Abstract
Climate change is one of the major threats to biodiversity, but its impact varies among the species. Bark beetles (Ips spp.), as well as other wood-boring pests of European forests, show escalating numbers in response to the changes driven by climate change and seriously affect the survival of the forests through the massive killing of trees. Many methods were developed to control these wood-boring beetles, however, their implementation can be detrimental for other forest specialists. Ants are widely used for biological pest-control, so in our study, we aimed to test the effect of Formica polyctena on the control of the wood-boring beetles. The results show that the proportion of infested trees is significantly reduced by the increase of the number of F. polyctena nests, with a strong effect on those infested by Ips species. We also show that the boring beetle community is shaped by different biotic and abiotic factors, including the presence of F. polyctena nests. However, the boring beetle infestation was not related to the latitude, altitude and age of the forests. Based on our results, we assert the effectiveness of the red wood ants as biological pest control and the importance of their conservation to keep the health of the forests.
Similar content being viewed by others
Introduction
Beetles are the most diverse group of insects on Earth with about 400,000 described species1. This large group is distributed worldwide and their species are present in all the ecosystems2,3, being involved in many different types of interactions. Some species belonging to the group of wood-boring beetles destroy the wood either to obtain food (xylophagous) or to protect their eggs, larvae, and pupae4. Wood-boring beetles play an important role in forests due to their contribution to dead wood degradation, involvement in the nutrient cycle, and facilitation of the arrival and establishment of other rare insect species5,6,7. However, some of the xylophagous beetle species are known to cause serious damages in fruit plantations and forests8,9,10.
Wood-boring beetle species belong mainly to the families Cerambycidae, Buprestidae, and Curculionidae, which altogether encompass a vast list of the species considered main forest pests11. Wood-boring beetles complete their life cycle by establishing a parasitic interaction with the tree11,12. Some of the longhorn beetles (Cerambycidae), jewel beetles (Buprestidae), and bark beetles (Curculionidae, Scolitynae) are monophagous or oligophagous, meaning that they are dependent on one or a few tree species of the same genera13. Wood-boring beetles are very important components of the nutrient cycle in forests as they decompose the decaying wood, contribute to the regeneration of soil nutrients and facilitate the establishment of other insects14. Usually, they infest living but weakened trees or untreated lumber, in which females (attracted by volatiles produced by dying or recently dead trees) place their eggs in crevices of trees affected by water stress or roots damaged by biotic or abiotic factors8,11. Moreover, they can also kill conifer seedlings by feeding on their stem bark15,16,17. In temperate coniferous forests, since infestations can potentially kill the trees, these wood-boring species constitute serious pests5 and can cause enormous ecological18,19 and economic losses8,20.
The most harmful wood-boring beetle species throughout the world belong to the subfamily Scolytinae (Latreille, 1804), which are considered the most destructive forest pests21,22,23. In Europe, the bark beetle Ips typographus, known as the European spruce bark beetle, is the cause of many major economic losses in the forest industry12,24 and forest degradation25,26,27. The massive loss of trees is caused by the bark beetle tunnelling under the trees’ bark and chewing on the cambium layer in the middle and lower parts of the trees that reduce the capacity of the tree to produce new cells that allow the growth of the trunk, branches and roots; but also by the indirect negative effect of fungi, bacteria, and mites associated with bark beetles28. Moreover, in European spruce forests, large-scale windthrow is usually connected with outbreaks of I. typophagus and results in extensive loss of trees7. Such outbreaks of the pest species are caused mostly by changes in the natural or semi-natural environment that can be linked to anthropogenic activities, having a strong impact also on the insect communities29,30,31,32.
Human activities lead to changes in many environmental factors, such as the increase of the global temperature linked to the climate change that threatens the survival of many insect species18,33. The short generation time and the close relation between temperatures and life-history traits18 make insects especially sensitive to temperature changes, like is the case of the specialist ants in the Formica rufa group, oligotope species of coniferous and mixed forests34. Besides the changes in temperatures, human impact also induces habitat loss and other changes in the natural environment, such as alterations in the precipitation regimes that affect the forest growth and production35,36,37. However, climate change can have an opposite effect in some invasive and pest species38, like the bark beetles, and influence positively their populations as it can shorten their generation time and facilitate their spread that leads to the outbreak of their native populations25,27. To try to overcome the negative effects, different management techniques have been developed in order to facilitate an early detection to minimalize the damage caused by different pests24,25. However, these techniques do not always take into consideration the long term effects and the well-being of many other forest-dwelling species, like birds (as woodpeckers39), parasitoids40 or other arthropods (as the clerid beetle Thanasimus dubius41) including wood ants37.
