Abstract
Strenuous exercise increases circulating cell free DNA (cfDNA) and stimulates blood phagocytes to generate reactive oxygen species (ROS) which may induce DNA strand breaks. We tested whether: (A) elevated cfDNA in response to three repeated bouts of exhaustive exercise has decreased integrity; (B) each bout of exercise increases luminol enhanced whole blood chemiluminescence (LBCL) as a measure of ROS production by polymorphonuclear leukocytes. Eleven men performed three treadmill exercise tests to exhaustion separated by 72 hours of resting. Pre- and post-exercise concentrations and integrity of cf nuclear and mitochondrial DNA (cf n-DNA, cf mt-DNA) and resting (r) and fMLP (n-formyl-methionyl-leucyl-phenylalanine)-stimulated LBCL were determined. Each bout increased concentrations of cf n-DNA by more than 10-times which was accompanied by about 2-times elevated post-exercise rLBCL and fMLP-LBCL. Post-exercise cf n-DNA integrity (integrity index, I229/97) decreased after the first (0.59 ± 0.19 vs. 0.48 ± 0.18) and second (0.53 ± 0.14 vs. 0.44 ± 0.17) bout of exercise. There were negative correlations between I229/97 and rLBCL (ƍ = –0.37), and I229/97 and fMLP-LBCL (ƍ = –0.40) – analysis of pooled pre- and post-exercise data (n = 66). cf mt- DNA integrity (I218/78) did not alter in response to exercise. This suggests an involvement of phagocyte ROS in cf n-DNA strand breaks in response to exhaustive exercise.
Similar content being viewed by others
Introduction
There are rising evidences suggesting that besides, health promoting effects, exhaustive exercises might have potential adverse effects on immune system1,2. Therefore, explanation of this issue is essential for training recommendations or customization of individual training load. Interestingly, bouts of strenuous exercise caused rapid increase in cell free DNA (cfDNA) concentration in plasma and, moreover, it was suggested to be a promising marker of acute exercise induced-metabolic changes in human body3. Exercise resulted in the surge of plasma cfDNA independently of workout associated with various sport disciplines and types of training such as weightlifting4, running5, soccer6, cycling7, rowing8, strength training9 or repeated sprint6. Concentration of post-exercise cfDNA positively correlated with the duration and the intensity of aerobic running10 as well as with selected markers of muscle damage11. Moreover, the ratio of post- to pre-exercise cfDNA associated with the distance covered by soccer players during the game6. In another study, increased cfDNA in response to heavy resistance exercise was described to have possible predictive value of muscle-performance decrease within 2 days after the bout12. Therefore, measurements of exercise-related variations of cfDNA can be used for monitoring of training load or diagnosis and prevention of overtraining syndrome11.
The common process following exhaustive exercises is leukocyte demargination from the vascular, hepatic, pulmonary or spleen reservoirs in parallel with exercise-induced increase in cardiac output and blood flow. This elevates the white blood cell count, however, it seems to be not the main contributor to the exercise-induced increase in cfDNA because the rise in the latter is many times higher than post-exercise leukocytosis3. Therefore, mechanisms responsible for this phenomenon could include at least the elevated number of circulating leukocytes accompanied by the increase in their activity leading to the release of a variety of biomolecules including DNA3. This is in line with the recent observation of increased formation of intravascular neutrophil extracellular traps (NETs) that could be responsible for the rise in cfDNA in subjects after exhaustive exercise13,14.
NETs formation involves disintegration of nuclear and granular membranes, diffusion of decondensed chromatin into the cytoplasm and its mixing with various proteins. After rupture of neutrophil plasma membrane, this chromatin associated with granular proteins such as myeloperoxidase, neutrophil elastase or cathepsin G is released into the extracellular space15,16 leading to the increase in plasma cf n-DNA.
Respiratory burst of circulating phagocytes (mainly polymorphonuclear – PMNs) in combination with phagocytosis and NETs formation is essential for innate immunity17,18. Increased generation of reactive oxygen species (ROS) related to activation of circulating PMNs NADPH oxidase complex was reported in healthy subjects after exhaustive exercise19,20.
Luminol-enhanced whole blood chemiluminescence technique (LBCL), which reflects mainly superoxide radicals production by circulating PMNs21,22, was used for evaluation of pre- and post-exercise ROS generation23,24,25. This is a rapid test which due to its low day-to-day variability and relative simplicity is suitable in epidemiological and clinical studies26. It can be executed in two settings: (a) – blood samples without stimulation – resting LBCL (rLBCL) and (b) – blood samples after stimulation with n-formyl-methionyl-leucyl-phenylalanine (fMLP), a chemotactic peptide which induces PMNs respiratory burst via activation of NADPH oxidase26 – fMLP- stimulated LBCL (fMLP-LBCL). Both rLBCL and fMLP-LBCL were increased in patients suffering from diseases accompanied by systemic inflammatory response such as systemic sclerosis, infective endocarditis and chronic renal failure27,28,29. Moreover, LBCL was attenuated in healthy subjects in response to regular consumption of fruits rich in anti-oxidant and anti-inflammatory polyphenols30,31.
ROS can induce damage to avariety of biomolecules including modification of DNA bases and DNA strand breaks. It is possible that simultaneous activation of respiratory burst of circulating PMNs and NETs formation in response to exhaustive exercise lead to strand breaks of released DNA and thus formation of cfDNA with decreased integrity. cfDNA can be divided in two pools: cell free nuclear DNA (cf n-DNA) and cell free mitochondrial DNA (cf mt-DNA), deriving from nucleus or cytoplasmic mitochondria’s, respectively. Because of lack of nucleosomal structure, cf mt-DNA fragments present different pattern of integrity than cf n-DNA32. Moreover, mt-DNA seems to be more sensitive to oxidative stress and other genotoxic damages due to the lack of protective proteins and efficient DNA repair mechanism33. Therefore, it seems that integrity of cf mt-DNA could be more affected by ROS increase following exhaustive exercise than that of cf n-DNA. To solve this question, we compared the pre- and post-exercise plasma concentrations and integrity of cf n-DNA and cf mt-DNA in relation to rLBCL and fMLP-LBCL (reflecting ROS production by circulating PMNs) in healthy physically active men subjected to three repeated exhaustive treadmill runs.
Results
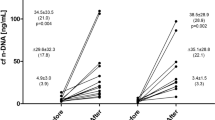
All included men successfully completed the study protocol of three repeated exhaustive treadmill exercises. Mean concentration of plasma cf n-DNA increased 11.3-, 11.8- and 17.3- times (p < 0.05) after the 1st, 2nd, and 3rd exercise, while cf mt-DNA increased significantly by about 2- and 2.4-times only after the 2nd and 3rd exhaustive treadmill run, respectively. Data of plasma concentrations of cf nDNA and cf mt DNA, other parameters related to exercise tests, changes of selected markers of muscle damage as well as metabolic and cardiovascular responses to exercise have been described elsewhere3.
