appropriate. Variables with less than 20% missing were considered as candidates for explanatory variables in analyses. Pairwise correlation was estimated for all combinations of variables. The least relevant variable, from the perspective of our main hypothesis, was dropped from analysis if Pearson correlation coefficient was over 0.75 or less than -0.75. Skewed continuous explanatory variables were transformed to normal distribution, choosing the method yielding the lowest Pearson P statistic/degrees of freedom22. All continuous variables were scaled to standard deviations (SD) and centered22. Analysis of variables with more than 5% missing did not reveal any evidence for systemic mechanism of missingness. Final analyses were based on the pooled estimates, using Rubin’s rules, from 24 datasets imputed by chained equations with predictive mean matching for continuous variables and logistic regression for categorical data, using all modelled variables and outcomes. Imputation was done under the assumption of missing at random. The association between circulatory shock and outcome was estimated using generalized additive methods (GAM) with smooth functions fitting continuous data to cubic restricted splines using 5 knots. Fit of the smooth functions for continuous data were visually checked by plotting residuals for the explanatory variables versus the log odds of the dependent variable, and by estimating the k-index. Models were adjusted for: first monitored rhythm: Pulseless Electrical Activity (PEA), asystole or either pulseless ventricular tachycardia or ventricular fibrillation (VT/VF); Presence of ST-Elevation Myocardial Infarction (yes/no); witnessed arrest (yes/no); bystander cardiopulmonary resuscitation (yes/no); time to start of ALS (square root transformed minutes); sex (male/female); time to ROSC (ordered quantiles transformed minutes); age (ordered quantiles transformed years); circulatory shock on presentation at hospital (yes/no), presence of the following comorbidities(present/not present): Neuro-vascular disease, Chronic obstructive pulmonary disease, obesity, diabetes, chronic kidney disease, hypertension, coronary artery disease, congestive heart failure, arrhythmia; interaction between comorbidities and circulatory shock on presentation. Results from GAM models are presented as odds ratios (OR) with 95% confidence interval (CI). To estimate the association with outcome for the continuous variables in our model, subgroup analysis was performed on a patient group defined by continuous variables linearly associated with neurologic outcome at hospital discharge. Cutoffs for the variables in subgroup analysis was empirically tested and confirmed linear. Complete cases models confirmed concurvity for continuous data < 0.01, and variance inflation index (VIF) for categorical data < 3, indicating no significant concurvity or multicollinearity. Goodness of fit was confirmed with Hosmer–Lemeshow test. Sensitivity analysis was performed comparing our results with that of data imputed with a span of 75–125% of originally imputed using a calibrated-∂ adjustment23 data for variables with missingness more than 5%. Because of the exploratory nature of this analysis, no correction was done for multitesting.
Results
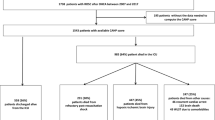
Out of 5943 enrolled patients in the INTCAR registry 4004 were eligible for analysis (Fig. 1). Of the included patients in final analysis, 1506 (38%) patients were in circulatory shock at hospital admission and 1298 (32%) patients had good neurologic outcome (CPC 1–2) at hospital discharge. Circulatory shock was more frequent in women, 42.0 versus 35.6% in men. Patients with circulatory shock at hospital admission were older 64 [54–73] versus 62 [51–72] years, had less witnessed cardiac arrest 75.8 versus 79.4%, higher prevalence of non-shockable rhythm (asystole or PEA) 52.3 versus 47.7%, longer median time to ROSC 29 [18–42] versus 20 [13–30] minutes, higher burden of comorbidities, and presented with worse neurologic clinical status. The use of a mechanical chest compression device was more common in the circulatory shock population, 22.9 versus 19.5% (Table 1).
The explanatory model revealed a nonlinear association between age, ROSC and outcome. The association with time to ALS was linear (Supplementary Fig. S1). For the full population, the adjusted OR for circulatory shock at hospital admission as an explanatory factor for good neurologic outcome, in the setting of no preexisting comorbidity, was 0.60 [0.46–0.79]. No significant interaction with preexisting comorbidities was detected (Fig. 2). The explained variance of the model was 32.6%.