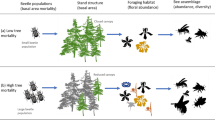
The natural ecosystems tend to find the balance among all the levels by keeping an equilibrium between preys and predators42, a concept that has become the basis of biological pest control. Under such circumstances, the biological control of wood-boring pest species can be more efficient with the use of native enemies together with the current management practices. Ants are known as natural enemies of insect pests worldwide43,44. In European mixed forests, red wood ants shape the invertebrate communities through different interactions at multi-trophic levels45,46 and have been also used as a biological control for pests in plantations since they are considered to be able to prey on or deter arthropod pests14. Moreover, they feature a series of characteristics that make them very efficient in this matter, such as their vast abundance in many ecosystems, a quick reaction to the increasing prey abundance, and the effective retrieval of prey individuals. In the case of red wood ants, the large size of their nests and their organization in polydomous systems allows them to host an enormous number of individuals34 and broods in the inhabited area, increasing considerably the protein consumption of the population. Considering the former, in our study, we investigated whether the presence of the red wood ant Formica polyctena contributes to the forest ecosystem balance by indirectly reducing the death of trees through decreasing the parasite infestation of trees. We hypothesized that the presence of F. polyctena in forests will influence the proportion of infested trees by bark beetles and other wood-boring beetle species, but also the fungal infestation linked to these beetles. To test the role of this wood ant species as a biological control agent, we carried out a large-scale study in which we measured the size and number of F. polyctena nests, the number of infested trees and the cause of their infestation (type of wood-boring beetles or fungus), as well as the physical damages of the trees, and we expected a reduced negative impact of wood-boring beetles in forests with larger populations of wood ants. Additionally, we made an attempt to contribute to the knowledge about the relevant ecological role of red wood ants in forest ecosystems and highlight the importance of the Formica rufa group in habitat conservation and forest pest management.
Materials and methods
The sampling was performed during the summer months (June-July) of 2017 and 2019, and was carried out in 31 forest patches from 12 regions located across a latitudinal gradient (46.215283° N–54.069650° N) crossing three different Central-European countries (Hungary, Slovakia, Poland). The lowest latitude corresponded to Hungary, where two regions (Ásotthalom and Kiskunság) were sampled. At the middle latitude of our gradient, we sampled the Carpathians, with Mátra and Bükk Mountains in the Hungarian area, Fatra Mountains in the Slovakian area, and Tatra, Pieniny, and Gorce Mountains in the Polish area. The other sampled areas encompassed the middle of Poland to the Baltic Sea and corresponded to Świętokrzyska, Kampinos, Białowieza, and Koszalin at the highest latitude.
In each region, we selected mixed forests that are under forestry management. These study sites are composed of Picea abies, Pinus sylvestris, P. nigra, Abies alba and Larix decidua as the most common coniferous species, and Betula pendula, Robinia pseudoacacia and Quercus spp. as the most common non-coniferous ones (according to the local forestry information: NÉBIH, Mapový portal KIMS, Bank Danych o Lasach).
To carry out the sampling, we used the colonies located haphazardly after intensive searching in the study regions. The location of the central point was marked with GPS (GARMIN Oregon 700t), and the 150 × 150 m plots around them were assigned in a random manner using Google Earth Pro. This plot size was determined to be a good representation of the territory of F. polyctena47. In accordance with the presence of F. polyctena populations, in most regions (Ásotthalom, Kiskunság, Bükk, Fatra, Pieniny, Świętokrzyska, Kampinos, Białowieza), we could sample three plots, while in Gorce, Tatra and Mátra Mountains we found only two plots, and in Koszalin only one plot. Within the plots, we recorded the GPS location of each F. polyctena nest and measured their dimensions (two perpendicular diameters and the height). The above-ground nest volume (i.e., semi-ellipsoid) was determined using the following equation:
where h is the height of the nest, and r1 and r2 are the two perpendicular nest radii. We used this equation because the above-ground nest volume is closely related to the red wood ant colony size48,49,50.
To test the relationship between the presence of F. polyctena nests and the tree infestation, we recorded the number and GPS location of every infested tree within a plot and noted down the type of parasite infestation (bark beetle, longhorn beetle, jewel beetle, or fungus). For a more precise characterization of the health status of forests, we also recorded the status of the infested trees (Alive or Dead) and their position (Standing or Laying). Finally, we determined the latitude and altitude of the sampling sites with the help of Google Earth (Google 2019) and established a “latitude value” as an increasing distance value starting from the southernmost study region (Ásotthalom: value 1) and added + 1 value with every 20 km (in beeline) passing to the north, finishing in Koszalin (North Poland, value 61; 28). In a previous study, we found a negative correlation between the latitude and the large-scale environmental variables like irradiation and temperature48. The average age of the forests was gathered from local forestry databases (NÉBIH, Mapový portal KIMS, Bank Danych o Lasach).
