Abstract
Dongting Lake is one of the most important inland freshwater lakes in China. To investigate the spatial distribution and seasonal variation characteristics of heavy metals (Cr, Co, Cu, Zn, Cd, and Pb) in the lake, 53 surface sediment samples were collected in the East Dongting Lake (ED Lake) in the wet and dry seasons. Results show Cr, Co, Cu, Zn, Cd, and Pb contents were 1.7 (1.9), 1.8 (2.0), 2.9 (3.0), 1.9 (1.9), 11.7 (13.1), and 2.0 (2.2)-fold of their geochemical soil background values of Hunan province (China) in the wet (dry) season. Spatial and seasonal heterogeneity could be found in the distribution of Cr, Co, Cu, Zn, and Pb in the surface sediments. The enrichment factor (EF) suggested that Cd has reached severe enrichment in the sediment. The result of the geo-accumulation index (\({I}_{geo}\)) indicated that Cr, Co, Cu, Zn, and Pb were at levels corresponding to low contamination, and moderately to highly polluted with Cd. Multivariate statistical analysis including pearson correlation analysis and principal component analysis was used for the identification of potential sources of the heavy metals in the sediments. The results showed that Cu, Zn, and Pb from the sediments of the East Dongting Lake in the wet and dry seasons were possibly anthropogenic sources, such as emissions from mining and smelting while Al, Fe, and Cr are attributed for natural sources. Cd enrichment in the sediments is influenced by both natural factors, and human activities in local areas.
Similar content being viewed by others
Introduction
Due to their toxic behavior, persistence, non-biodegradability, and bioaccumulation, heavy metals are considered as key environmental pollutants that need to be controlled1,2. Once absorbed by living organisms, heavy metals are difficult to degrade and can be transferred and accumulated along the food chain, ultimately posing a significant threat to human health3,4. The two main sources of heavy metals in the environment are geochemical reactions and anthropogenic activities. The main anthropogenic sources are industrial discharge, agricultural chemicals, and municipal sewage discharge. Residual heavy metals from human activities enter aquatic ecosystems through surface runoff, groundwater, and atmospheric deposition. The identification of anthropogenic or natural sources of heavy metals is important and can be used to assess the impact of human activities on ecosystems and to predict changes in the biological effects of heavy metals5. Studies have shown that only a small proportion of the heavy metals entering aquatic systems exist in the water column in a dissolved state, and most of them are adsorbed by the sediment in various ways (physical adsorption, chemical precipitation, etc.) and enriched in the sediment, resulting in a much higher concentration of heavy metals in the sediment than in the water column. There is the potential for heavy metals in the sediment to be re-released into the water column, which can lead to secondary contamination of the water column when hydrodynamic conditions or environmental factors (pH, redox potential, etc.) change6,7,8. Therefore, sediment can be also considered as a potential source of heavy metals in the aquatic environment9,10. The seasonal variation of climate characteristics like temperature, rainfall, and humidity activities and production change significantly, which contributes to seasonally variable distribution characteristics and sources of heavy metals11,12. Hence, monitoring programs covering different seasons depict temporal variations in aquatic environment and may offer representative data for assessing the environmental status of the lake system13.
