Abstract
The aim of this study is to compare the scleral thickness of central serous chorioretinopathy (CSC) eyes with controls using anterior segment optical coherence tomography (AS OCT). This prospective case control study included 15 patients (15 eyes) with CSC and 15 age and gender matched healthy subjects. All subjects underwent spectral domain OCT with enhanced depth imaging and swept source AS OCT of temporal sclera. We investigated difference in scleral thickness between the two groups and relationship between choroidal and scleral thickness. Among the 15 eyes in the study group, 1 eye had acute CSC, 4 had recurrent CSC, 7 had inactive CSC, and 3 had chronic CSC. There was no significant difference in terms of age, gender, axial length and spherical equivalent between the two groups. The choroidal and scleral thickness of the study group were significantly greater than those of the control group (P < 0.001, P = 0.034). Choroidal thickness was positively correlated with scleral thickness (P = 0.031). A thick sclera along with a thick choroid were demonstrated in CSC eyes using AS OCT. Scleral characteristics might be involved in the pathogenesis of CSC by affecting outflow resistance of venous drainage in choroidal circulation.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is a disease characterized by a localized exudative detachment of the neurosensory retina and retinal pigment epithelium (RPE)1. While the underlying pathophysiology of CSC remains unclear, choroidal vascular disturbance demonstrated on indocyanine green angiography (ICGA)—for instance, increased permeability of the choriocapillaris, vascular congestion, and venous dilation—is thought to be a principal mechanism behind the detachment of the neurosensory retina and RPE2,3. Recent studies using enhanced-depth imaging (EDI) optical coherence tomography (OCT) provided additional evidence supporting the role of the choroid in CSC4. Compared with healthy eyes, CSC eyes have increased subfoveal choroidal thickness (SFCT) (that is, pachychoroid) and dilated choroidal vessels (that is, pachyvessels)4,5.
As the choroid of the eye is primarily a vascular structure lacking in autoregulation, impairment in drainage of choroidal flow could cause congestion of the choroidal venous system, leading to pachychoroid and choroidal hyperpermeability, as seen in uveal effusion syndrome (UES)6,7. The pathogenesis of UES has been explained as outflow obstruction due to decreased transscleral outflow and compression of the vortex vein by an abnormal sclera (i.e., a thick and rigid sclera)7,8. Although the severity of choroidal congestion and exudative retinal detachment in CSC differs from that in UES, both are common findings in these conditions9. However, little information is available regarding the characteristics of sclera in CSC eyes. In this study, we aimed to investigate the scleral thickness of CSC eyes using anterior segment (AS) OCT and compare the results with those of normal eyes.
Results
We enrolled 15 eyes from 15 patients diagnosed as CSC as the study group, and 15 eyes from 15 age- and gender-matched subjects as the control group. Among the 15 eyes in the study group, 1 eye had acute CSC, 4 had recurrent CSC, 7 had inactive CSC, and 3 had chronic CSC. At the time of enrollment, 6 out of 15 eyes in the study had no prior treatment, while 9 eyes had received different treatments (Table 1). The study group comprised 11 men and 4 women with a mean age of 50.27 ± 14.42 years (range 28–76 years), and the control group comprised 13 men and 2 women with a mean age of 54.73 ± 11.82 years (range 30–72 years). The two groups did not differ significantly in terms of age, gender, AL and spherical equivalent (P = 0.361, P = 0.651, P = 0.125, P = 0.878, respectively) (Table 2). The main systemic disease involving taking of medication in both the groups was cardiovascular diseases, including hypertension and coronary heart diseases, and no significant difference was noted between the two groups in terms of their presence (P = 0.143).