Association with good neurological outcome at hospital discharge. Forest plot illustrating the odds ratios for Cerebral Performance Category 1–2, in a multivariate generalized additive methods model. Analysis was performed in the full cohort, n = 4004. The reference category for first monitored rhythm is shockable rhythm (Ventricular fibrillation or ventricular tachycardia). Time to ALS time to ALS has been square root transformed scaled to standard deviations and centered. Interaction between circulatory shock and preexisting comorbidities are included in model. Point estimates are presented as odds ratios (OR) with 95% confidence intervals. Time to return of spontaneous circulation and age are included in model but not presented due to nonlinearity. PEA Pulseless electrical activity; STEMI ST-Elevation myocardial infarction; ECG Electrocardiogram; CPR Cardiopulmonary resuscitation; SD Standard deviation; COPD Chronic pulmonary obstructive disease; BMI Body mass index; TTM Targeted temperature management.
To investigate the relative contribution on outcome for of all the variables in the model, we defined a subgroup of patients with linear continuous variables by empiric testing of different cutoffs for these. We found that in the group aged 42–92 years with time to ROSC 9–87 min constituting 3030 patients (76% of the total cohort), explanatory continuous variables were linear (Supplementary Fig. S2). For the patients in the subgroup analysis the adjusted OR for good outcome with circulatory shock at hospital admission and none of the defined preexisting comorbidities, was 0.65 [0.47–0.90]. The OR for the interaction between circulatory shock and preexisting hypertensive disease, as the only comorbidity, was 0.64 [0.42–0.98], indicating 36% worse odds for good outcome in patients with circulatory shock and a history of hypertension compared to circulatory shock without previous hypertension. Contrary, in circulatory shock and preexisting arrhythmia the OR for the interaction was 1.96 [1.03–3.71], indicating roughly double the odds for good outcome compared to circulatory shock alone (Fig. 3).
Association with good neurological outcome at hospital discharge in subgroup analysis. Forest plot illustrating the odds ratios for Cerebral Performance Category 1–2, in a multivariate generalized additive methods model. Analysis was performed in a subgroup of patients with linear continuous explanatory variables, aged 42–92 years, with time to ROSC 9–87 min. The reference category for first monitored rhythm is shockable rhythm (Ventricular fibrillation or ventricular tachycardia). Age (years) and time to ROSC (minutes) have been transformed to normality by ordered quantiles, time to ALS has been square root transformed. After transformation, the variables have been scaled to standard deviations and centered. Interaction between circulatory shock and preexisting comorbidities are included in the model. Point estimates are presented as odds ratios (OR) with 95% confidence intervals. PEA Pulseless electrical activity; STEMI ST-Elevation myocardial infarction; ECG Electrocardiogram; ROSC Return of spontaneous circulation; CPR Cardiopulmonary resuscitation; SD Standard deviation; COPD Chronic pulmonary obstructive disease; BMI Body mass index; TTM Targeted temperature management.
In sensitivity analysis of the impact of missing data, the point estimate for circulatory shock at hospital admission as a predictor for neurologic outcome at hospital discharge remained very similar to that of the imputed dataset (data not shown).
Discussion
In this large retrospective cohort study of unconscious adult survivors of OHCA admitted to the intensive care unit, the main finding is that circulatory shock at hospital admission is independently associated with poor neurologic outcome at hospital discharge.
The use of hypotension as a surrogate definition of circulatory shock, complicates the interpretation of the underlying cause(s), since blood pressure is the product of cardiac output and systemic vascular resistance. A number of observational studies have previously investigated blood pressure levels during the early phase of critical care, leading up to the European resuscitation council 2021 guidelines recommendation of a MAP > 65 mmHg for unconscious survivors of cardiac arrest8,10,12,13,14,18,19,24,25,26,27,28,29,30,31,32,33,34. Seven of these studies analyzed blood pressure levels within the first hour after hospital admission17,18,19,28,30,34,35, three of them reporting conflicting evidence regarding blood pressure as an independent predictor of outcome17,19,34. Contrary to our findings, a single center study consisting of a mixed cohort of IHCA/OHCA cases not treated with targeted temperature management (TTM), found that MAP five minutes after ROSC was not associated with neurologic outcome in adjusted analysis34. Less than 15% of patients in this study had recorded 5-min MAP levels < 65 mmHg, which might reflect a lingering adrenaline effect, which could have confounded the results. In an Australian registry study, systolic arterial blood pressure < 90 mmHg at admission after OHCA was found to be independently associated with lower hospital survival only in the subgroup of patients with an initial shockable rhythm17. Patients in that study were aggressively resuscitated with fluid loading and prehospital vasopressors, suggesting that the relative contribution of circulatory shock on outcome might decrease in patients with the worst physiologic characteristics. In a study of a mixed cohort of IHCA/OHCA patients, a systolic arterial blood pressure < 90 mmHg within one hour of ICU admission was found to be independently associated with higher in-hospital mortality19. This is in line with our findings, but the authors found a higher relative contribution on outcome, possibly due patient selection and model not adjusted for time to ROSC.