Dongting Lake is one of the most important inland freshwater lakes in China. Located in the eastern part of the Dongting Lake, East Dongting Lake (ED Lake) receives a constant flow of water and material input from the Yangtze River through the mouth of Ouchi and out through Chenglingji. The Dongting Lake is surrounded by a large number of industrial enterprises including metallurgical and chemical industries, as well as large areas of agricultural land14, and a large amount of industrial and agricultural wastewater, as well as domestic sewage, is discharged into the Dongting Lake every year15,16. Anthropogenic activities have greatly influenced the content and distribution of heavy metals in the lake. Many studies have been done on the investigation, monitoring, and assessment of the pollution status of Dongting Lake17,18,19. Annual precipitation in the Dongting Lake basin is 1100–1400 mm, which descends from the outer hills to the inner plains. The precipitation of mostly heavy rain and rainstorm from April to July provides over 50% of the total annual precipitation. April to August is the wet season in the area, with the maximum water level occurring from July to August. By contrast, November to March is the dry season20. The grain size and sedimentation rate of particles carried by the incoming water, and flow rate of the water body in the Dongting Lake during the wet season are different from those during the dry season, which can affect the content, distribution, and geochemical behavior of heavy metal elements in the Dongting Lake in different seasons5,21. However, most studies of heavy metals in Dongting Lake focused only on one season. Li et al. investigated heavy metals in the surface sediments of Dongting Lake in January and studied their spatial risk assessment and source apportionment22,23. Similarly, Peng et al. assessed the spatial distribution and ecological risk of heavy metals in West Dongting Lake in the dry season24. Few studies have compared the heavy metal content of Dongting Lake in the different seasons. In this study, ecological risk assessment and source apportionment of the sediments of the East Dongting Lake were carried out, and the spatial distribution of heavy metal levels in different seasons was compared.
The objectives of this study are (1) to investigate the seasonal variations (wet and dry seasons) of heavy metals content in the sediment of the ED Lake, (2) to conduct a source identification of heavy metals in the sediment of the ED Lake by using multivariate statistical analysis.
Materials and methods
Sample collection and analysis
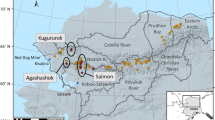
Three cruises (A, B, and C) were designed for sampling to investigate the spatial distribution characteristics of heavy metals and for seasonal comparisons. Sampling was carried out along cruises A, B, and C in August 2012 (wet season) and along cruises A and B in January 2013 (dry season) (Fig. 1). A total of 53 surface sediment samples (31 samples in the wet season and 22 samples in the dry season) were collected using a homemade mud collector and placed in clean plastic bags. Samples collected in the wet and dry seasons were abbreviated as ED-W (including samples from cruises A, B, and C) and ED-D (including samples from A and B), respectively. The sampling sites were kept at the same latitude and longitude coordinates during the dry season as during the wet season.
The sediment samples were naturally dried and ground through a 200 mesh sieve. All samples were stored at 4 °C and analyzed at the Beijing Research Institute of Uranium Geology (BRIUG). Cr, Co, Cu, Zn, Cd, and Pb in the sediments were determined by inductively coupled plasma mass spectrometry (ICP-MS, ELEMENT XR, Thermo, USA). Al and Fe were determined by X-ray fluorescence spectrometer (XRF, AB-104 L, PW2404, Ltd., the Netherlands).
QA/QC
Sample duplicates, method blanks, and standard reference materials GBW07309 (GSD-9) and GBW07312 (GSD-12) were used for quality assurance and quality control. A duplicate experiment was done for every four samples and the pass rate of duplicate samples was 100%. The recoveries of the two standard reference materials were 96–106% and 103–106% respectively.
Enrichment factor and geo-accumulation
The enrichment factor (EF) was calculated to estimate the enrichment and possible anthropogenic impact of heavy metals on sediments using the following Eq. 25:
where \({(\frac{{C}_{i}}{{C}_{Al}})}_{Sediment}\) is the ratio of the concentration of a particular metal i (Ci) to the Al concentration (CAl) in the sediment sample; and \({(\frac{{C}_{i}}{{C}_{Al}})}_{Background}\) is the ratio of the background concentration of a particular metal i (Ci) to the reference background concentration of Al (CAl). Either Al or Fe has been used as a conservative element for EF calculations in many studies to distinguish the source of heavy metals26,27. In this study, Al was selected as a reference metal. The value of enrichment factor < 1 indicates no enrichment; 1 to 3 is minor; 3 to 5 is moderate; 5 to 10 is moderately severe; 10 to 25 is severe; 25 to 50 is very severe, and > 50 is extremely severe enrichment28.