Statistical analysis
First, we were interested in whether the degree of the beetle infestation is correlated with the latitudinal and altitudinal gradient of the study sites and the forest age. To test this, we built separate GLM models (negative binomial error, maximum likelihood fit) for each parasite group with the proportion of trees infested by Ips spp., Cerambycidae Buprestidae and fungi as dependent variables, whereas the log + 1 transformed latitude value, altitude, and age of the forest as explanatory variables (N = 12).We used a Redundancy Analysis with Hellinger transformation of species data (RDA, N = 31) to test the association between the number of trees affected by both biotic and abiotic factors. In the model, a matrix built with the average frequency of the trees affected by each beetle and fungi species from each study plot within the study sites was used as a dependent variable. The matrix was built with log-transformed averages of the following explanatory variables: number of nests (Nests number), nests size (Nest volumes), the perimeter of the tree trunks (Perimeter), the ratio of alive trees to the total number of affected trees (Alive), the ratio of the standing trees to the total number of affected trees (Stand), and the ratio of conifers to the total number of affected trees (Conifer). The significance tests for the general model, in order to calculate the effect of the explanatory variables on the dissimilarity matrix of the dependent variables (number of Ips spp., Buprestidae, Cerambycidae and fungal infested trees), were based on permutational ANOVA tests. .
We also checked whether the infestation by bark beetles varies depending on the status and characteristics of the trees. In the GLM model (Poisson error, maximum likelihood, N = 31), the number of infested trees by Ips spp. was used as a dependent variable, whereas the log + 1 transformed number of alive trees and the perimeter of the trees served as explanatory variables. The same approach was used to test the relationship between the Cerambycidae , Buprestidae and fungal infestation and the characteristics of the trees. In the models, the number of infested trees by Cerambycidae, by Buprestidae and by fungi were used as dependent variables.
A GLM model (Poisson error, maximum likelihood, N = 31) was built to check whether the beetle or the Ips spp. parasitism facilitates the fungal parasitism. In the models, the number of trees affected by fungi was used as the dependent variable and the number of trees affected by beetles was used as an explanatory variable in the first model, whereas the number of trees affected by Ips spp. was used as an explanatory variable in the second model.
We were interested in whether a larger number of F. polyctena nests provides stronger pest control in the forests by a reduction of the number of infested trees. We used Generalized Linear Mixed Models (GLMMs; binomial error, maximum-likelihood fit, N = 31) to check whether the number of F. polyctena nests exert an effect on the number of trees parasitized by each beetle group or fungi. In the models, the ratio of parasitized trees with the Ips spp., Cerambycidae, Buprestidae or fungi to the total number of affected trees were used as dependent variables, whereas the number of F. polyctena nests as an explanatory variable.
We were also interested in whether the number of F. polyctena nests decreases while increasing the dimensions of the nests present within the study plots, and whether larger F. polyctena nests reduce more effectively the infestation of the trees by either beetles or fungi. To test the effect of the nest size on the infestation of the trees with the Ips spp., Cerambycidae Buprestidae, fungi or by all beetle groups in general, we carried out GLMMs (binomial error, maximum-likelihood fit, N = 31). In the models, the ratio of the parasitized trees (Ips spp., Cerambycidae, Buprestidae, fungi or by all beetle groups) to the total number of affected trees were used as dependent variables and the nest size (m3) as the explanatory variable. Additionally, we checked whether the number of F. polyctena nests influences their size. To test it, we carried out a GLMM (Poisson error, maximum likelihood, N = 31) in which the number of F. polyctena nests was used as a dependent variable and the size of the nests (measured in volume, m3) as an explanatory variable.
Finally, to test whether the number of the wood ant nests or size have an influence on the number of parasite groups, we used a GLMM (Poisson error, maximum likelihood, N = 31) with the number of parasite groups as a dependent variable and the number and size of the nests as explanatory variables.
The statistical analyses were carried out by using the R statistical software51. The RDA (carried out by using the function rda) and the PERMANOVA were carried out by using the adonis2 function in the vegan package52 and the Hellinger data transformation was performed by using the function hellinger from the labdsv package53. Generalized Linear Mixed Models were built with the glmer function from lme4 package54. In all the models, we used the identity of the plots and the study area as nested random factors. All models were tested for model overdispersion by using the DHARMa package55. If model overdispersion occurred, negative binomial error distribution was used56. In these latter cases, the models were built with the glmer.nb function. The graphical representation of the results was performed by using the function ggplot in the R package ggplot257.
Results
Our study covered a total of 63 ha and was distributed in 12 regions located in a latitudinal gradient of 900 km in beeline crossing three countries. A total of 393 F. polyctena nests and 3021 trees were measured and searched for parasite infection (Supplementary Table 1). Considering all the affected trees, 74.9% were parasitized by beetle species. The most common pest species belonged to the bark beetle Ips spp., which parasitized 51.3% of the trees.
Our results show that the infestation by Ips spp. was not influenced by the increasing latitude, altitude, or the average age of the forests under study. The same was found for the infestation by the Cerambycidae, Buprestidae and fungi species (Table 1).