Our results suggest that hypotension and/or circulatory shock may contribute to subsequent neurologic outcome. However, the association between circulatory shock (using both the pragmatic and physiological definition of circulatory shock) and neurologic outcome has, to our knowledge, not been studied. A few small observational studies report the influence of low cardiac output in the setting of the subacute phase of OHCA. Huang et al. report increased hospital survival, but no difference in neurologic outcome among patients with a cardiac index (CI) > 2.5 l/min/m2 at 12 h post ROSC10. In two other studies, no such association was found8,9; another study found an association between higher CI time integral and poor neurologic outcome at 28 days11. Two randomized controlled trials have published neutral results on surrogate markers for neurologic outcome using circulatory interventions to optimize cerebral oxygen delivery in the acute phase post OHCA15,16. The results of our subgroup analysis suggest an interaction between circulatory shock on admission and pre-arrest hypertensive disease. This finding is in line with previous studies14,36; the effect was not significant in the full cohort. The results of the exploratory subgroup analysis should be cautiously interpreted in the context of multi-testing and should be seen as hypothesis generating.
This study adds to the previous conflicting evidence that early circulatory shock in the context of OHCA is independently associated with poor neurologic outcome. Autoregulation is the innate mechanism regulating cerebral blood flow over a wide range of perfusion pressures, mitigating ischemia and hyper perfusion37. The lower limit of autoregulation, however, is right shifted after cardiac arrest38. This leaves cerebral blood flow reliant on blood pressure, rendering cerebral oxygenation vulnerable in hypotension. This process is further aggravated by cerebral hypoperfusion caused by microcirculatory injury39, potentially explaining our findings. Results also suggest that individually optimized blood pressure targets should be investigated in future studies, as some patients may benefit from higher post-arrest blood pressure targets. This could explain why hemodynamic interventional trials have failed to improve outcome in the context of cardiac arrest. Comparing the point estimates in our study, the relative influence of the presence of circulatory shock at the time of hospital admission on neurologic outcome at hospital discharge is minor as compared to some of the other explanatory variables in our model, specifically first documented rhythm, age, and time to ROSC. Explained variance is low, indicating the need for further studies targeting the pathophysiologic mechanism of circulatory shock in this context and how individual physiological characteristics may affect the trajectory of circulatory shock and outcomes.
Limitations
Definitions of circulatory shock, timing and duration of circulatory shock, patient selection, sample size, TTM-levels and timing/choice of endpoints make comparison of studies cumbersome. We acknowledge a number of limiting factors in our study, the most obvious ones being: (1) The lack of a universally accepted definition of circulatory shock; (2) The sensitivity and specificity for circulatory shock with the predefined definition used in the INTCAR database has not been published. (3) Additional signs normally associated with the clinical diagnosis of circulatory shock, e.g. lactate, was not included in the INTCAR database. (4) Neurologic outcome in this study was evaluated at hospital discharge, which may be too early for complete neurologic recovery. (5) Additional outcome measures of neurologic recovery or neurologic injury, eg neuropsychiatric/cognitive testing and biomarkers indicating cerebral injury (eg. NSE, NFL and S-100), was not available in the predefined dataset. Due to the correlation between dichotomized neurologic outcome with mortality, our results also should be interpreted within the context of survival. Further, the study population represents a convenience sample and registered data were not monitored, which introduces potential bias. The influence of circulatory shock on outcome in the setting of cardiac arrest is inherently difficult because of competing risk of poor outcome due to the initial hypoxic/ischemic insult. We lack data on cause of arrest, biomarkers and physiological parameters, which could account for the low explained variance in our models. Analyses were made on a large predefined dataset, and although the sensitivity analysis over a wide range was similar to the results of the imputed dataset, there is no guarantee that missing data did not affect the results. By virtue of design, causal inferences cannot be made.
Conclusion
Circulatory shock at hospital admission after OHCA is independently associated with poor neurologic outcome at hospital discharge.
Data availability
The data that support the findings of this study are available from corresponding author, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request.
References
Dragancea, I., Rundgren, M., Englund, E., Friberg, H. & Cronberg, T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 84, 337–342. https://doi.org/10.1016/j.resuscitation.2012.09.015 (2013).
Nolan, J. P. et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79, 350–379. https://doi.org/10.1016/j.resuscitation.2008.09.017 (2008).
Nielsen, N. et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 369, 2197–2206. https://doi.org/10.1056/NEJMoa1310519 (2013).
Lascarrou, J. B. et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 381, 2327–2337. https://doi.org/10.1056/NEJMoa1906661 (2019).