The geo-accumulation (\({I}_{geo}\)) method is a quantitative index for the study of heavy metal pollution in sediments29. \({I}_{geo}\) was calculated as:
where \({C}_{i}\) is the concentration of heavy metal i in the samples, mg/kg; \({B}_{i}\) is the soil background value of heavy metal i in Hunan province, China30. A factor of 1.5 is used to amend the possible variation in the background value due to lithogenic effects. The classification of \({I}_{geo}\) was as follows: unpolluted (\({I}_{geo}\le 0\)), lowly polluted (\({0<I}_{geo}\le 1\)), moderately polluted (\({1<I}_{geo}\le 2\)), moderately to highly polluted (\({2<I}_{geo}\le 3\)), highly polluted (\({3<I}_{geo}\le 4\)), highly to extremely polluted (\({4<I}_{geo}\le 5\)), and extremely polluted (\({I}_{geo}>5\)).
Statistical analysis
Pearson correlation analysis and principal component analysis were performed by SPSS 22.0 (IBM SPSS Inc.). Pearson correlation analysis was subjected to a two-tailed significance test. Kaiser–Meyer–Olkin (KMO) and Bartlett's sphericity test were used to assess the validity of the principal component analysis31. The varimax method was used for rotation. Extraction of principal components based on eigenvalues (> 1) and cumulative variance (> 80%) in principal component analysis.
Results and discussion
Heavy metals contents
As can be seen from Table 1, in the ED-W, the concentrations of Zn varied the most between sites, ranging from 133.0 to 214.0 μg/g, while Co and Cd varied the least, with SD values of 1.34 and 0.47, respectively. In the ED-D, Zn and Cu had larger SD values than the other heavy metals (23.37 and 20.18, respectively), while Cd ranged from 0.48 to 2.94 μg/g with an SD value of 0.51. The contents of all six heavy metals in the sediments were higher than their background values to varying degrees. In the ED-W, Cr, Co, Cu, Zn, Cd, and Pb concentrations in the sediments were 1.7, 1.8, 2.9, 1.9, 11.7, and 2.0 times higher than those in the background values, respectively, while in the ED-D, Cr, Co, Cu, Zn, Cd, and Pb concentrations in the sediments were 1.9, 2.0, 3.0, 1.9, 13.1, and 2.2 times higher than those in the background values, respectively (Table 1).
A comparison of the heavy metal contents of the sediments in the East Dongting Lake with those in some other lakes is shown in Table 1. High concentrations of Cr, Cu, Zn, Cd, and Pb can be found in some lakes, possibly closely related to local anthropogenic activities. Another study on the East Dongting Lake showed a significant decrease of concentrations in Cr, Cu, Zn, and Pb compared to this study, except for a significant increase of concentrations in Cd from 0.92 mg/kg and 1.03 to 2.74 mg/kg in the ED-W and ED-D.
Spatial distribution of heavy metals in different seasons
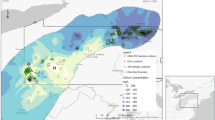
According to Fig. 2, The peak concentrations of heavy metals Cr, Co, Cu, Zn, and Pb in the dry and wet seasons were found at sites B1, B2, and B3 (except Cr in the wet season), while the valley values were found at sites A10, A9, A8, and B12 (except Co in the wet season). In cruise B (from B1 to B12), a clear trend of variation of Cu, Zn, and Pb contents with spatial distribution can be observed. In cruise A, this variation can only be clearly seen in Cu. As can be seen in Fig. 1, the sites B1, B2, and B3 are close to the left shore of the lake and are located near the outlet of Ouchi, from which the water flow from the Yangtze River is discharged into the East Dongting Lake. Therefore, this spatial distribution of heavy metal content was most likely to be affected by hydrodynamic conditions39. During the flow of water from the outlet of Ouchi to the center of the lake, the velocity of the water gradually decreases, and the sediment carried by the water is gradually deposited on the bottom of the lake, together with the dilution effect of the lake water, thus the heavy metal content decreases with the increase of the offshore distance. Some elements, such as Pb and Zn, showed an increasing trend of content from A1 to A10 in the wet season samples. It might be attributed to the fact that aquatic plants are more abundant near the shore due to the shallow water depth. Some plants have an enrichment behavior towards heavy metals in the sediment and this may have an impact on the heavy metal content in the sediment. Differences in the growth of aquatic plants at different sites were observed during the field survey. Additionally, as for the cruise C, however, unfortunately, no samples were collected in the dry season. Only the concentration of the heavy metals in the wet season was summarized here briefly. The variation of heavy metal content with spatial location can be observed from C1 to C9. There is an increasing trend of Cd, Pb, and Zn content from C3 to C9. The heavy metal content in the cruise C samples is close to that of the wet season samples from cruises A and B. However, the spatial distribution pattern of heavy metal contents of the cruise C samples is somewhat different from that of the wet season samples from cruises A and B. The distribution of Co, Zn, and Pb contents in cruise C tended to be similar to that of the wet season samples from cruise A rather than the wet season samples from cruise B.