Overall the results of the Redundance Analysis (RDA) showed that there is a significant relationship (F = 2.29, p = 0.001; Fig. 1) between the number of trees affected by the parasites (beetle groups and fungi) and the explanatory variables (Nests Number, Nest Volume, Conifers, Alive, Stand, and Tree Perimeter). The first two RDA axis explained in total 96.44% of the variation of the number of trees affected: the first one explained 86.29%, the second 10.15%. The Nest Number (F = 2.96, p = 0.019) and the number of Standing trees (F = 2.46, p = 0.051) were significantly associated with the number of trees affected by beetles and fungi, whereas the frequency of Conifer trees, Alive trees, the Perimeter of the trunks or the Nests volume of F. polyctena had no significant effect in this respect (Table 2).
Moreover, the number of trees infested by Ips spp. showed to be negatively related to the proportion of Alive trees but no relationship was found with the Perimeter of the trunk of the infested trees (Table 3). In the trees infested by the Cerambycidae and Buprestidae groups, we found a positive relationship with the Perimeter of the tree trunks, as well as a negative relationship between the infestation by these groups and the number of Alive trees, which was significant only in the Buprestidae infestation. However, no significant results were found in fungal infestations (Table 3). Notwithstanding, our results do not show any significant relationship between the beetle infestation and the fungal infestation in the studied plots (z = 0.29, p = 0.775).
The increasing number of F. polyctena nests present in the study sites was significantly correlated with a reduction in the number of infested trees (z = − 2.19, p = 0.028; Fig. 2a). Moreover, the number of infested trees by Ips spp. showed to be significantly reduced by the increasing number of F. polyctena nests (z = − 2.13, p = 0.033, Fig. 2b). However, no significant negative effect of the number of F. polyctena nests was found on the Cerambycidae (z = − 0.03, p = 0.975; Fig. 2c) or Buprestidae (z = − 0.01, p = 0.989; Fig. 2d) infestations, on the number of beetle groups infesting the trees (z = 0.05, p = 0.961) and on the fungal infestation (z = − 1.72, p = 0.086, Fig. 2e). Moreover, the increase in the number of F. polyctena nests was correlated with a decrease in their size (z = − 2.60, p = 0.007, Fig. 3).
Scatterplot with fitted regression line illustrating the relationship between the number of F. polyctena nests and the proportion of infested trees by (a) all the studied pest groups, and separately for (b) bark beetles (Ips spp.), (c) boring beetles of the Cerambycidae group, (d) boring beetles of the Buprestidae group, and (e) fungi. Significant results are with bold font.
The size of the nests did not have any significant effect on the number of trees affected by Ips spp. (z = 1.58, p = 0.115), Cerambycidae (z = − 0.98, p = 0.329) and Buprestidae (z = − 1.16, p = 0.247) groups separately, nor on the total number of infested trees (z = 0.99, p = 0.321). Similarly, an increase in the nest size did not affect the number of trees infested by fungi (z = 0.09, p = 0.923). Finally, the number of parasite groups infesting the trees was not affected by the number (z = 0.05, p = 0.961) or size (z = − 0.38, p = 0.698) of the F. polyctena nests.
Discussion
In our study, we tested the role of F. polyctena as pest control for wood-boring beetles and fungi in 12 regions located along a latitudinal and altitudinal gradient from South-Hungary to North-Poland. Our results show that the beetle community was mainly shaped by the number of F. polyctena nests, the percentage of alive affected trees and the perimeter of the affected tree trunks present within the studys. The increasing number of F. polyctena nests seems to lead to a reduction in the number of trees infected by bark beetles and, despite the fungal infestation was not significantly reduced, it also seems to be affected. However, the number of wood ant nests did not affect the number of trees infected by other species than Ips spp. Moreover, neither the nest size, the age of the forests, the altitude or the latitudinal location of the study sites showed to be related to the degree of beetle or fungal infestation.
The infestation by bark beetles is often associated with assemblages of fungi, bacteria, and mites which determine their successful tree colonization and reproduction28,58. The death of trees is the result of a double effect: a direct one by the bark beetle boring action and an indirect one by the inoculation with phytopathogenic fungi used to feed their broods22. Notwithstanding, the degree of the infestation by these wood-boring beetles can be determined by their local community structure and composition, as found also in other arthropod groups59,60. Moreover, the wood-boring beetles, in general, can be regulated also by different habitat characteristics like the health status of the trees, the abundance of host plant species, natural predators or even interspecific competition61. From these possible effects, our results indicate that the infestation by the wood-boring species parasitizing the studied forests was shaped to a different extent by the number of red wood ant nests, percentage of alive infested trees and the perimeter of the affected trees.