Changes of integrity of circulating cell free nuclear and mitochondrial DNA in response to three repeated bouts of exhaustive treadmill exercise
Pre-exercise integrity of cf n-DNA expressed as I229/97 did not alter significantly over the study period. However, the tendency to a gradual decrease in I229/97 was noted e.g. I229/97 = 0.59 ± 0.19 before the 1st bout in comparison to I229/97 = 0.50 ± 0.22 before the 3rd one (Table 1). Exhaustive exercise resulted in the decrease in cf n-DNA integrity especially after the 1st (pre-exercise I229/97 = 0.59 ± 0.19 vs post-exercise I229/97 = 0.48 ± 0.18, p < 0.05) and the 2nd bout (pre-exercise I229/97 = 0.53 ± 0.14 vs post-exercise I229/97 = 0.44 ± 0.17, p < 0.05). In contrast to cf n-DNA integrity, the pre- and post-exercise-integrity of cf mt-DNA (I218/78) was relatively stable over the study period and did not alter in response to exercise (Table 1).
Changes of resting and fMLP-stimulated luminol enhanced whole blood chemiluminescence in response to three repeated bouts of exhaustive treadmill exercise
Mean rLBCL increased by about 2.1-, 2.6- and 2.4-times (p < 0.05) in response to the 1st, 2nd and the 3rd bout of exhaustive exercise (Table 2). The values of pre-exercise rLBCL were relatively stable over the study period and the same behavior was noted for the post-rLBCL. Similarly, behaved mean fMLP-LBCL: 1.7-, 1.8- and 1.5-fold increase (p < 0.05) after each exhaustive treadmill run (Table 2). Mutual comparisons of pre-exercise fMLP-LBCL values at consecutive days did not reveal any significant differences, as well for post-exercise fMLP-LBCL values. Consequently, the mean ratio of pre-exercise fMLP-LBCL to pre-exercise rLBCL values was similar and ranged from 2.7 at the 1st bout to 3.0 at the 3rd bout. The mean ratio of post-exercise fMLP-LBCL to post-exercise rLBCL was also stable and reached 2.2, 1.8 and 1.9 after the 1st, 2nd and 3rd bout of exercise, respectively.
Correlations between integrity of cf n-DNA and whole blood chemiluminescence before and after three repeated bouts of exhaustive exercise
Because we studied a relatively low group of men (n = 11), the direct analysis of correlations between variables obtained during one bout was inconclusive. Therefore, to overcome (at least partially) this problem, we analyzed Spearman’s (ƍ) correlations between integrity of cf n-DNA (I229/97) and LBCL by using the following datasets from three bouts of exhaustive exercise: (A)- pooled individual pre-exercise data (n = 33); (B)- pooled individual post-exercise data (n = 33); and (C)- pooled pre- and post-exercise data (n = 66) (Table 3). There was a negative correlation of moderate strength between pre-exercise I229/97 and pre-exercise fMLP-LBCL (ƍ = −0.36, p < 0.05), while correlation between post-exercise I229/97 and post-exercise rLBCL or fMLP-LBCL reached borderline significance (ƍ = −0.31, p = 0.08 and ƍ = −0.32, p = 0.06). In the case of analysis of dataset involving pre- and post-exercise data together, I229/97 negatively correlated with rLBCL (ƍ = −0.37, p < 0.05) and fMLP-LBCL (ƍ = −0.40, p < 0.05) (Table 3). The same analyses performed between integrity of cf mt-DNA (I218/78) and whole blood chemiluminescence (rLBCL, fMLP-LBCL) revealed no significant correlations (ƍ ranged between 0.11 and 0.38).
Discussion
It is generally believed that regular physical activity has important health promoting effect. However, long term, repeated exhaustive exercises can evoke pathological reactions leading to the development of overtraining syndrome34,35,36. High intensity exercise related to various sports disciplines4,5 was reported to cause fast, transient increases in circulating cfDNA reaching concentrations comparable to those observed in trauma or sepsis37,38. Some studies suggest that elevated plasma cfDNA levels may by associated with the increased risk of occurrence of overtraining syndrome in athletes39,40. Recently, we found that three repeated bouts of exhaustive treadmill exercise induced increase in cf n-DNA and cf mt-DNA in average trained healthy men without development of tolerance3. In the present study (which is the continuation of the afore-mentioned one), we found that the increase in cf n-DNA in response to each of three repeated bouts of exhaustive treadmill exercise is accompanied by the decrease in cf n-DNA integrity. In parallel to this phenomenon, each bout of exercise caused significant increase in rLBCL and fMLP-LBCL and that reflects an increased post-exercise ROS production by circulating phagocytes, namely PMNs. Moreover, analysis of pooled data from all bouts revealed a few significant negative correlations between integrity index of cf n-DNA and LBCL. These considerations suggest the involvement of ROS generated from circulating phagocytes in fragmentation of post-exercise cf n-DNA. Although, cf mt-DNA raised in response to the 2nd and 3rd bout of exercise, very surprisingly, no changes of the integrity index of cf mt-DNA were noted over the study period.
Effect of three repeated bouts of exhaustive treadmill exercise on luminol enhanced whole blood chemiluminescence
Each bout of exercise increased mean rLBCL and fMLP-LBCL in healthy men. Moreover, individual results revealed substantial increase in post-exercise rLBCL and fMLP-LBCL in each volunteer at any occasion. These observations indicate that exhaustive run increased basic generation of ROS by circulating PMNs and primed these cells for increased ROS production after stimulation with fMLP. Our results are consistent with the previous studies showing increased zymosan-induced luminol enhanced chemiluminescence of neutrophils isolated from blood collected after intensive aerobic exercise in healthy men23,24,25.
Moreover, neutrophils of subjects who completed 100 km marathon run revealed increased ROS production as reflected by the afore-mentioned method41. On the other hand, other studies did not confirm these observations and even reported suppression of ROS production by neutrophils in the group of well-trained men after marathon run or after acute bout of moderate intensity running and cycling using the same stimulator of phagocyte oxidative burst42,43. These differences may result from various study protocols, including exercise load and duration, time of blood collection, usage of whole blood or isolated cells for determination of ROS generation after stimulation with various cell activators. For instance, a continuous 90 min. exercise at the intensity of 50% VO2max caused an increase of phorbol 12-myristate 13-acetate (PMA)-induced neutrophils chemiluminescence while zymosan-induced chemiluminescence remained unchanged in healthy men44.