The mean concentrations of Cr, Co, Cu, Cd, and Pb were higher in the dry season than in the wet season probably due to the dilution by rainwater (Table 1), which influences concentration and heavy metal mobility. Furthermore, the mobility of heavy metals depends not only on the total concentrations in the sediment, but also on the soil or sediment properties themselves, metal properties, and environmental factors40. In addition, aquatic plants flourish during the wet season and aquatic plants can have an impact on the heavy metal content of the sediment. Some aquatic plants can take up heavy metals from the sediment, which can lead to a reduction in the heavy metal content of the sediment41. On the contrary, Zn has lower mean values in the dry season than in the wet season, which can be attributed to the large amount of rain in the wet season, precipitation causes more pollutants on the land to flow into the river and settle in the sediment39. In addition, higher SD values of Cr, Co, and Cu are observed in the wet season than in dry seasons, indicating the greater variations in the contents of Cr, Co, and Cu.
Assessment of sediment risk
Based on EF values (Table 2), the majority of elements (Cr, Co, Cu, Zn, and Pb) showed no or minor enrichment at all sites, indicating lighter anthropogenic pollution of the sediment. Only Cd show higher EF values. The maximal EF value for Cd in ED-W and ED-D are 14.15 and 18.87, respectively; according to the EF scale, they belong to severe enriched sediments. Hence, the significant enrichment of Cd may pose a more serious potential ecological risk to the surrounding environment.
As can be seen from Table 3, all the average \({I}_{geo}\) values of Cr, Co, Cu, Zn, Cd, and Pb were above zero, indicating that sediment of the wet and dry seasons was in polluted status. From the classification criteria, the sediments could be categorized as lowly polluted with Cr, Co, Cu, Zn, and Pb (0 < mean values < 1), and moderately to highly and highly polluted with Cd in the wet and dry seasons (mean values = 2.77 and 3.01), respectively.
Multivariate statistical analysis
Pearson’s correlation
The values of the pearson correlation coefficient matrix are listed in Table 4. The results show that Al, Fe, and Cr were significantly positively correlated with each other (p < 0.01), and Cu, Zn, and Pb were significantly positively correlated with each other (p < 0.01) in the ED-W. In addition, Co was significantly correlated with Fe and Cr (p < 0.01). In the ED-D, Cr was significantly positively correlated with Cu, and Pb (p < 0.01), and Co was significantly positively correlated with Cu and Pb (p < 0.01). Cu, Zn, and Pb were significantly positively correlated with each other (p < 0.01). In addition, Fe was significantly correlated with Al (p < 0.01). Al and Fe are mainly found in fine-grained sediments and clastic minerals, and the strong correlation of heavy metals with Al and Fe suggests that these heavy metals are less influenced by anthropogenic activities and that they may be of the same origin as Al and Fe42.