In our study, though the highest percentage of infestation was carried out by the bark beetles (Ips spp.), other wood-boring species belonging to the families Cerambycidae (Latreille, 1802) and Buprestidae (Leach, 1815) were also present. Due to the higher abundance of Ips species, the other wood-boring species would be more successful by avoiding nesting close to the bark beetles to reduce the larval competition for resources61, which might result in niche partitioning by the species in their search for an optimal reproduction rate. If we take into account that conifers are the main nesting target for bark beetles53,62, and the most abundant trees in our studied forests, this partitioning can be even more accentuated. Furthermore, wood-boring beetles mainly attack dead and weakened trees. Notwithstanding, healthy alive trees and shrubs can be also attacked when the beetle population reaches a high abundance receiving the nomination of “primary invaders”22,63,64. For example, Dendroctonus frontalis, a Curculionidae that produces galleries with an “S” pattern, quickly disrupts the cambium63. Moreover, our results show that boring beetles (belonging to Cerambycidae and Buprestidae groups) are benefited by trees with a larger perimeter. Larger trees can offer a more suitable nesting place (higher amount of older tissues) for the boring beetle larvae and adults are more attracted to carry out their oviposition in them. For example, the species of the genera Agrilus tend to lay their eggs on the south side of large living trees with thick bark8.
The worldwide concept of ants as natural enemies of insect pests43,44 is mainly based on the fact that besides their predatory habits, they also disturb pests during their oviposition and feeding43. Moreover, they also possess the capability of switching to the most abundant prey and of modifying their foraging behaviour to increase the contact with prey species65. In forests, red wood ants can shape the invertebrate community of trees by reducing their species richness through different interactions at multi-trophic levels that also includes the predation on forest pests21,29. Additionally, the supercolonies of the red wood ants show characteristics (such as large biomass of supercolonies, long-term stability, colonization of large areas and high predation on herbivores) that make these species even more suitable as biocontrol agents45,61. Our results show that the presence of F. polyctena nests reduced the number of infected trees by bark beetles. In our scenario, the reduction of this arthropod group might be the result of a combination of a direct effect linked with their predation habits and an indirect effect of the monopolization of resources, the aphid colonies (constituting their stable source of carbohydrates, important in maintaining their large colony size), by the red wood ants66.
The complex polydomous system of F. polyctena requires a large amount of food that is provided by foraging in mass29,32, covering an extensive area of the forest. Moreover, the nuptial flight of bark beetles occurs in spring67, coinciding with the presence of the sexual larvae that determines the peak of predation in red wood ants, and the presence of small populations of lachnids, the main food source of red wood ants later on the season68. In this scenario, bark beetles can easily become the target prey species of red wood ants, mostly in the spring period. For example, in Białowieża forest has been observed a F. polyctena worker with a bark beetle individual in its mandibles (I. Sondej, pers. comm.). Moreover, due to the effects of the climate change (mostly the elevated temperatures), instead of two bark beetle generations in lower latitudes and one in higher latitudes, three and two generations appear, respectively69. This can lead to a higher abundance of bark beetles in the spring period, being more available as a food source for red wood ants. Additionally, the vast number of workers moving to the tree canopy to collect the honeydew from the aphids cover almost totality the tree trunks (34, authors pers. observ.), making extremely difficult for other arthropods to perch. Moreover, the F. polyctena workers tend also to attack insects when they perceive their movement70. As a result, the number of available trees for the oviposition of the bark beetle is strongly reduced, so their abundance is as well negatively affected. This can lead to an increase in tree survival and forest health. However, this forest protection effect is local and the protected area would be more accentuated in the springtime and limited to the area covered by the supercolony of F. polyctena. The mentioned indirect protection from pests has been already used in agricultural practices, e.g. F. polyctena has been used for pest control in apple plantations in Denmark, where its presence led to greater production in the first year61.
The presence of red wood ants is strongly linked to habitat features, which is the main reason why the changes in their habitat had strongly affected their populations resulting in their current threatened status23. Besides factors like the availability of proper food sources, nesting places, and the material for mound construction37, also the temperature and isolation properties are important determinants of the presence of the red wood ant nests both at local and at larger scales48,49,50. Moreover, the combination of the large- and small-scale factors, like the reduction of the insolation on a local scale due to the shading by the tree canopy has been demonstrated to exert an important effect on the F. polyctena nests size and distribution48. However, the latitudinal and altitudinal location as well as the average age of the forests were not related to the infestation of trees by beetles or fungi. This could be due to anthropogenic climate change which promotes higher generation rates in bark beetle populations69 and make possible to these species to become highly abundant in a wide range of habitats.