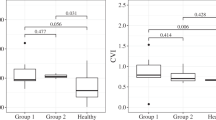
The median SFCT in the study and control groups was 443.0 µm (interquartile range 389.0–474.0 µm; range 319.0–748.0 µm) and 294.0 µm (interquartile range 281.0–330.0 µm; range 212.0–333.0 µm), respectively (Table 2; Fig. 1). The median sub-LR scleral thickness measured in the study group was 418.5 µm (interquartile range 370.5–461.5 µm; range 343.0–597.0 µm) while that in the control group was 374.0 µm (interquartile range 352.5–405.5 µm; range 309.0–486.0 µm) (Table 2; Fig. 1). We observed a statistically significant difference in both the SFCT and the sub-LR scleral thickness of the two groups (P < 0.001, P = 0.034, respectively). Correlation analysis showed a moderate positive linear relationship between SFCT and the sub-LR scleral thickness (r = 0.394, P = 0.031) (Fig. 2). The intraclass correlation coefficient (ICC) values for the intra-observer and inter-observer reliability for the measurement of the scleral thickness demonstrated high levels of repeatability (ICC = 0.916, P < 0.001, ICC = 0.950, P < 0.001, respectively). Figure 3 displays these representative cases.
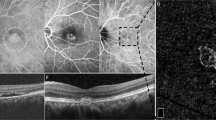
Representative cases of CSC eyes (A–E) and normal eyes (F,G). Color fundus photographs of CSC eyes show serous macular detachment (A) and fluorescein angiography shows typical smoke stack pattern of leakage (B). Late phase image of indocyanine angiography shows multiple areas of choroidal hyperpermeability. We observed very thick choroid in enhanced-depth imaging (EDI) of optical coherence tomography (OCT) and relatively thick sclera in swept source anterior segment (AS) OCT in CSC eyes (D,E), compared with those of normal eyes (F,G).
Discussion
In this study, we examined the scleral thickness of CSC eyes to investigate the possible role of the sclera in the pathogenesis of pachychoroid. We found that both the scleral and the choroidal thickness of CSC eyes were significantly greater than those of healthy eyes. Additionally, choroidal thickness and scleral thickness were positively correlated. These results are consistent with those of Imanaga et al., who first reported thick sclera in eyes with CSC10. They measured scleral thickness in four quadrants using AS OCT with more cases, providing further strong evidence for the hypothesis of thick sclera in CSC eyes.
Venous drainage of the choroidal circulation is thought to occur primarily through the four vortex veins. Pang et al., using ultra-wide field ICGA, observed dilated choroidal vessels in CSC eyes and identified these dilated vessels as macular branches of the vortex vein, noting that the entire course of these vessel from the distal ends to ampullas of the vortex veins were markedly dilated, suggesting outflow congestion of the draining vortex vein11. The large vessels located in the outer choroidal layer are known to be the major contributors to the increase in the choroidal thickness seen with EDI OCT in CSC5,12,13. Morphologic features of these large vessels were visualized using en face swept source OCT, and corresponded with the macular branch of the vortex veins observed in ICGA14,15,16. Based on these results, the engorgement of the macular branch of the vortex vein associated with outflow congestion is thought to be a major contributing factor to increased choroidal thickness in CSC.
Because the vortex vein emerges from the eyeball only through the narrow scleral tunnel, its outflow pathway could be affected by the characteristics of the sclera, which is a relatively rigid structure in the eye17. Eyes with thick sclerae may have narrower and longer scleral tunnels, which would increase the outflow resistance of the vortex vein, causing a bottleneck phenomenon. The primary pathogenic mechanism behind the development of UES is believed to be a thickened and rigid sclera, which impedes transscleral outflow and causes congestion of the vortex vein9,18. Successful results of surgical procedures such as subscleral sclerectomy and vortex vein decompression in UES have further strengthened the evidence of the abnormal sclera as the primary pathology7,19. Interestingly, Venkatesh et al. reported a similar case in a CSC patient20. In eyes with chronic CSC complicated by exudative retinal detachment, they performed partial thickness scleral resection with mitomycin C, which resulted in improved visual acuity and resolution of subretinal fluid. In contrast, several reports showed temporary thickening of the SFCT after scleral buckling21,22,23. Iwase et al. suggested that in such cases venous drainage obstruction induced by the compression force of the scleral buckle leads to choroidal thickening22. These cases are believed to be good examples reflecting the relationship among the sclera, the vortex vein, and choroidal congestion.