Factor analysis and source identification of heavy metals
Principal component analysis (PCA) was performed on Al, Fe, Cr, Co, Cu, Zn, Cd, and Pb. Data for the eight elements can be reflected by three principal components. In the ED-W, the three principal components (PCs) accounted for 90.37% of the variability of all the studied variables. The result of PCA was represented in Fig. 3. Factors with high loadings (> 0.8) in PC1 are Cd, Pb, and Zn, and factors with high loadings (> 0.6) in PC2 are Fe, Co, Cr, and Al, and factor with high loading (> 0.9) in PC3 is Cu. Figure 4 shows the loading plots of the PCs in the ED-D, which have captured most of the variation from the data (88.744%). The loading plots show that the elements were discriminated into three clusters. Factors with higher loadings (> 0.6) in PC1 are Cr, Cu, Zn, Pb, and Co, and factor with higher loading (> 0.7) in PC2 is Cd, and factors with higher loadings (> 0.6) in PC3 are Al and Fe. Hunan province is known as the home of non-ferrous metals, with reserves of Pb and Zn among the highest in China, and considerable reserves of Cu. Activities such as mining and smelting can result in the accumulation of heavy metals in the surrounding environment43, and the significant positive correlation of Cd with Zn and Pb may be related to non-ferrous minerals such as Pb–Zn ores in the Yangtze River basin44. Some studies have shown the presence of Cd in pesticides and fertilizers, so Cd may be associated with agricultural production45. Municipal waste and domestic sewage are anthropogenic sources of Cu46. The significant positive correlation of Co with Cu and Pb in the ED-D suggests that Co may be of the same source as Cu and Pb. In addition, Cr released from the burning of coal in industrial production could enter the soil and sediment by way of deposition and the use of fertilizers and pesticides is also an essential way in which Cr and Cu enter the environment47,48. The results of a geochemical investigation on the Yangtze River Basin since 1999 also show that there are geochemical anomalies of Cd and other heavy metals in the alluvial deposits along the river banks, possibly mainly due to the weathering and denudation of Cd-rich rock layers49,50. For example, black shale enriched in Cd is widely distributed in the Yangtze River basin51,52. This also explains the moderate positive correlation of Cd with Al and Fe. In addition, industrial and agricultural activities also contribute to the enrichment of Cd53. In the ED-W, due to the significant positive correlation of Fe with Al and Co, and the strong positive correlation of Cr with Co (r = 0.625), Cr and Co may be from the same source as Al and Fe, i.e. natural erosion. Therefore, in the ED-W, PC1 may be interpreted as anthropogenic sources (like Cu, Zn, and Pb), including industrial activities such as mining and smelting and the emission and use of chemicals such as fertilizers and pesticides, PC2 may be interpreted as natural source (like Fe, Co, Cr, and Al), and PC3 may be interpreted as domestic sewage, like Cu. In the ED-D, PC1 may be interpreted as mining and agricultural production sources (like Zn, Pb, Cu, Cr, and Co). PC2 may be interpreted as natural weathering and agricultural activities, like Cd. PC3 may be interpreted as a natural source, like Al and Fe.
Conclusion
This study analyzed the spatial distribution and identified the sources of six heavy metals (Cr, Co, Cu, Zn, Cd, and Pb) in the surface sediment of ED Lake. All the heavy metals (Cr, Co, Cu, Zn, Cd, and Pb) studied show higher contents than the corresponding soil background values of Hunan province (China) in varying degrees. The obvious spatial heterogeneity of the distribution of Cu, Zn, and Pb contents in ED-W and ED-D may result from the hydrodynamic conditions of water flow and biological effects. Moreover, seasonal variation had significant effects on the concentrations of heavy metals in the surface sediment, and differences in precipitation between the wet and dry seasons, as well as the growth of aquatic plants, may be responsible. The metal enrichment factor (EF) and geo-accumulation index (\({I}_{geo}\)) indicated a low contamination level of Cr, Co, Cu, Zn, and Pb in the study area and a moderate to severe enrichment of Cd in some sediment samples. The results of pearson’s correlation analysis and principal component analysis indicate that the heavy metals in ED-W and ED-D may be from anthropogenic sources (like Cu, Zn, Co, and Pb), and natural sources (like Al, Fe, Co, and Cr), while Cd may derive from natural weathering and some human activities.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Fu, J., Hu, X., Tao, X. C., Yu, H. X. & Zhang, X. W. Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere 93, 1887–1895 (2013).
Liao, J. et al. Heavy metals in river surface sediments affected with multiple pollution sources, South China: distribution, enrichment and source apportionment. J. Geochem. Explor. 176, 9–19 (2017).