PMA is a direct activator of protein kinase C with following activation of NADPH oxidase and neutrophils oxidative burst while stimulation with opsonized zymosan (insoluble cell-wall preparation from the fungi Saccharomyces cerevisiae) involves phagocytosis of these particles, cell degranulation and massive production of ROS45. In our study, we used fMLP (an analog of N-formylated bacterial chemotactic peptides). In our study, we used fMLP (an analog of N-formylated bacterial chemotactic peptides). This peptide activates signal transduction pathways (phospholipase C–dependent generation of diacyl glycerol and inositol 1,4,5-triphosphate, rise in the intracellular Ca2+ concentration, protein kinase C activation) upon binding to specific G protein–coupled receptors on the PMNs plasma membrane. This, in turn, leads to the formation of the active NADPH oxidase complex and ROS production45. Thus, the peak time – time from agonist addition to appearance of maximal chemiluminescence – is about 7-times shorter for fMLP than for opsonized zymosan23,46. This remark may explain the discrepancies between results of our study and some previous investigations42,43 about the effect of exercise on ROS production by isolated neutrophils after stimulation with opsonized zymosan.
Moreover, we studied the effect of exercise on LBCL, thus neutrophils and monocytes were not subjected to any process of isolation that could change the cell ability to respond to stimulations with various agonists. Elimination of the risk of cell priming or de-priming during the isolation procedure is the important advantage of this method21,31. Such technique allows monitoring of oxidants release by circulating phagocytes under conditions that more closely resemble the in vivo situation than the analysis of isolated cells31,47. Severe exercise resulted in essential increase in plasma pro-inflammatory cytokines, namely IL-6, IL-8, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and granulocyte macrophage colony stimulating factor (GM-CSF)48,49. Although these pro-inflammatory cytokines alone induced rather weak oxidative response of PMNs, they strongly enhanced ROS generation in response to secondary stimulation with fMLP50,51,52,53, and that could be an explanation of increased rLBCL and fMLP-LBCL in healthy men after exhaustive exercise.
Effect of repeated bouts of exhaustive treadmill exercise on the integrity of plasma cell free DNA
Each bout of exhaustive exercise increased several times the concentration of circulating cf n-DNA, accompanied by the decrease in I229/97 of post-exercise cf n-DNA. This indicates that apool of cf n-DNA released in response to exhaustive exercise is subjected to factors leading to its enhanced fragmentation. Conversely, no changes in I218/78 were noted over the study period, although cf mt-DNA raised significantly after the 2nd and 3rd bout. Thus, one may conclude that exercise-induced release of cf mt-DNA was not accompanied by parallel processes resulting in its additional fragmentation. It is believed that the source of exercise-induced circulating cf n-DNA are NETs15. Various factors related to strenuous exercise including heat stress, catecholamines, pro-inflammatory cytokines (e.g IL-6, IL-8) can induce formation of NETs15. Intracellular generation of ROS by NADPH oxidase together with their elaboration by myeloperoxidase in intracellular granules are involved in triggering NETs formation54. Thus, nuclear DNA mixed with some components of granules before expulsion of NET fibers from PMNs can be exposed to and damaged by ROS, including breaking of DNA strands into shorter pieces. This could be a plausible explanation of increased post-exercise plasma levels of cf n-DNA with decreased integrity and is in line with observed moderate negative correlations between I229/97 and rLBCL and fMLP-LBCL. Moreover, previous studies showing increased concentration of 8-oxo-7,8-dihydro-2-deoxyguanosine (an oxidized derivative of deoxyguanosine - marker of cellular oxidative stress and oxidative damage to DNA) in leukocytes and urine of athletes after intense exercise55 support this explanation.
The exercise-induced rise in plasma cf n-DNA is transient8,56,57 probably due to simultaneous increase in the activity of circulating deoxyribonuclease I8. Thus, the post-exercise cf n-DNA decreased back to baseline within 0.5 to 2 hours of recovery8.
The bouts of exhaustive treadmill exercise were separated by 3 days of rest, and all healthy volunteers did not perform any additional strenuous exercise during the whole 13-day study period. Therefore, one may conclude that the contribution of NETs in maintaining the pre-exercise cf n-DNA levels was low (and even negligible) and other processes with the unchanging intensities (e.g. necrosis, apoptosis) were the source of abaseline pool of circulating cf n-DNA. This may explain the relatively stable concentrations of pre-exercise cf n-DNA and its integrity over the study period. Circulating cf mt-DNA increased in response to exhaustive exercise at the same time as cf n-DNA. However, pre- and post-exercise I218/78 of cf mt-DNA did not differ at any occasion. This is quite surprising considering the release of cf mt-DNA from NETs58 and formation of NETs from pure mt-DNA in response to skeletal injuries and orthopedic surgery in humans59. On the other hand, neutrophils infected with bacteria formed NETs with extracellular fibers containing n-DNA as the main structural component in vitro18. It cannot be excluded that neutrophils in response to exhaustive exercise extrude cf n-DNA alone or with very low admixture of cf mt-DNA. Thus, the exercise-induced increase in cf mt-DNA was about 5- to 6-times lower than that of cf n-DNA and no changes of cf mt-DNA integrity were noted.
Acute severe exercise on cycle ergometer until exhaustion increased mitochondrial ROS production in neutrophils of sedentary young males while the same physical exertion had no stimulatory effect on neutrophils mitochondrial ROS after 2 months of regular exercise training program in these subjects60.
Aerobic strenuous exercise resulted in an increase in superoxide radical activity in contracting muscles. However, the contribution of muscle mitochondria to this increase was small61,62, and even mitochondria generated less ROS during exercise than at rest61. We studied average trained healthy men who regularly performed recreational training where some of them participated in sport disciplines in the past. Therefore, it seems that exhaustive treadmill run did not induce increased generation of mitochondrial ROS and subsequent oxidative damage to mt-DNA.
Platelets are able to release microparticles containing functional mitochondria as well as free mitochondria63,64. Circulating phospholipase A2 can digest mitochondrial membranes with subsequent release of mt-DNA into extracellular space63. Exhaustive exercise (e.g. marathon run) can induce platelets activation and their degranulation65. Additionally, short term lifestyle intervention (moderate energy restriction and aerobic training for 6 weeks) increased serum phospholipase A2 activity in overweight or obese subjects66.
Consequently, it is possible that platelets could be another source of cf mt-DNA and encapsulating membranes can protect mt-DNA from oxidative attack. On the other hand, even mt-DNA released in response to exercise could be damaged by ROS originated from other sources (e.g. NADPH oxidase), and its amount seems to be too little to decrease the integrity of total post-exercise pool of circulating cf mt-DNA. These considerations may also explain no differences between pre- and post-exercise integrity of plasma cf mt-DNA.