Various studies have implicated several risk factors associated with CSC development. Recently, two large case–control studies simultaneously reported hyperopia as an independent risk factor for CSC24,25. In both studies, consistent with the study by Manayath et al., myopia was found to protect from CSC26. Hyperopia |usually indicates that the eyes have a short AL; the AL of unilateral idiopathic CSC and fellow eyes was also reported to be shorter than that of control eyes27. Scleral thickness and choroidal thickness are well known to decrease with axial elongation of the globe28,29. Conversely, in small hyperopic eyes, the sclera is relatively thick. Therefore, we postulated that the underlying pathology of small hyperopic eyes as a risk factor for CSC might be associated with relatively thick sclerae, which would have increased choroidal circulation resistance outflow by the same mechanism as that in the case of UES which occurs primarily in short eyes.
Based on previous studies and our findings, we concluded that the pathogenesis of CSC might be characterized by an imbalance between the inflow and outflow of choroidal circulation. In other words, in eyes with decreased outflow capacity, increased choroidal inflow would cause choroidal congestion, leading to serous RPE and retinal detachment. In contrast, eyes with adequate outflow capacity could readily dispose of increased choroidal inflow without causing choroidal congestion. Scleral characteristics and their relationship with the vortex vein may be important factors affecting the outflow capacity. Tittle et al. reported a significant increase in choroidal blood flow after isometric exercise in chronic CSC patients compared with that in healthy subjects30. Compared with normal controls, patients with CSC also exhibited dynamic choroidal thickening in response to increased perfusion pressure induced by postural changes31. These results in CSC eyes might represent impairment of choroidal outflow preventing the ready disposal of rapidly increasing choroidal inflow.
This study has the following limitations. First, it involved a small sample size, which limited the statistical strength of the analysis. Second, because there is no anatomical reference point in the measurement of scleral thickness (as, for instance, the foveolar in the case of choroidal thickness), it was difficult to compare the thickness of the sclera at any particular point. Moreover, we only measured the temporal quadrant because it was difficult to expose the sclera for accurate measurements in other quadrants. However, we cannot rule out the possibility that confounding factors specific to temporal area may have affected our results. Nevertheless, the findings by Imanaga et al. suggest that these effects are minimal10. To Third, since it is hard to capture the entire sclera under the posterior pole using currently available imaging modalities, we measured scleral thickness at the insertion site of the lateral rectus muscle using AS OCT. However, in UES, the entire sclera from its anterior to its posterior area is thickened, and CSC is suggested to be an extensive disease not limited to the posterior pole7,11. One of our cases did indeed show anterior choroidal effusion, which was demonstrated by AS OCT (Fig. 4). The vortex vein is also located at the periphery, not the posterior pole17; it is therefore an unsuitable location for observing the difference between CSC eyes and normal eyes. By investigating the sclera in CSC, we have proposed another aspect for consideration regarding the pathogenesis of CSC. Further study with a larger sample size and imaging modalities that facilitate more accurate measurement of scleral thickness will allow for confirmation of our results.
In conclusion, in eyes with CSC, both the sclera and the choroid were thicker than those in normal eyes. Scleral characteristics, by affecting outflow resistance, may be another contributing factor in the development of CSC. Therefore, the balance between inflow and outflow of choroidal circulation should be considered and novel treatment strategies aimed at relieving outflow obstruction are indicated in the management of CSC.
Methods
We performed this prospective, cross-sectional, case–control study at Kangwon National University Hospital, Chuncheon, Korea between October 2019 and November 2019. The institutional review board of Kangwon National University Hospital approved the study, which adhered to the tenets of the Declaration of Helsinki. All patients gave their written informed consent prior to their enrollment in the study. We consecutively enrolled patients diagnosed with CSC as the study group, and formed the control group from age- and gender-matched healthy subjects. We based diagnosis of CSC on ocular examination including spectral-domain (SD) OCT and fluorescein angiography (FA) results. CSC is classified in accordance with the suggestion of Daruich et al., and all subtypes of CSC were included in this study1. We excluded patients with (1) a choroidal thickness < 300 µm; (2) an axial length > 25 mm; (3) any other retinal abnormalities, including diabetic retinopathy, retinal vein occlusion, choroidal neovascularization, and posterior uveitis; (4) previous vitreoretinal surgery and trauma; (5) a history of taking medications that could induce CSC-like retinopathy, such as mitogen-activated protein kinase inhibitors32; (6) poor-quality images that could not be evaluated because of low resolution.