Barlas, N., Akbulut, N. & Aydoğan, M. Assessment of heavy metal residues in the sediment and water samples of Uluabat Lake, Turkey. Bull. Environ. Contam. Toxicol. 74(28), 6–293 (2005).
Batvari, B. P. D. et al. Heavy metals accumulation in crab and shrimps from Pulicat lake, north Chennai coastal region, southeast coast of India. Toxicol. Ind. Health. 32, 1–6 (2016).
Chen, Y. et al. Major ion and dissolved heavy metal geochemistry, distribution, and relationship in the overlying water of Dongting Lake, China. Environ. Geochem. Health. 41, 1091–1104 (2019).
Huang, F. W. et al. Assessment of pollutions and identification of sources of heavy metals in sediments from west coast of Shenzhen, China. Environ. Sci. Pollut. Res. 25, 3647–3656 (2018).
Mamat, Z., Haximu, S., Zhang, Z. Y. & Aji, R. An ecological risk assessment of heavy metal contamination in the surface sediments of Bosten Lake, northwest China. Environ. Sci. Pollut. Res. 23, 7255–7265 (2016).
Xu, Y. F., Wu, Y., Han, J. G. & Li, P. P. The current status of heavy metal in lake sediments from China: pollution and ecological risk assessment. Ecol. Evol. 7, 5454–5466 (2017).
Ginger, L. J. et al. Watershed vs. within-lake drivers of nitrogen: phosphorus dynamics in shallow lakes. Ecol. Appl. 27, 2155–2169 (2017).
Shinohara, R. et al. Dynamics of particulate phosphorus in a shallow eutrophic lake. Sci. Total Environ. 563–564, 413–423 (2016).
Nuel, M., Laurent, J., Bois, P., Heintz, D. & Wanko, A. Seasonal and ageing effect on the behaviour of 86 drugs in a full-scale surface treatment wetland: Removal efficiencies and distribution in plants and sediments. Sci. Total Environ. 615, 1099–1109 (2018).
Men, C., Liu, R. M., Wan, G. Q. R., Guo, L. J. & Shen, Z. Y. The impact of seasonal varied human activity on characteristics and sources of heavy metals in metropolitan road dusts. Sci. Total Environ. 637, 844–854 (2018).
Swarnalatha, K., Letha, J. & Ayoob, S. Effect of seasonal variations on the surface sediment heavy metal enrichment of a lake in South India. Environ. Monit. Assess. 186, 4153–4168 (2014).
Zhang, Y. X., Tian, Y., Shen, M. C. & Zeng, G. M. Heavy metals in soils and sediments from Dongting Lake in China: Occurrence, sources, and spatial distribution by multivariate statistical analysis. Environ. Sci. Pollut. Res. 25, 13687–13696 (2018).
Li, L. Q., Wang, C. M., Zhang, Y., Huang, D. Z. & Tian, Q. Study of macrozoobenthos and heavy metals of surface sediment in Dongting Lake. Ecol. Environ. Sci. 25, 286–291 (2016) ((in Chinese)).
Zhang, G. G., Tian, Q. & Guo, J. Heavy metal ecological risk of surface sediments in Dongting Lake and its trend. Asian J. Ecotoxicol. 10, 184–191 (2015) ((in Chinese)).
Feng, Y., Bao, Q., Xiao, X. & Lin, M. Geo-accumulation vector model for evaluating the heavy metal pollution in the sediments of Western Dongting Lake. J. Hydrol. 573, 40–48 (2019).
Lian, H. et al. Variation trend and risk assessment of heavy metals in surface sediments of Dongting Lake. Res. Environ. Sci. 32, 126–134 (2019) ((in Chinese)).
Liu, J. Y. et al. An integrated model for assessing heavy metal exposure risk to migratory birds in wetland ecosystem: a case study in Dongting Lake wetland, China. Chemosphere 135, 14–19 (2015).