Lemiale, V. et al. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 39, 1972–1980. https://doi.org/10.1007/s00134-013-3043-4 (2013).
Annborn, M. et al. The association of targeted temperature management at 33 and 36 °C with outcome in patients with moderate shock on admission after out-of-hospital cardiac arrest: A post hoc analysis of the target temperature management trial. Intensive Care Med. 40, 1210–1219. https://doi.org/10.1007/s00134-014-3375-8 (2014).
Perkins, G. D. et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 96, 328–340. https://doi.org/10.1016/j.resuscitation.2014.11.002 (2015).
Grand, J. et al. Cardiac output, heart rate and stroke volume during targeted temperature management after out-of-hospital cardiac arrest: Association with mortality and cause of death. Resuscitation 142, 136–143. https://doi.org/10.1016/j.resuscitation.2019.07.024 (2019).
Oksanen, T. et al. Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: A retrospective study. Resuscitation 85, 1018–1024. https://doi.org/10.1016/j.resuscitation.2014.04.026 (2014).
Huang, C. H. et al. Association of hemodynamic variables with in-hospital mortality and favorable neurological outcomes in post-cardiac arrest care with targeted temperature management. Resuscitation 120, 146–152. https://doi.org/10.1016/j.resuscitation.2017.07.009 (2017).
Torgersen, C. et al. Haemodynamic variables and functional outcome in hypothermic patients following out-of-hospital cardiac arrest. Resuscitation 84, 798–804. https://doi.org/10.1016/j.resuscitation.2012.10.012 (2013).
Nolan, J. P. et al. European resuscitation council and European society of intensive care medicine guidelines 2021: Post-resuscitation care. Resuscitation 161, 220–269. https://doi.org/10.1016/j.resuscitation.2021.02.012 (2021).
Nolan, J. P. et al. European resuscitation council and European society of intensive care medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 47, 369–421. https://doi.org/10.1007/s00134-021-06368-4 (2021).
Ameloot, K. et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: Time to drop “one-size-fits-all” hemodynamic targets?. Resuscitation 90, 121–126. https://doi.org/10.1016/j.resuscitation.2015.03.001 (2015).
Ameloot, K. et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: The Neuroprotect post-cardiac arrest trial. Eur. Heart J. 40, 1804–1814. https://doi.org/10.1093/eurheartj/ehz120 (2019).
Jakkula, P. et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 44, 2091–2101. https://doi.org/10.1007/s00134-018-5446-8 (2018).
Bray, J. E. et al. The association between systolic blood pressure on arrival at hospital and outcome in adults surviving from out-of-hospital cardiac arrests of presumed cardiac aetiology. Resuscitation 85, 509–515. https://doi.org/10.1016/j.resuscitation.2013.12.005 (2014).
Young, M. N. et al. Higher achieved mean arterial pressure during therapeutic hypothermia is not associated with neurologically intact survival following cardiac arrest. Resuscitation 88, 158–164. https://doi.org/10.1016/j.resuscitation.2014.12.008 (2015).
Trzeciak, S. et al. Significance of arterial hypotension after resuscitation from cardiac arrest*. Crit. Care Med. 37, 2895–2903. https://doi.org/10.1097/CCM.0b013e3181b01d8c (2009).
Grasner, J. T. et al. European resuscitation council guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 161, 61–79. https://doi.org/10.1016/j.resuscitation.2021.02.007 (2021).
Brain Resuscitation Clinical Trial I Study Group. A randomized clinical study of cardiopulmonary-cerebral resuscitation: Design, methods, and patient characteristics. Brain Resuscitation Clinical Trial I Study Group. Am. J. Emerg. Med. 4, 72–86 (1986).
Peterson, R. A. & Cavanaugh, J. E. Ordered quantile normalization: A semiparametric transformation built for the cross-validation era. J. Appl. Stat. 47, 2312–2327. https://doi.org/10.1080/02664763.2019.1630372 (2020).
Pham, T. M., Carpenter, J. R., Morris, T. P., Wood, A. M. & Petersen, I. Population-calibrated multiple imputation for a binary/categorical covariate in categorical regression models. Stat. Med. 38, 792–808. https://doi.org/10.1002/sim.8004 (2019).
Kilgannon, J. H. et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation 79, 410–416. https://doi.org/10.1016/j.resuscitation.2008.07.019 (2008).
Gaieski, D. F. et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation 80, 418–424. https://doi.org/10.1016/j.resuscitation.2008.12.015 (2009).
Sunde, K. et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 73, 29–39. https://doi.org/10.1016/j.resuscitation.2006.08.016 (2007).