There are very scant and unconvincing data on the integrity of exercise-induced cf DNA in humans. Incremental treadmill test until volitional exhaustion induced significant increase in circulating cf n-DNA but without changes of its integrity compared to pre-exercise cf n-DNA in male athletes67. On the other hand, 10 km relay race resulted in a significant decrease in the integrity of post-exercise cf n-DNA in a group of recreational runners68. Moreover, neither of these studies evaluated circulating concentrations and integrity of pre- and post-exercise cf mt-DNA. Our results showed that repeated exhaustive exercise decreased integrity of circulating cf n-DNA but did not change the integrity of cf mt-DNA.
Correlations between integrity of cf n-DNA and luminol enhanced whole blood chemiluminescence
Because of the low number of studied volunteers, we pooled pre- and post-exercise data from three consecutive bouts of exhaustive treadmill run and calculated correlations between cf n-DNA integrity and rLBCL and fMLP-LBCL. Such approach has some limitations and can increase the risk of bias. Nonetheless, for instance, we found significant negative correlations between I229/97 and rLBCL, and I229/97 and fMLP-LBCL for pooled individual pre- and post- exercise data from three bouts. Moreover, all values of Spearman’s ƍ (n = 6) ranged between −0.40 to −0.29 and that suggests that there is some negative relationship between integrity of cf n-DNA and generation of ROS by circulating phagocytes.
However, according to values of ƍ, these correlations can be interpreted as weak or moderate. This information suggests indirectly that other factors could be responsible for decreased integrity of post-exercise cf n-DNA, or post-exercise LBCL did not reflect precisely the generation of ROS by PMNs during the exhaustive run when NETs were formed and cf n-DNA released. Circulating cf n-DNA increased already after 9 min. from the onset of incremental treadmill run test (at the end of 10 km/h stage corresponding to 63% VO2max)68. In our study, mean run time was 47 min., 57 min. and 56 min. at the 1st, 2nd and 3rd bout, respectively3. Thus, pre- and post-exercise LBCL (rLBCL and fMLP-LBCL) reflected the activity of PMNs just before and after the exercise but not during the last 30 min. of run accompanied by the formation of NETs. Moreover, luminol crosses the cellular membrane of phagocytes (PMNs and monocytes); in this way, LBCL can mirror the extra- and intracellular production of ROS69. Hence, additional blood sampling during exercise followed by measurements of LBCL and cf n-DNA integrity (to estimate the dynamics of changes) would give more valuable results of the analysis of associations between cf n-DNA fragmentation and ROS production by blood phagocytes.
It is possible that numerous plasma antioxidants can inactivate extracellular ROS (in the close vicinity of PMNs) before their reaction with n-DNA. In consequence, intracellular activity of ROS during formation of NETs seems to be crucial for the decrease of cf n-DNA integrity. These aforementioned reasons may explain the low strength of observed correlations between pooled data of cf n-DNA integrity and LBCL.
Acute exercise of different duration and intensity increased plasma antioxidant activity in men70. Exhaustive cycling raised the activity of antioxidant enzymes e.g. superoxide dismutase and glutathione peroxidase in lymphocytes from peripheral blood71. Moreover, young athletes had higher Trolox-equivalent antioxidant capacity of plasma than their sedentary counterparts72. Thus, exercise-induced increase in circulating antioxidants (as adaptive response to muscle workout) may explain no effect of the third bout of exercise on cf n-DNA integrity and be an additional elucidation of weak correlations between post-exercise cf n-DNA integrity and LBCL.
It should be mentioned that one subject had no decrease in cf n-DNA integrity in response to each of the three bouts of exhaustive exercise (Table 5, supplementary file). Although, he presented an increase in post-exercise rLBCL and fMLP-LBCL (Tables 7 and 8, supplementary file) along with many-fold increase in post-exercise cf n-DNA3, the integrity indexes of pre- and post-exercise cf n-DNA were similar over the study period. It is possible that this volunteer had high activity of circulating and intracellular antioxidants which inactivated ROS before their reaction with n-DNA released from NETs. Circulating neutrophils are functionally a heterogeneous cell population16,73. Certain neutrophil subpopulations could have increased capacity to form NETs and release cf n-DNA16,74, and some of them can also release more ROS spontaneously or upon stimulation by various mediators75,76. It cannot be excluded that in some persons these neutrophil subpopulations do not overlap, thus released cf n-DNA in response to exhaustive exercise would not be exposed to ROS and undergo subsequent fragmentation. Moreover, NETosis can occur without activation of NADPH oxidase and ROS generation16. These processes may also be a possible explanation of the afore-mentioned observations. Nevertheless, it is interesting that some subjects can increase cf n-DNA in response to exhaustive exercise without changes of its integrity.
Concomitant increase in the serum activity of DNase I was recognized as a factor responsible for transient nature of cf n-DNA response to strenuous exercise77. Therefore, decreased integrity of post-exercise cf n-DNA could be the result of cleaving NETs DNA by this endonuclease. However, we did not observe any changes of post-exercise cf mt-DNA integrity. For that reason, the contribution of DNase I to the decreased integrity of post-exercise cf n-DNA seems to be not important under the conditions of our study. Increased post-exercise cf n-DNA and its integrity index as well as LBCL returned to baseline levels within 72 hour interval between bouts of exercise. Therefore, it is difficult to conclude about the clinical significance of these phenomena. Perhaps, in athletes with very high training- and competition-load, these changes may persist for longer time and be an additional marker of the risk of injury or over-training syndrome. However, it requires further more extensive studies.
Limitations of the study
Relatively low number of studied subjects (n = 11) and exclusion of female volunteers from the trial could be recognized as a weakness of the present study. Because this report is the extension of our previous one, all these afore-mentioned limitations were discussed in details elsewhere3. In addition, the inhibitory effect of progesterone and estradiol on ROS production by human PMNs in vitro78 supported our decision on participation of only male volunteers in the study. The low number of studied subjects was counterbalanced by three repeated bouts of exercise. We found that all bouts induced asignificant increase in cf n-DNA, rLBCL and fMLP-LBCL, while two bouts were accompanied by a decrease in cf n-DNA integrity and increase in circulating cf mt-DNA without changes of its integrity. This indicates the repeatability of these phenomena despite the small studied group of volunteers.
Conclusions
We found that repeated bouts of exhaustive exercise separated by three days of resting caused an increase in luminol enhanced whole blood chemiluminescence and in concentrations of circulating cf n-DNA. Post exercise cf n-DNA revealed decreased integrity which negatively correlated with LBCL. Because whole blood chemiluminescence reflects ROS production by circulating phagocytes, one may conclude that oxidants may be involved in the release of cf n-DNA and cf n-DNA strand breaks in response to exhaustive exercise. Although exercise caused moderate increase in plasma levels of cf mt-DNA, its integrity was stable and did not associate with blood chemiluminescence. This suggests a minor role of ROS in exercise-induced changes of cf mt-DNA. Further studies involving larger groups of male and female volunteers should be performed, especially bearing in mind the analysis of correlation and determination of causality between decreased of post-exercise cf n-DNA integrity and enhanced LBCL.