Long, X. T. et al. Estimation of spatial distribution and health risk by arsenic and heavy metals in shallow groundwater around Dongting Lake plain using GIS mapping. Chemosphere 269, 128698. https://doi.org/10.1016/j.chemosphere.2020.128698 (2021).
Lian, H. et al. Influence of river-lake relation evolution on spatio-temporal variation of heavy metal pollution in sediments in Dongting Lake. J. Hydroecol. 40, 8–17 (2019) ((in Chinese)).
Li, F. et al. Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J. Geochem. Explor. 132, 75–83 (2013).
Li, F. et al. Integrated source apportionment, screening risk assessment, and risk mapping of heavy metals in surface sediments: a case study of the Dongting Lake, Middle China. Hum. Ecol. Risk Assess. Int. J. 20, 1213–1230 (2014).
Peng, D. et al. Spatial distribution of heavy metals in the West Dongting Lake floodplain, China. Environ. Sci.: Process. Impacts. 22, 1256–1265 (2020).
Xu, J. Y. et al. Assessment of heavy metal pollution in the sediment of the main tributaries of Dongting Lake, China. Water 10, 1060. https://doi.org/10.3390/w10081060 (2018).
Wu, Y. H., Liu, E. F., Yao, S. C., Zhu, Y. X. & Xia, W. L. Recent heavy metal accumulation in Dongjiu and Xijiu lakes, East China. J. Paleolimnol. 43, 385–392 (2010).
Erol, S., Neven, C., Stanislav, F. B., Ali, K. M. & Mihri, H. Contamination assessment of ecotoxic metals in recent sediments from the Ergene River, Turkey. Environ. Earth Sci. 75, 1051. https://doi.org/10.1007/s12665-016-5855-3 (2016).
Birth, G. A scheme for assessment human impacts on coastal aquatic environments using sediments (eds. Woodcoffe C. D. et al.) 14 (Wollongong University Papers in Center for Maritime Policy, 2003).
Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geol. J. 2, 108–118 (1969).
China National Environmental Monitoring Center. The Background Values of Chinese Soils. 329–497 (Beijing: China Environmental Science Press, 1990). (in Chinese)
Zhang, Z. X. et al. Assessment of heavy metal contamination, distribution and source identification in the sediments from the Zijiang River, China. Sci. Total Environ. 645, 235–243 (2018).
Makokha, V. A., Qi, Y. L., Shen, Y. & Wang, J. Concentrations, distribution, and ecological risk assessment of heavy metals in the East Dongting and Honghu Lake, China. Expos Health. 8, 31–41 (2016).
Bai, J. H. et al. Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol. Modell. 222, 301–306 (2011).
Suresh, G., Sutharsan, P., Ramasamy, V. & Venkatachalapathy, R. Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol. Environ. Saf. 84, 117–124 (2012).
Ayyanar, A. & Thatikonda, S. Distribution and ecological risks of heavy metals in Lake Hussain Sagar, India. Acta Geochimica. 39, 255–270 (2020).
Kachoosangi, F. T., Karbassi, A., Sarang, A. & Noori, R. Sedimentation rate determination and heavy metal pollution assessment in Zariwar Lake, Iran. SN Appl. Sci. 2, 1483. https://doi.org/10.1007/s42452-020-03279-9 (2020).
Erenturk, S. et al. Spatial distribution and risk assessment of radioactivity and heavy metal levels of sediment, surface water and fish samples from Lake Van, Turkey. J. Radioanal. Nucl. Chem. 300, 919–931 (2014).
Skordas, K., Kelepertzis, E., Kosmidis, D., Panagiotaki, P. & Vafidis, D. Assessment of nutrients and heavy metals in the surface sediments of the artificially lake water reservoir Karla, Thessaly, Greece. Environ. Earth Sci. 73, 4483–4493 (2015).
Yuan, P. et al. Spatial and seasonal variations and risk assessment for heavy metals in surface sediments of the largest river-embedded reservoir in China. Environ. Sci. Pollut. R. 27, 35556–35566 (2020).
Nehme, N. et al. The distribution of heavy metals in the Lower River Basin, Lebanon. Phys. Procedia. 55, 456–463 (2014).