Ameloot, K. et al. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: A prospective observational study. Resuscitation 91, 56–62. https://doi.org/10.1016/j.resuscitation.2015.03.016 (2015).
Annoni, F. et al. The impact of diastolic blood pressure values on the neurological outcome of cardiac arrest patients. Resuscitation 130, 167–173. https://doi.org/10.1016/j.resuscitation.2018.07.017 (2018).
Bro-Jeppesen, J. et al. Hemodynamics and vasopressor support during targeted temperature management at 33 °C versus 36 °C after out-of-hospital cardiac arrest: A post hoc study of the target temperature management trial*. Crit. Care Med. 43, 318–327. https://doi.org/10.1097/CCM.0000000000000691 (2015).
Chiu, Y. K., Lui, C. T. & Tsui, K. L. Impact of hypotension after return of spontaneous circulation on survival in patients of out-of-hospital cardiac arrest. Am. J. Emerg. Med. 36, 79–83. https://doi.org/10.1016/j.ajem.2017.07.019 (2018).
Laurikkala, J. et al. Mean arterial pressure and vasopressor load after out-of-hospital cardiac arrest: Associations with one-year neurologic outcome. Resuscitation 105, 116–122. https://doi.org/10.1016/j.resuscitation.2016.05.026 (2016).
Russo, J. J. et al. Optimal mean arterial pressure in comatose survivors of out-of-hospital cardiac arrest: An analysis of area below blood pressure thresholds. Resuscitation 128, 175–180. https://doi.org/10.1016/j.resuscitation.2018.04.028 (2018).
Janiczek, J. A. et al. Hemodynamic resuscitation characteristics associated with improved survival and shock resolution after cardiac arrest. Shock 45, 613–619. https://doi.org/10.1097/SHK.0000000000000554 (2016).
Mullner, M. et al. Arterial blood pressure after human cardiac arrest and neurological recovery. Stroke 27, 59–62. https://doi.org/10.1161/01.str.27.1.59 (1996).
Beylin, M. E. et al. Higher mean arterial pressure with or without vasoactive agents is associated with increased survival and better neurological outcomes in comatose survivors of cardiac arrest. Intensive Care Med. 39, 1981–1988. https://doi.org/10.1007/s00134-013-3075-9 (2013).
Roberts, B. W. et al. Association between elevated mean arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest: Results from a multicenter prospective cohort study. Crit. Care Med. 47, 93–100. https://doi.org/10.1097/CCM.0000000000003474 (2019).
Lassen, N. A. Autoregulation of cerebral blood flow. Circ. Res. 15(Suppl), 201–204 (1964).
Sundgreen, C. et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 32, 128–132. https://doi.org/10.1161/01.str.32.1.128 (2001).
Sekhon, M. S., Ainslie, P. N. & Griesdale, D. E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 21, 90. https://doi.org/10.1186/s13054-017-1670-9 (2017).
Funding
Open access funding provided by Lund University. The INTCAR registry was supported by research grants from Stig and Ragna Gorthon Foundation, Hans-Gabriel & Alice Trolle-Wachtmeisters Foundation for Medical Research, Regional research grants from the council of Skåne and government funding of clinical research within the Swedish National Health Services (ALF), and in part by NIH U54GM115516: nne-ctr.net». The funding organizations did not have any access to the data, nor did they have any influence on data analysis or interpretation.
Author information
Authors and Affiliations
Contributions
J.D.: Designed study, analyzed and interpreted the results, prepared and approved final version of this manuscript. M.A.: Substantially revised and approved final version of this manuscript. J.D.: Data manager/maintainer of INTCAR registry. Prepared data for analysis. Substantially revised and approved final version of this manuscript. A.D.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. S.F.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. H.F.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. K.B.K.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. T.L.M.: Data manager/maintainer of INTCAR registry. Substantially revised and approved final version of this manuscript. J.M.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. N.P.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. D.S.: Conception of the INTCAR registry. Substantially revised and approved final version of this manuscript. P.S.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. K.S.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. E.S.: Principal investigator in INTCAR registry. Substantially revised and approved final version of this manuscript. S.U.: Substantially revised and approved final version of this manuscript. N.N.: Conception of the INTCAR registry. Design of study. Analyzed and interpreted results, substantially revised and approved final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Düring, J., Annborn, M., Dankiewicz, J. et al. Influence of circulatory shock at hospital admission on outcome after out-of-hospital cardiac arrest. Sci Rep 12, 8293 (2022). https://doi.org/10.1038/s41598-022-12310-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12310-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.