Methods
Studied group
The studied group of eleven, average-trained, non-smoking healthy men as well as the inclusion/exclusion criteria were the same as in our previous article3. Volunteers had mean age 34.0 ± 5.2 years, mean body mass index 26.2 ± 3.1 kg/m2 and mean maximal oxygen consumption VO2max 49.6 ± 4.5 ml/kg x min. They were free of any pharmacological treatment including vitamins and food supplements for 3 months preceding the study. They signed informed consent and did not change their dietary habits during the study period.
The study design
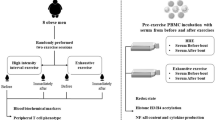
The study design was described in details in our previous report3. Briefly, the study consisted of 4 visits at the 1st, 7th, 10th and 13th day of observation. At the first visit (day 1st) 11 average-trained men underwent treadmill VO2max test and afterwards, at the three consecutive visits (day 7th, 10th and 13th), participants performed treadmill exercise to volitional exhaustion at speed corresponding to 70% of their personal VO2max (Fig. 1). Venous blood (15 ml) was collected into vacutainer tubes with EDTA (Becton Dickinson, Franklin Lakes, NJ) within 5 min. before and after each bout of exercise. One milliliter thereof was placed into a separate tube for resting (rLBCL) and n-formyl-methionyl-leucyl-phenylalanine (fMLP) – stimulated LBCL as well as blood cell count (Micros Analyzer OT 45,ABX, Montpellier, France). The rest was used for determination of concentration and integrity of cell free nuclear (cf n-DNA) and mitochondrial DNA (cf mt-DNA). During the whole study period (13 days) volunteers did not perform any exhaustive exercise besides those related to the study protocol. The study was conducted according to the Declaration of Helsinki. The protocol was reviewed and approved by The Medical University of Lodz Ethics Committee (RNN/95/14/KB), and all volunteers provided a written informed consent.
Overview of the study design. ECG -electrocardiography, FVC – forced vital capacity, FEV1 – forced exhaled volume in the first second, VO2max – maximal oxygen consumption, fMLP –n-formyl-methionyl-leucyl-phenylalanine, cfDNA – cell free DNA, PCR – polymerase chain reaction. VO2max was measured by a continuous incremental maximal exercise test using programmable treadmill with gas exchange analysis system and 12-lead wireless ECG at day 1st. Afterwards volunteers performed treadmill run to volitional exhaustion at speed corresponding to 70% of their personal VO2 max at days 7th, 10th and 13th. Volitional exhaustion was defined as volunteer inability to maintain the required speed of running (exercise intensity) or its wish to stop the test.
Blood processing and measurement of cfDNA
EDTA blood samples were centrifuged (1600 × g, 4 °C, 10 min.) immediately after collection. The obtained plasma samples were subjected to the second centrifugation (16 000 × g, 4 °C, 5 min.) to remove the cell debris and were stored at −80 °C for no longer than 4 weeks until isolation of cfDNA with QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany) and measurement of plasma concentrations of cf n-DNA and cf m-DNA with real time quantitative PCR as described previously3. Individual results were obtained as a mean from two separate runs and expressed in ng/mL for cf n-DNA and as genome equivalents (GE)/ml plasma (1 GE = 6.6 pg DNA) for cf mt-DNA79.
Determination of plasma cell free DNA integrity
The integrity of circulating cf n-DNA was evaluated by a quantitative real-time PCR (qPCR) targeting the human GAPDH (glyceraldehyde 3-phosphate dehydrogenase) gene (gene ID 2597). The length of the amplicons selected for this assay was 97 and 229 bp, respectively. The ratio of the concentration of the longer amplicon (GAPDH229) to the concentration of the shorter one (GAPDH97) (ranging from 0 to 1) defined the integrity index 229/97 (I229/97), which was used to estimate the fragmentation of cf n-DNA. Higher values of I229/97 (e.g.I229/97 = 1) indicate that all the cf n-DNA molecules are at least 229 bp in length in the GAPDH gene while lower values show that cf n-DNA contains fragments below 229 bp in the same target gene sequence. Similarly two amplicons, one of 218 bp length (encoding part of mitochondrial ATPase 6 gene, ID 4508, and mitochondrial ATPase 8 gene, ID 4509) and the second one of 78 bp length (encoding part of mitochondrial ATPase 8 gene) for calculation of cf mt-DNA integrity index 218/78 (I218/78) were chosen. The assay was designed in a way that the forward primer and the probe were the same for each pair of amplicons, whereas two different reverse primers were used. Both, GAPDH97 and MTATP78 primers and corresponding probes were described and successfully used in previous studies79. The primers for longer sequences (GAPDH229 primer and MTATP218 primer) were planned using Primer3 software (Table 4). qPCR was carried out in 20 μL of total reaction volume containing 6.5 μL H2O, 10 μL TaqMan® Universal PCR Master Mix (Applied Biosystems, Branchburg, New Jersey, USA), 0.25 μL of each of the two matched primers (one forward and one reverse primer for longer amplicon or one forward and one reverse primer for shorter amplicon) (Sigma-Aldrich), 1 μL of a FAM-labeled MT-ATP 8-probe or 1 μL of a MVIC-labeled GAPDH-probe (both probes from Applied Biosystems, Branchburg, New Jersey, USA), and 2 μL of TE buffer containing cfDNA isolated from plasma. The final concentrations of primers and probes were 0.6 µmol/L and 0.4 µmol/L, respectively. Negative control samples received 2 μL of TE buffer without cfDNA from plasma. Reaction was done in duplicate and performed using the 7900 HT Real-time PCR System (Applied Biosystems) under the following conditions: an initiation step at 50 °C for 2 min., followed by a first denaturation at 95 °C for 10 min., then 40 cycles at 95 °C for 15 s. and annealing at 60 °C for 1 min. Serial dilutions of human genomic DNA (Roche) (final concentrations from 0.5 ng/mL to 5000 ng/mL) were used to construct the calibration curve (r2 = 0.9996) for measurement of PCR products.
Resting and fMLP-induced luminol enhanced whole blood chemiluminescence
The resting (rLBCL) and fMLP-induced LBCL (fMLP-LBCL) reflecting ROS production by circulating phagocytes were measured according to the method of Kukovetz et al.21 with some modifications31.