In conclusion, our study highlights the high effectiveness of red wood ants as pest control agents in mixed coniferous forests. The increasing number of F. polyctena nests, regardless of their size, leads to a severe reduction of the number of infested trees, demonstrating the protective role of F. polyctena nests mostly against bark beetles. Moreover, it seems that this effect is species-specific and is also influenced by other habitat characteristics. Based on these results we can tell that red wood ants can be valuable tools in the development of forest management plans for the control of pest species, such as Ips spp. that are globally a major cause of tree mortality27,53,71. Considering that wood ants can be translocated with good success rate (see72), their colonies can be reintroduced after restoring the lost habitats where F. polyctena was previously present, so they can contribute to their maintenance, or perhaps in not too degraded forest habitats where they might contribute to the recovery of the perturbed forests. Moreover, our study also highlights the importance of the conservation plans for red wood ant species due to their important role in preserving the health of the forests, essential for the forest communities in the light of the threat of global climate change.
Data availability
Raw data were generated at Department of Social and Myrmecophilous Insects, at the Museum and Institute of Zoology (PAS). Derived data supporting the findings of this study are available from the corresponding author (GTP) or the senior author (IM) on request.
References
Stork, N. E., McBroom, J., Gely, C. & Hamilton, A. J. New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. PNAS 112, 7519–7752 (2015).
Sømme, L. & Block, W. Adaptations to alpine and polar environments in insects and other terrestrial arthropods. In Insects at Low Temperature (eds Lee, R. E. & Denlinger, D. L.) (Springer, 1991).
Springer, M. Marine insects. In: Marine Biodiversity of Costa Rica, Central America. (ed. Wehrtmann, I. S. & Cortés, J.) Monographiae Biologicae, vol 86. (Springer, 2009).
Cebeci, H. H. et al. The wood boring insects (Coleoptera: Cerambycidae and Buprestidae) recorded as the new pests for acer Undulatum Pojark from the Babadag mountain (SW Turkey). Fresenius Environ. Bull. 27, 9325–9328 (2018).
Becker, G. Untersuchungen über die Ernährungsphysiologie der Hausbockkäferlarven. Z. Vgl. Physiol. 29, 315–388 (1942).
Nardi, J. B., Mackie, R. I. & Dawson, J. O. Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems?. J. Insect Physiol. 48, 751–763 (2002).
Wermelinger, B., Duelli, P. & Obrist, M. K. Dynamics of saproxylic beetles (Coleoptera) in windthrow areas in alpine spruce forests. For. Snow Landsc. 77, 133–148 (2002).
Evans, H. F., Moraal, L. G. & Pajares, J. A. Biology, ecology and economic importance of Buprestidae and Cerambycidae. In: Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis (ed. Lieutier, F., Day, K. R., Battisti, A., Grégoire, J. C. & Evans, H. F.) 447–474 (Springer, 2004).
FAO (Food and Agriculture Organization of the United Nations) Global review of forest pests and diseases. FAO Forestry Paper 156 (2009).
Offenberg, J., Nielsen, J. S. & Damgaard, V. Wood Ant (Formica polyctena) services and disservices in a danish apple plantation. Sociobiology 66, 247–256 (2019).
Cline, A., Ivie, M. A., Bellamy, C. L. & Scher, J. A Resource for Wood Boring Beetles of the World: Wood Boring Beetle Families, Lucid v. 3.4. USDA/APHIS/PPQ Center for Plant Health Science and Technology, Montana State University, and California Department of Food and Agriculture. http://www.lucidcentral.org/keys/v3/WBB (2009).
Rudinski, J. A. Ecology of Scolytidae. Annu. Rev. Entomol. 7, 327–348 (1962).
Huber, D. P. W., Aukema, B. H., Hodgkinson, R. S. & Lindgren, B. S. Successful colonization, reproduction, and new generation emergence in live interior hybrid spruce, Picea engelmannii x glauca, by mountain pine beetle, Dendroctonus ponderosae. Agric. For. Entomol. 11, 83–89 (2009).
O’neill, K. M., Fultz, J. E. & Ivie, M. A. Distribution of adult Cerambycidae and Buprestidae (Coleoptera) in a subalpine forest under shelterwood management. Coleopt. Bull. 62(1), 27–36 (2008).
Leather, S. R., Day, K. R. & Salisbury, A. N. The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): A problem of dispersal?. Bull. Entomol. Res. 89, 3–16 (1999).
Nordlander, G., Bylund, H., Örlander, G. & Wallertz, K. Pine weevil population density and damage to coniferous seedlings in a regeneration area with and without shelterwood. Scand. J. For. Res. 18, 438–448 (2003).
Nordlander, G., Hellqvist, C., Johansson, K. & Nordenhem, H. Regeneration of European boreal forests: Effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. For. Ecol. Manag. 262(12), 2354–2363 (2011).
Bale, J. S. et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16 (2002).
Kahuthia-Gathu, R., Kirubi, D. T. & Gitonga, D. Composition and abundance ofwood-boring beetles of Acacia xanthophloea and their associated natural enemies in Thika, Kenya. J. Asia Pac. Biodivers. 11, 248–254 (2018).
Holmes, T. & Koch, F. Bark beetle epidemics, life satisfaction, and economic well-being. Forests 10, 696 (2019).