Al-Afify, A. D. & Abdel-Satar, A. M. Risk assessment of heavy metal pollution in water, sediment and plants in the Nile River in the Cairo region, Egypt. Oceanol. Hydrobiol. Stud. 49, 1–12 (2020).
Wang, X., Liu, B. L. & Zhang, W. S. Distribution and risk analysis of heavy metals in sediments from the Yangtze River Estuary, China. Environ. Sci. Pollut. Res. 27, 10802–10810 (2020).
Zhang, Z. X. et al. Risk assessment, spatial distribution, and source identification of heavy metal(loid)s in paddy soils along the Zijiang River basin, in Hunan Province, China. J. Soils Sediments. 19, 4042–4051 (2019).
Zhao, C. D. et al. Methodology of tracking source of cadmium anomalies and their quantitative estimation in the Yangtze River Basin. Earth Sci. Front. 15, 179–194 (2008).
Wang, X., Wang, W. X., Pan, B., Zhou, C. C. & Liu, G. J. Spatial distribution, contamination assessments and sources of heavy metals in surface water from the Nanfei River. Resour. Environ. Yangtze Basin. 26, 297–303 (2017) ((in Chinese)).
Zhang, G. G. Pollution characteristics, sources and ecological risk of heavy metals in surface sediments from Dongting Lake. Environ. Monit. China. 6, 58–64 (2015) ((in Chinese)).
Li, Y. Y. et al. Heavy metals in soil of an urban industrial zone in a metropolis: risk assessment and source apportionment. Stoch. Environ. Res. Risk A. 34, 435–446 (2020) ((in Chinese)).
Luo, L., Ma, Y. B., Zhang, S. Z., Wei, D. P. & Zhu, Y. G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 90, 2524–2530 (2009).
Cheng, H. X. et al. A research framework for source tracking and quantitative assessment of the Cd anomalies along the Yangtze River Basin. Earth Sci Front. 12, 261–272 (2005) ((in Chinese)).
Xu, X. H. et al. Spatial distribution and source apportionment of agricultural soil heavy metals in a rapidly developing area in east China. Bull. Environ. Contam. Toxicol. 106, 3–39 (2021).
Perkins, R. B. & Mason, C. E. The relative mobility of trace elements from short-term weathering of a black shale. Appl. Geochem. 56, 67–79 (2015).
Yu, C. X., Peng, B., Peltola, P., Tang, X. Y. & Xie, S. R. Effect of weathering on abundance and release of potentially toxic elements in soils developed on Lower Cambrian black shales. Proc. R. China. Environ. Geochem. Health. 34, 375–390 (2012).
Yin, G. Y., Liu, L. M. & Yuan, C. C. Assessing environmental risks for high intensity agriculture using the material flow analysis method—A case study of the Dongting Lake basin in South Central China. Environ. Monit. Assess. 187, 472 (2015).
Acknowledgements
This work was funded by Important National Science & Technology Specific Projects of China (2011ZX05023-002-005).
Author information
Authors and Affiliations
Contributions
Y.Y. performed the data analysis and wrote the manuscript. B.L. helped perform the analysis with constructive discussions and modified the manuscript. H.L. contributed to the conception of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, Y., Liu, B. & Liu, H. Spatial distribution and source identification for heavy metals in surface sediments of East Dongting Lake, China. Sci Rep 12, 7940 (2022). https://doi.org/10.1038/s41598-022-12148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12148-x
This article is cited by
-
Spatiotemporal ecological risk evaluation and source identification of heavy metals and nutrients in the water and lake surface sediment in a protected catchment area of a volcanic lake
Environmental Monitoring and Assessment (2024)
-
The environmental impact of heavy metals in sediments of main valleys in the eastern side of Mosul City, Iraq
Environmental Monitoring and Assessment (2024)
-
Recent advances in instrumental techniques for heavy metal quantification
Environmental Monitoring and Assessment (2023)
-
Ecological risk assessment of potentially toxic elements in the bottom sediments of a stream in Oke-Ere, Kogi State, North Central Nigeria
International Journal of Environmental Science and Technology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.