Briefly, 30 µL of venous blood (collected into vacutainer tubes with EDTA) was initially diluted with 1 mL of mixture solution prepared fresh before the assay and containing 127.5 µg/mL of luminol31. Then 103 µL of diluted blood was added to 797 µL of mixture solution (to obtain a final blood dilution of 300 times), placed in a multitube luminometer (AutoLumat Plus LB 953, Berthold, Germany) equipped with a Peltier-cooled detector to ensure high sensitivity, and a low and stable background noise signal, and incubated for 15 min. at 37 °C in the dark. Then 100 µL of the control solution (solvent for fMLP) or 100 µL of fMLP solution (final agonist concentration in the sample of 2 × 10−5 mol/L) for measurement of rLBCL and fMLP-LBCL was added by automatic dispensers and the total light emission was automatically measured for 120 seconds. Individual results were obtained as the mean of triplicate experiments and rLBCL and fMLP-LBCL was expressed in relative light units (RLU) per 104 phagocytes (PMNs and monocytes) present in the assayed sample31.
Statistical analysis
Results are expressed as a mean (SD) and median (interquartile range). Analysis of variance (ANOVA) for repeated observations (parametric test) or Friedman’s ANOVA (non parametric test) was applied for the assessment of changes in variables over time (before and after three consecutive exercise bouts) depending on data distribution which was tested with Shapiro-Wilk’s W test. In case of statistically significant ANOVA, the post hoc analyses were done with Scheffe’s test or post hoc analysis for Friedman’s ANOVA (multiple comparisons at 2 different time points). Correlations between variables were determined using Spearman’s ƍ. A p value < 0.05 was considered significant.
Change history
13 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Brown, W. M., Davison, G. W., McClean, C. M. & Murphy, M. H. A Systematic Review of the Acute Effects of Exercise on Immune and Inflammatory Indices in Untrained Adults. Sports medicine - open 1, 35, https://doi.org/10.1186/s40798-015-0032-x (2015).
Saito, D. et al. Effects of long-distance running on serum opsonic activity measured by chemiluminescence. Luminescence: the journal of biological and chemical luminescence 18, 122–124, https://doi.org/10.1002/bio.438 (2003).
Stawski, R. et al. Repeated bouts of exhaustive exercise increase circulating cell free nuclear and mitochondrial DNA without development of tolerance in healthy men. PloS one 12, e0178216, https://doi.org/10.1371/journal.pone.0178216 (2017).
Atamaniuk, J. et al. Cell-free plasma DNA and purine nucleotide degradation markers following weightlifting exercise. Eur J Appl Physiol 110, 695–701, https://doi.org/10.1007/s00421-010-1532-5 (2010).
Fatouros, I. G. et al. Time of sampling is crucial for measurement of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin Biochem 43, 1368–1370, https://doi.org/10.1016/j.clinbiochem.2010.08.020 (2010).
Haller, N. et al. Circulating, cell-free DNA as a marker for exercise load in intermittent sports. 13, e0191915, https://doi.org/10.1371/journal.pone.0191915 (2018).
Tug, S. et al. Exploring the Potential of Cell-Free-DNA Measurements After an Exhaustive Cycle-Ergometer Test as a Marker for Performance-Related Parameters. International journal of sports physiology and performance 12, 597–604, https://doi.org/10.1123/ijspp.2016-0157 (2017).
Velders, M. et al. Exercise is a potent stimulus for enhancing circulating DNase activity. Clinical biochemistry 47, 471–474, https://doi.org/10.1016/j.clinbiochem.2013.12.017 (2014).
Tug, S. et al. Acute effects of strength exercises and effects of regular strength training on cell free DNA concentrations in blood plasma. PloS one 12, e0184668, https://doi.org/10.1371/journal.pone.0184668 (2017).
Haller, N., Tug, S., Breitbach, S., Jorgensen, A. & Simon, P. Increases in Circulating Cell-Free DNA During Aerobic Running Depend on Intensity and Duration. International journal of sports physiology and performance 12, 455–462, https://doi.org/10.1123/ijspp.2015-0540 (2017).
Vittori, L. N., Tarozzi, A. & Latessa, P. M. Circulating Cell-Free DNA in Physical Activities. Methods in molecular biology (Clifton, N.J.) 1909, 183–197, https://doi.org/10.1007/978-1-4939-8973-7_14 (2019).
Andreatta, M. V. et al. Cell-Free DNA as an Earlier Predictor of Exercise-Induced Performance Decrement Related to Muscle Damage. International journal of sports physiology and performance 13, 953–956, https://doi.org/10.1123/ijspp.2017-0421 (2018).
Syu, G. D., Chen, H. I. & Jen, C. J. Acute severe exercise facilitates neutrophil extracellular trap formation in sedentary but not active subjects. Medicine and science in sports and exercise 45, 238–244, https://doi.org/10.1249/MSS.0b013e31826df4a1 (2013).
Beiter, T., Fragasso, A., Hudemann, J., Niess, A. M. & Simon, P. Short-term treadmill running as a model for studying cell-free DNA kinetics in vivo. Clinical chemistry 57, 633–636, https://doi.org/10.1373/clinchem.2010.158030 (2011).
Beiter, T., Fragasso, A., Hartl, D. & Niess, A. M. Neutrophil extracellular traps: a walk on the wild side of exercise immunology. Sports medicine (Auckland, N.Z.) 45, 625–640, https://doi.org/10.1007/s40279-014-0296-1 (2015).
Sollberger, G., Tilley, D. O. & Zychlinsky, A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Developmental cell 44, 542–553, https://doi.org/10.1016/j.devcel.2018.01.019 (2018).
Babior, B. M. NADPH oxidase: an update. Blood 93, 1464–1476 (1999).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology 176, 231–241, https://doi.org/10.1083/jcb.200606027 (2007).
Mochida, N. et al. The main neutrophil and neutrophil-related functions may compensate for each other following exercise-a finding from training in university judoists. Luminescence: the journal of biological and chemical luminescence 22, 20–28, https://doi.org/10.1002/bio.922 (2007).
Peake, J. et al. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. Journal of applied physiology (Bethesda, Md.: 1985) 97, 612–618, https://doi.org/10.1152/japplphysiol.01331.2003 (2004).
Kukovetz, E. M., Bratschitsch, G., Hofer, H. P., Egger, G. & Schaur, R. J. Influence of age on the release of reactive oxygen species by phagocytes as measured by a whole blood chemiluminescence assay. Free Radic Biol Med 22, 433–438 (1997).
Bedouhene, S., Moulti-Mati, F., Hurtado-Nedelec, M., Dang, P. M. & El-Benna, J. Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. American journal of blood research 7, 41–48 (2017).
Suzuki, K. et al. Effects of exhaustive endurance exercise and its one-week daily repetition on neutrophil count and functional status in untrained men. International journal of sports medicine 17, 205–212, https://doi.org/10.1055/s-2007-972833 (1996).