Adlung, K. G. A critical evaluation of the European research on use of red wood ants (Formica rufa group) for the protection of forests against harmful insects. Z. Angew. Entomol. 57, 167–189 (1966).
Goldazarena, A., Romón, P. & López, S. Bark beetles control in forests of Northern Spain. Integrated Pest Management and Pest Control—Current and Future Tactics, Dr. Sonia Soloneski (Ed), ISBN: 978-953-51-0050-8, In Tech. http://www.intechopen.com/books/integrated-pest-management-and-pest-control-current-and-future-tactics/bark-beetles-control-in-forests-of-northern-spain (2012).
IUCN 2020. The IUCN Red List of Threatened Species. Version 2020-1. https://www.iucnredlist.org (2020).
Abdullah, H., Darvishzadeh, R., Skidmore, A. K., Groen, T. A. & Heurich, M. European spruce bark beetle (Ips typographus, L.) green attack affects foliar reflectance and biochemical properties. Int. J. Appl. Earth Obs. 64, 199–209 (2018).
Bentz, B. J. & Jӧnsson, A. M. Modeling bark beetle responses to climate change. In Bark Beetles: Biology and Ecology of Native and Invasive Species (eds Vega, F. & Hofstetter, R.) 533–553 (Elsevier Academic Press, 2015).
Linder, M. et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol. Manag. 259, 698–709 (2010).
Marini, L. et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40, 1426–1435 (2017).
Schowalter, T. D. Insect ecology: an ecosystem approach, 3rd ed. (ed. Schowalter, T. D.) (Academic Press, 2011).
Frizzi, F., Masoni, A., Quilghini, G., Ciampelli, P. & Santini, G. Chronicle of an impact foretold: The fate and effect of the introduced Formica paralugubris ant. Biol. Invasions 20, 3575–3589 (2018).
Leong, M. et al. The habitats humans provide: Factors affecting the diversity and composition of arthropods in houses. Sci. Rep. 7, 15347 (2017).
Schweiger, O. et al. Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organisational levels and spatial scales. J. Appl. Ecol. 42, 1129–1139 (2005).
Trigos-Peral, G. et al. Three categories of urban green areas and the effect of their different management on the communities of ants, spiders and harvestmen. Urban Ecosyst. 23, 803–818 (2020).
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377 (2012).
Czechowski, W., Radchenko, A., Czechowska, W. & Vepsäläinen, K. The ants of Poland with reference to the myrmecofauna of Europe. Fauna Poloniae 4 (Natura optima dux Foundation, 2012).
Seidl, R. et al. Forest disturbances under climate change. Nat. Clim. Chang. 7, 395–402 (2017).
Sommerfeld, A. et al. Patterns and drivers of recent disturbances across the temperate forest biome. Nat. Commun. 9, 4355 (2018).
Sorvari, J. & Hakkarainen, H. Wood ants are wood ants: Deforestation causes population declines in the polydomous wood ant Formica aquilonia. Ecol. Entomol. 32, 707–711 (2007).
Roura-Pascual, N. et al. Geographic potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc. R. Soc. Lond. B. 271(1557), 2527–2535 (2004).
Kroll, J. C. & Fleet, R. R. Impact of woodpecker predation on over-wintering within-tree populations of the southern pine beetle (Dendroctonus frontalis). In The Role of Insectivorous Birds in Forest Ecosystems (eds Dickson, J. G. et al.) 269–281 (Academic Press, 1979).
Khanday, A. L., Sureshan, P. M., Buhroo, A. A., Ranjith, A. P. & Tselikh, E. Pteromalid wasps (Hymenoptera: Chalcidoidea) associated with bark beetles, with the description of a new species from Kashmir, India. J. Asia Pac. Biodivers. 12, 262–272 (2019).
Reeve, J. D. Predation and bark beetle dynamics. Oecologia 112, 48–54 (1997).
Curtsdotter, A. et al. Ecosystem function in predator–prey food webs confronting dynamic models with empirical data. J. Anim. Ecol. 88, 196–210 (2018).
Choate, B. & Drummond, F. A. Ants as biological control agents in agricultural cropping systems. Terr. Arthropod Rev. 4, 157–180 (2011).
Maňák, V., Björklund, N., Lenoir, L. & Nordlander, G. The effect of red wood ant abundance on feeding damage by the pine weevil Hylobius abietis. Agric. For. Entomol. 17, 57–63 (2015).
Robinson, E. J. H., Stockan, J. A. & Glenn, R. I. Wood ants and their interaction with other organisms. In Wood Ant Ecology and Conservation (eds Stockan, A. & Robinson, E. J. H.) 177–206 (Cambridge Universsity Press, 2016).
Sorvari, J. Foraging distances and potentiality in forest pest insect control: An example with two candidate ants (Hymenoptera: Formicidae). Myrmecol. News 12, 211–215 (2009).