All participants underwent a comprehensive ophthalmologic examination, including measurement of best-corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy, and dilated fundus examination. We measured axial length (AL) with a partial coherence laser interferometer (IOL master 500; Carl Zeiss Meditec AG, Jena, Germany), and determined choroidal thickness from images obtained using the EDI mode of a Spectralis OCT device (Heidelberg Engineering Inc, Heidelberg, Germany), analyzing them with the associated software (Version 6.0). We obtained the horizontal and vertical sections, comprising 100 averaged scans passing directly through the center of the fovea, in a 5° × 30° rectangle centered on the macula. We measured the SFCT from the outer portion of the hyperreflective line corresponding to the RPE to the inner surface of the choroid/scleral boundary. Two independent graders measured the SFCT and we used the average measurements in statistical analysis.
Measurement of scleral thickness
We imaged the eyes of all participants with a swept source AS OCT (CASIA2; Tomey Corporation, Nagoya, Japan). We captured images of temporal sclera during 45° nasal gaze position using ‘bleb wide’ mode (Fig. 5). Raster scans of ‘bleb wide’ mode comprised 256 high-quality cross-sectional images of horizontal and vertical lines over the sclera with 400 A-scans per line sampling. Of all scanned images, we selected one best-quality image of the horizontal section perpendicular to the corneal limbus for analysis. We manually measured scleral thickness at the insertion site of the lateral rectus (LR) using built-in 2D Analysis software (Version 3E.2), measuring perpendicularly from the LR muscle/scleral interface to the scleral/choroidal boundary. Two examiners (YJ Lee and JY Lee) independently performed the measurements of sub-LR scleral thickness and we used the average measurements in statistical analysis.
Statistical analysis
We used SPSS version 22.0 software (SPSS, Inc., Chicago, IL, USA) to perform the statistical analysis, and considered P < 0.05 to be statistically significant. We performed the Shapiro–Wilk test on various continuous variables for the assessment of Gaussian distribution. We present descriptive data as mean (standard deviation) or median (interquartile range), and we used the Student t test or Mann–Whitney U test as statistical methods of comparing variables, as determined based on the above results. We performed Spearman’s correlation analysis to examine the relationship between choroidal thickness and scleral thickness. We expressed the inter-observer reliability of the scleral thickness measured by the two observers as the ICC.
Change history
06 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-93183-y
References
Daruich, A. et al. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 48, 82–118 (2015).
Piccolino, F. C., Borgia, L., Zinicola, E. & Zingirian, M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye (Lond.) 9(Pt 3), 324–332 (1995).
Prunte, C. & Flammer, J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am. J. Ophthalmol. 121, 26–34 (1996).
Imamura, Y., Fujiwara, T., Margolis, R. & Spaide, R. F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29, 1469–1473 (2009).
Yang, L., Jonas, J. B. & Wei, W. Choroidal vessel diameter in central serous chorioretinopathy. Acta Ophthalmol. 91, e358–e362 (2013).
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168 (2010).
Uyama, M. et al. Uveal effusion syndrome: Clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology 107, 441–449 (2000).
Gass, J. D. Uveal effusion syndrome. A new hypothesis concerning pathogenesis and technique of surgical treatment. Retina 3, 159–163 (1983).
Kumar, V., Azad, S. V., Vohra, R. & Venkatesh, P. Serous macular detachment in nanophthalmos: A manifestation of pachychoroid spectrum. Am. J. Ophthalmol. Case Rep. 15, 100522 (2019).
Imanaga, N. et al. Scleral thickness in central serous chorioretinopathy. Ophthalmology. Retina 5, 285–291 (2021).
Pang, C. E., Shah, V. P., Sarraf, D. & Freund, K. B. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am. J. Ophthalmol. 158, 362-371.e362 (2014).