Smith, J. A., Telford, R. D., Mason, I. B. & Weidemann, M. J. Exercise, training and neutrophil microbicidal activity. International journal of sports medicine 11, 179–187, https://doi.org/10.1055/s-2007-1024788 (1990).
Yamada, M. et al. Effect of exhaustive exercise on human neutrophils in athletes. Luminescence: the journal of biological and chemical luminescence 15, 15–20, 10.1002/(sici)1522-7243(200001/02)15:1<15::aid-bio570>3.0.co;2-o (2000).
Ristola, M. & Repo, H. Luminol-enhanced chemiluminescence of whole blood. Statistical analysis, and comparison of the responses of different subjects. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 97, 503–512 (1989).
Luczynska, M. et al. Elevated whole blood chemiluminescence in patients with systemic sclerosis. Clinical and experimental rheumatology 23, 173–179 (2005).
Ostrowski, S., Kasielski, M., Kordiak, J. & Nowak, D. Elevated resting and agonist-induced whole blood chemiluminescence in patients with active infective endocarditis. Interactive cardiovascular and thoracic surgery 8, 12–16, https://doi.org/10.1510/icvts.2008.183285 (2009).
Rysz, J. et al. Increased whole blood chemiluminescence in patients with chronic renal failure independent of hemodialysis treatment. Archivum immunologiae et therapiae experimentalis 54, 347–355, https://doi.org/10.1007/s00005-006-0040-0 (2006).
Bialasiewicz, P. et al. Addition of strawberries to the usual diet decreases resting chemiluminescence of fasting blood in healthy subjects-possible health-promoting effect of these fruits consumption. Journal of the American College of Nutrition 33, 274–287, https://doi.org/10.1080/07315724.2013.870502 (2014).
Bialasiewicz, P. et al. Sour Cherries but Not Apples Added to the Regular Diet Decrease Resting and fMLP-Stimulated Chemiluminescence of Fasting Whole Blood in Healthy Subjects. Journal of the American College of Nutrition 37, 24–33, https://doi.org/10.1080/07315724.2017.1354739 (2018).
Tepper, C. G. & Studzinski, G. P. Resistance of mitochondrial DNA to degradation characterizes the apoptotic but not the necrotic mode of human leukemia cell death. Journal of cellular biochemistry 52, 352–361, https://doi.org/10.1002/jcb.240520311 (1993).
Alexeyev, M. F. Is there more to aging than mitochondrial DNA and reactive oxygen species? The FEBS journal 276, 5768–5787, https://doi.org/10.1111/j.1742-4658.2009.07269.x (2009).
Kreher, J. B. Diagnosis and prevention of overtraining syndrome: an opinion on education strategies. Open access journal of sports medicine 7, 115–122, https://doi.org/10.2147/oajsm.s91657 (2016).
Schwellnus, M. & Soligard, T. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. 50, 1043–1052, https://doi.org/10.1136/bjsports-2016-096572 (2016).
Meeusen, R. et al. Diagnosing overtraining in athletes using the two-bout exercise protocol. British journal of sports medicine 44, 642–648, https://doi.org/10.1136/bjsm.2008.049981 (2010).
Rhodes, A. & Cecconi, M. Cell-free DNA and outcome in sepsis. Critical care (London, England) 16, 170, https://doi.org/10.1186/cc11508 (2012).
Gogenur, M., Burcharth, J. & Gogenur, I. The role of total cell-free DNA in predicting outcomes among trauma patients in the intensive care unit: a systematic review. Critical care (London, England) 21, 14, https://doi.org/10.1186/s13054-016-1578-9 (2017).
Breitbach, S., Tug, S. & Simon, P. Circulating cell-free DNA: an up-coming molecular marker in exercise physiology. Sports medicine (Auckland, N.Z.) 42, 565–586, https://doi.org/10.2165/11631380-000000000-00000 (2012).
Fatouros, I. G. et al. Cell-free plasma DNA as a novel marker of aseptic inflammation severity related to exercise overtraining. Clinical chemistry 52, 1820–1824, https://doi.org/10.1373/clinchem.2006.070417 (2006).
Sato, H. et al. Changes in the production of reactive oxygen species from neutrophils following a 100-km marathon. Nihon eiseigaku zasshi. Japanese journal of hygiene 51, 612–616 (1996).
Chinda, D. et al. A competitive marathon race decreases neutrophil functions in athletes. Luminescence: the journal of biological and chemical luminescence 18, 324–329, https://doi.org/10.1002/bio.744 (2003).
Pyne, D. B., Baker, M. S., Smith, J. A., Telford, R. D. & Weidemann, M. J. Exercise and the neutrophil oxidative burst: biological and experimental variability. European journal of applied physiology and occupational physiology 74, 564–571 (1996).
Sato, H. et al. Effects of acute endurance exercise and 8 week training on the production of reactive oxygen species from neutrophils in untrained men. Nihon eiseigaku zasshi. Japanese journal of hygiene 53, 431–440 (1998).
Makni-Maalej, K. et al. Zymosan induces NADPH oxidase activation in human neutrophils by inducing the phosphorylation of p47phox and the activation of Rac2: involvement of protein tyrosine kinases, PI3Kinase, PKC, ERK1/2 and p38MAPkinase. Biochemical pharmacology 85, 92–100, https://doi.org/10.1016/j.bcp.2012.10.010 (2013).
Szkudlarek, U., Maria, L., Kasielski, M., Kaucka, S. & Nowak, D. Exhaled hydrogen peroxide correlates with the release of reactive oxygen species by blood phagocytes in healthy subjects. Respiratory medicine 97, 718–725 (2003).
Kopprasch, S., Graessler, J., Kohl, M., Bergmann, S. & Schroder, H. E. Comparison of circulating phagocyte oxidative activity measured by chemiluminescence in whole blood and isolated polymorphonuclear leukocytes. Clinica chimica acta; international journal of clinical chemistry 253, 145–157 (1996).
Nielsen, H. G., Oktedalen, O., Opstad, P. K. & Lyberg, T. Plasma Cytokine Profiles in Long-Term Strenuous Exercise. Journal of sports medicine (Hindawi Publishing Corporation) 2016, 7186137, https://doi.org/10.1155/2016/7186137 (2016).
Suzuki, K. et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exercise immunology review 8, 6–48 (2002).
Borish, L., Rosenbaum, R., Albury, L. & Clark, S. Activation of neutrophils by recombinant interleukin 6. Cellular immunology 121, 280–289 (1989).
Elbim, C., Bailly, S., Chollet-Martin, S., Hakim, J. & Gougerot-Pocidalo, M. A. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infection and immunity 62, 2195–2201 (1994).
El-Benna, J., Dang, P. M. & Gougerot-Pocidalo, M. A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Seminars in immunopathology 30, 279–289, https://doi.org/10.1007/s00281-008-0118-3 (2008).