Gößwald, K. Die Waldameise im Ökosystem Wald, ihr Nutzen und ihre Hege. Aula Verlag, Wiesbaden, 510 pp (1990). (shortened editon is 978-3-89104-755-2 (ISBN)).
Juhász, O. et al. Large- and small-scale environmental factors drive distributions of ant mound size across a latitudinal gradient. Insects 11, 350 (2020).
Juhász, O. et al. Consequences of climate change-induced habitat conversions on red wood ants in a Central European mountain: a case study. Animals 10, 1677 (2020).
Risch, A., Ellis, S. & Wiswell, H. Where and why? Wood ant population ecology. In Wood Ant Ecology and Conservation (eds Stockan, A. & Robinson, E. J. H.) 81–105 (Cambridge Universsity Press, 2016).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2020).
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P. R., O'Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., Szoecs, E., Wagner, H. Vegan: Community Ecology Package (2019).
Roberts, D.W. (2010) labdsv: Ordination and Multivariate Analysis for Ecology. http://cran.r-project.org/package=labdsv
Bates, D., Maechler, M., Bolker, B. & Walke, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1), 1–48 (2015).
Hartig, F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.1. 0. CRAN/GitHub (2016).
Lindén, A. & Mäntyniemi, S. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 97, 1414–1421 (2011).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Schowalter, T. D. Ecology and management of bark beetles (Coleoptera: Curculionidae: Scolytinae) in Southern Pine Forests. J. Integ. Pest Mngmt. 3, 1–7 (2012).
Hölldobler, B. & Wilson, E. O. The Ants (Harvard University Press, 1990).
Siemann, E., Haarstad, J. & Tilman, D. Dynamics of plant and arthropod diversity during old field succession. Ecography 22, 406–414 (1999).
Schlyte, F. & Anderbrandt, O. Competition and niche separation between two bark beetles: Existence and mechanisms. Oikos 68, 437–447 (1993).
Raffa, K. F. et al. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioSci 58, 501–517 (2008).
Drees, B. M., Jackman, J. A. & Merchant, M. E. Wood-Boring Insects of Trees and Shrub. Texas Agricultural Extension Service. http://hdl.handle.net/1969.1/160397 (1994).
Ray, C. et al. Patterns of woodboring beetle activity following fires and bark beetle outbreaks in montane forests of California, USA. Fire Ecol. 15, 21 (2019).
Risch, S. J. & Carroll, C. R. The ecological role of ants in two Mexican agroecosystems. Oecologia 55, 114–119 (1982).
Maák, I. et al. Behaviours indicating cannibalistic necrophagy in ants are modulated by the perception of pathogen infection level. Sci. Rep. 10, 17906 (2020).
Doležal, P. & Sehnal, F. A simple method for the detection of imaginal diapause in beetles. J. Appl. Entomol. 131(3), 221–223 (2007).
Horstmann, K. Untersuchungen ueber den Nahrungserwerb der Waldameisen (Formica polyctena Foerster) im Eichenwald II Abhaengigkeit vom Jahresverlauf und vom Nahrungsangebot. Oecologia (Berlin) 8, 371–390 (1972).
Overbeck, M. & Schmidt, M. Modelling infestation risk of Norway spruce by Ips typographus (L.) in the Lower Saxon Harz Mountains (Germany). For. Ecol. Manag. 266, 115–125 (2012).
Ayre, G. Response to movement by Formica polyctena Forst. Nature 199, 405–406 (1963).
Thom, D., Seidl, R., Steyrer, G., Krehan, H. & Formayer, H. Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. For. Ecol. Manag. 307, 293–302 (2013).
Czechowski, W. & Vepsäläinen, K. Territory size of wood ants (Hymenoptera: Formicidae): A search for limits of existence of Formica polyctena Först., an inherently polygynic and polycalic species. Ann. Zool. 59, 179–187 (2009).
Acknowledgements
The authors would genuinely thank to the Forest Research Institute in Białowieża National Park, Gorce National Park, Tatra National Park and Museum and Institute of Zoology, Polish Academy of Sciences for their essential role in this research.
Funding
This research was supported by the Gwiazda grant of the Museum and Institute of Zoology, Polish Academy of Sciences. The Open Access Fund was supported by the University of Szeged (Grant Number: 5178).
Author information
Authors and Affiliations
Contributions
I.M. and O.J. designed the experiments; G.T.P., O.J., P.J.K., G.M., A.T. and I.M. carried out the field experiments and the data collection; G.T.P. and I.M. carried out the data analysis; G.T.P. prepared figures and tables; G.T.P., O.J. and I.M. wrote the manuscript. All authors participated in the revision of the final version and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trigos-Peral, G., Juhász, O., Kiss, P.J. et al. Wood ants as biological control of the forest pest beetles Ips spp.. Sci Rep 11, 17931 (2021). https://doi.org/10.1038/s41598-021-96990-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96990-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.