Chung, Y. R., Kim, J. W., Kim, S. W. & Lee, K. Choroidal thickness in patients with central serous chorioretinopathy: Assessment of Haller and Sattler layers. Retina 36, 1652–1657 (2016).
Yang, L., Jonas, J. B. & Wei, W. Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 54, 4659–4665 (2013).
Ferrara, D. et al. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology 121, 719–726 (2014).
Dansingani, K. K., Balaratnasingam, C., Naysan, J. & Freund, K. B. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 36, 499–516 (2016).
Hiroe, T. & Kishi, S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol. Retina 2, 152–161 (2018).
Kutoglu, T., Yalcin, B., Kocabiyik, N. & Ozan, H. Vortex veins: Anatomic investigations on human eyes. Clin. Anat. 18, 269–273 (2005).
Elagouz, M., Stanescu-Segall, D. & Jackson, T. L. Uveal effusion syndrome. Surv. Ophthalmol. 55, 134–145 (2010).
Brockhurst, R. J. Vortex vein decompression for nanophthalmic uveal effusion. Arch. Ophthalmol. 98, 1987–1990 (1980).
Venkatesh, P., Chawla, R., Tripathy, K., Singh, H. I. & Bypareddy, R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye Vis. 3, 23 (2016).
Miura, M. et al. Choroidal thickness after scleral buckling. Ophthalmology 119, 1497–1498 (2012).
Iwase, T. et al. Change in choroidal blood flow and choroidal morphology due to segmental scleral buckling in eyes with rhegmatogenous retinal detachment. Sci. Rep. 7, 5997 (2017).
Gama, I. et al. Macular choroidal thickness after vitreoretinal surgery: Long-term effect of pars plana vitrectomy with and without encircling scleral buckling surgery. Archivos de la Sociedad Espanola de Oftalmologia 92, 577–584 (2017).
Chatziralli, I. et al. Risk factors for central serous chorioretinopathy: Multivariate approach in a case–control study. Curr. Eye Res. 42, 1069–1073 (2017).
Ersoz, M. G., Arf, S., Hocaoglu, M., Sayman Muslubas, I. & Karacorlu, M. Patient characteristics and risk factors for central serous chorioretinopathy: An analysis of 811 patients. Br. J. Ophthalmol. 103, 725–729 (2019).
Manayath, G. J. et al. Is myopia a protective factor against central serous chorioretinopathy?. Int. J. Ophthalmol. 9, 266–270 (2016).
Moon, H., Lee, D. Y. & Nam, D. H. Axial length in unilateral idiopathic central serous chorioretinopathy. Int. J. Ophthalmol. 9, 717–720 (2016).
Shen, L. et al. Scleral thickness in Chinese eyes. Investig. Ophthalmol. Vis. Sci. 56, 2720–2727 (2015).
Shen, L. et al. Scleral and choroidal thickness in secondary high axial myopia. Retina 36, 1579–1585 (2016).
Tittl, M. et al. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 46, 4717–4721 (2005).
Nathaniel Roybal, C. et al. Dysfunctional autonomic regulation of the choroid in central serous chorioretinopathy. Retina 38, 1205–1210 (2018).
Francis, J. H. et al. Clinical and morphologic characteristics of MEK inhibitor-associated retinopathy: Differences from central serous chorioretinopathy. Ophthalmology 124, 1788–1798 (2017).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Discussion and Reference list. Modifications have been made to the Discussion and References. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, Y.J., Lee, Y.J., Lee, J.Y. et al. A pilot study of scleral thickness in central serous chorioretinopathy using anterior segment optical coherence tomography. Sci Rep 11, 5872 (2021). https://doi.org/10.1038/s41598-021-85229-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85229-y
This article is cited by
-
Biomechanical properties measured with dynamic Scheimpflug analyzer in central serous chorioretinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Pathomechanisms in central serous chorioretinopathy: A recent update
International Journal of Retina and Vitreous (2023)
-
Evaluation of scleral thickness in patients with Fuchs endothelial dystrophy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Assessment of the anterior scleral thickness in central serous chorioretinopathy patients by optical coherence tomography
Japanese Journal of Ophthalmology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.