Khwaja, A., Carver, J. E. & Linch, D. C. Interactions of granulocyte-macrophage colony-stimulating factor (CSF), granulocyte CSF, and tumor necrosis factor alpha in the priming of the neutrophil respiratory burst. Blood 79, 745–753 (1992).
Bjornsdottir, H. et al. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free radical biology & medicine 89, 1024–1035, https://doi.org/10.1016/j.freeradbiomed.2015.10.398 (2015).
Neubauer, O., Reichhold, S., Nersesyan, A., Konig, D. & Wagner, K. H. Exercise-induced DNA damage: is there a relationship with inflammatory responses? Exercise immunology review 14, 51–72 (2008).
Atamaniuk, J. et al. Increased concentrations of cell-free plasma DNA after exhaustive exercise. Clinical chemistry 50, 1668–1670, https://doi.org/10.1373/clinchem.2004.034553 (2004).
Atamaniuk, J. et al. Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. European journal of applied physiology 104, 711–717, https://doi.org/10.1007/s00421-008-0827-2 (2008).
Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I. & Simon, H. U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell death and differentiation 16, 1438–1444, https://doi.org/10.1038/cdd.2009.96 (2009).
McIlroy, D. J. et al. Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery. Journal of critical care 29(1133), e1131–1135, https://doi.org/10.1016/j.jcrc.2014.07.013 (2014).
Syu, G. D., Chen, H. I. & Jen, C. J. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PloS one 6, e24385, https://doi.org/10.1371/journal.pone.0024385 (2011).
Sakellariou, G. K. et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants & redox signaling 18, 603–621, https://doi.org/10.1089/ars.2012.4623 (2013).
Zuo, L., Zhou, T., Pannell, B. K., Ziegler, A. C. & Best, T. M. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta physiologica (Oxford, England) 214, 329–348, https://doi.org/10.1111/apha.12515 (2015).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183, https://doi.org/10.1182/blood-2014-05-573543 (2014).
Marcoux, G. et al. Microparticle and mitochondrial release during extended storage of different types of platelet concentrates. Platelets 28, 272–280, https://doi.org/10.1080/09537104.2016.1218455 (2017).
Kratz, A., Wood, M. J., Siegel, A. J., Hiers, J. R. & Van Cott, E. M. Effects of marathon running on platelet activation markers: direct evidence for in vivo platelet activation. American journal of clinical pathology 125, 296–300, https://doi.org/10.1309/prf5-n7p2-xm6e-243h (2006).
Rector, R. S. et al. Short-term lifestyle modification alters circulating biomarkers of endothelial health in sedentary, overweight adults. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 31, 512–517, https://doi.org/10.1139/h06-040 (2006).
Breitbach, S. et al. Direct quantification of cell-free, circulating DNA from unpurified plasma. PloS one 9, e87838, https://doi.org/10.1371/journal.pone.0087838 (2014).
Breitbach, S., Sterzing, B., Magallanes, C., Tug, S. & Simon, P. Direct measurement of cell-free DNA from serially collected capillary plasma during incremental exercise. Journal of applied physiology (Bethesda, Md.: 1985) 117, 119–130, https://doi.org/10.1152/japplphysiol.00002.2014 (2014).
Rajecky, M., Lojek, A. & Ciz, M. Differentiating between intra- and extracellular chemiluminescence in diluted whole-blood samples. International journal of laboratory hematology 34, 136–142, https://doi.org/10.1111/j.1751-553X.2011.01370.x (2012).
Berzosa, C. et al. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. Journal of biomedicine & biotechnology 2011, 540458, https://doi.org/10.1155/2011/540458 (2011).
Tauler, P. et al. Increased lymphocyte antioxidant defences in response to exhaustive exercise do not prevent oxidative damage. The Journal of nutritional biochemistry 17, 665–671, https://doi.org/10.1016/j.jnutbio.2005.10.013 (2006).
Carlsohn, A. et al. Exercise increases the plasma antioxidant capacity of adolescent athletes. Annals of nutrition & metabolism 53, 96–103, https://doi.org/10.1159/000165357 (2008).
Garley, M. & Jablonska, E. Heterogeneity Among Neutrophils. Archivum immunologiae et therapiae experimentalis 66, 21–30, https://doi.org/10.1007/s00005-017-0476-4 (2018).
Kobayashi, Y. Neutrophil biology: an update. EXCLI journal 14, 220–227, https://doi.org/10.17179/excli2015-102 (2015).
Elbim, C. & Lizard, G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry. Part A: the journal of the International Society for Analytical Cytology 75, 475–481, https://doi.org/10.1002/cyto.a.20726 (2009).
Leliefeld, P. H. C. & Pillay, J. Differential antibacterial control by neutrophil subsets. 2, 1344−1355, https://doi.org/10.1182/bloodadvances.2017015578 (2018).
Beiter, T. et al. Neutrophils release extracellular DNA traps in response to exercise. Journal of applied physiology (Bethesda, Md.: 1985) 117, 325–333, https://doi.org/10.1152/japplphysiol.00173.2014 (2014).
Buyon, J. P., Korchak, H. M., Rutherford, L. E., Ganguly, M. & Weissmann, G. Female hormones reduce neutrophil responsiveness in vitro. Arthritis and rheumatism 27, 623–630 (1984).
Kohler, C. et al. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Molecular cancer 8, 105, https://doi.org/10.1186/1476-4598-8-105 (2009).
Acknowledgements
This study was supported by a research grant from the Medical University of Lodz No: 503/1-079-01/503-11-001.
Author information
Authors and Affiliations
Contributions
R.S. D.N. K.W. were involved in conceptualization, study design and methodology, R.S. P.M. collected the data, R.S. E.P. A.S. A.W. conducted experiments, P.K. T.B. K.W. were responsible for participant safety during the execution of treadmill exhaustive exercise,. D.N. G.P. supply resources, R.S. performed data analysis, statistics and visualization, D.N. was responsible for project administration and supervision, R.S. D.N. wrote the main text, D.N. R.S. E.P. G.P. review and edit the manuscript. All authors approved final submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stawski, R., Walczak, K., Perdas, E. et al. Decreased integrity of exercise-induced plasma cell free nuclear DNA – negative association with the increased oxidants production by circulating phagocytes. Sci Rep 9, 15970 (2019). https://doi.org/10.1038/s41598-019-52409-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52409-w
This article is cited by
-
Guidelines for pre-analytical conditions for assessing the methylation of circulating cell-free DNA
Clinical Epigenetics (2021)
-
Evaluation of circulating cell-free DNA in cholestatic liver disease using liver-specific methylation markers
BMC Gastroenterology (2021)
-
Circulating cell free DNA response to exhaustive exercise in average trained men with type I diabetes mellitus
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.