Abstract
Piriformospora indica, a root endophytic fungus, promotes growth of the economically important chickpea plant (Cicer arietinum Linn.) and protects it against the pathogenic fungus Botrytis cinerea. Biomass and root development were found to be significantly improved in chickpea plants colonized with P. indica as compared to the plants grown without P. indica as well as from the plants infected with the B. cinerea. Our PCR analyses showed that gradual increase in the colonization of P. indica in the plants result in the inhibition of the colonization of B. cinerea. P. indica colonized plants showed increased antioxidant enzyme activities. Interestingly, there were pronounced decrease in the antioxidant enzyme activities in shoots infected with B. cinerea and colonized with P. indica in alternate and simultaneous mode as compared to plants infected with B. cinerea alone. We conclude that P. indica helps plants to overcome the disease load by enhancing antioxidant enzyme defense system. Our data suggest that, bio-protective action of P. indica might be mediated via systemic induction of antioxidant defense in the host plants.
Similar content being viewed by others
Introduction
Mycorrhizal associations are very important for a wide range of plants. They are broadly categorised into endomycorrhiza and ectomycorrhiza1. Arbuscular mycorrhiza (AM) association is found between a wide variety of plants e.g., in some bryophytes, gymnosperms, pteridophytes and angiosperms. Arbuscular Mycorrhizal Fungi (AMF) are obligate biotrophs and colonize root cells when they get a specific response from the plants. Through this association both partners fulfil their nutritional demands. In particular phosphate is given by the fungus to the host plant and in turn fungus gets reduced carbon from the plant2. The symbiotic interaction requires a balance between the plant defence and the nutritional requirements of both partners. Mycorrhized plants are also better protected against pathogens3,4,5.
It is generally believed that during early phases of beneficial plant/microbe interaction, plant activates defence responses and alters the antioxidant enzyme level, since it does not know yet whether it interacts with a friend or foe. The weak plant defence response includes defence gene activation and H2O2 production6. Stimulation of the anti-oxidative enzyme levels in the plant suggests that oxidative compounds are formed during the interaction with the fungus. Plants produce different types of antioxidative scavengers to cope with ROS7. The enzymatic ROS scavenging system includes sodium superoxide dismutases (SODs), glutathione peroxidases (GPXs), ascorbate peroxidases (APXs), catalases (CATs), glutathione-S-transferases (GSTs) and glutathione reductases (GRs).
B. cinerea is a necrotrophic pathogen that causes disease in many economically important cereal crops and medicinal plants all over the world. B. cinerea is the causative agent of Botrytis Grey Mould (BGM) disease in several plant species, including chickpea (Cicer arietinum Linn.). The asexual stage of B. cinerea is dominant in chickpea plant and the pathogen infects mainly the aerial part and the infection may lead to complete crop loss8,9,10.
Unlike AMF, P. indica is an axenically cultivable fungus that colonises a wide range of hosts without any host specificity11. P. indica colonizes plants belonging to bryophytes, pteridophytes, gymnosperms and a large number of monocots and dicots12,13,14. Colonization of P. indica in plants promotes biomass production, early flowering, seed production, nutrient uptake from the soil and provides tolerance against biotic and abiotic stresses15,16,17,18. It has been reported that P. indica protects barley from the adverse effects of the high salt concentration. P. indica associated plants showed a high level of antioxidant enzyme activities which protect plants from oxidative damage. P. indica inoculated plants showed an increase in SOD, GR, CAT and GST activities19,20 which help plants to overcome infections and stresses. They might also participate in promoting plant growth by keeping the ROS level below a critical threshold21. To know how P. indica help the infected chickpea plants to overcome disease load of B. cinerea, tripartite interaction of P. indica, B. cinerea and chickpea plants and the role of antioxidant enzymes have been investigated in this study.
Results
Role of P. indica in bioprotection against B. cinerea
We have observed that P. indica spores germinate and enter into the chickpea roots within 5 to 7 days. Further we have found that colonization of the roots depends on the size of the inoculum and duration of co-cultivation. We have observed minimum 7–9% of colonization by the P. indica in the roots of chickpea plants in 7 days and maximum 65% colonization after 30 days of interaction (Table 1 ). We have observed intracellular densely packed pear shaped chlamydospores in the plant roots (Fig. 1). We observed distinct morphological alterations in case of roots as well as in shoots of plants inoculated with P. indica as compared to the plants without P. indica (control) (Fig. 2A). In case of colonized plants by P. indica, we have found an increase in the length of primary, seminal, crown and lateral roots. We have also observed increased number of seminal and crown roots in case of plants inoculated with P. indica as compared to the plants grown alone (Fig. 2B). During later stages fine secondary and tertiary roots formed interwoven network (Fig. 2A,B). In contrast, plants infected with B. cinerea showed poor root and shoot growth as compared to non-infected plants (Fig. 2A). Significantly, more number of secondary roots and increased crown root length was observed in case of plants colonized with the P. indica as compared to the plants infected with the B. cinerea as well as with that of plants not inoculated with either of the fungus (P < 0.05) (Fig. 2C,D). Also crown root length as well as secondary roots number were found to be significantly more in case of control plants (not inoculated with either of the fungus) as compared to the plants only infected with the B. cinerea (P < 0.05). We have observed increased length of crown roots and more number of secondary roots in case of plants first inoculated with P. indica and 10 days later infected with B. cinerea as compared to chickpea plants which were not colonized either with B. cinerea or P. indica and this difference was found to be significant (P < 0.05) (Fig. 2C,D). A similar trend was also observed when the plants were first infected with B. cinerea and at day 10 inoculated with P. indica, or when both fungi were applied simultaneously as compared to the plants infected with the B. cinerea only (P < 0.05) (Fig. 2C,D).
Morphological shoot/root growth pattern analysis in case of chickpea plant inoculated with P. indica and infected with B. cinerea in different experimental condition. (A) Plant shoot and root phenotype demonstrating the bioprotection role of P. indica against B. cinerea. Chickpea plants were grown for 30 days without any fungus were used as control (C); Chickpea plants inoculated with P. indica alone at day 0 and grown for 30 days (P); Chickpea plants infected with B. cinerea alone at day 0 and grown for 30 days (B); Chickpea plants first inoculated with P. indica at day 0 and at day 10 infected with B. cinerea and grown for 30 days (P→B); Chickpea plants first infected with B. cinerea at day 0 and at day 10 inoculated with P. indica and grown for 30 days (B→P); Chickpea plants inoculated/infected simultaneously with both fungi at day 0 and grown for 30 days (P+B). (B) Root growth pattern in control plant without colonization (a); and in a plant colonized with P. indica (b). (C) Change in length of crown roots determined under similar condition mentioned above (D) Number of secondary roots determined under similar condition mentioned above; Six pots, each having four plantlets per pot were used to determine the secondary root numbers (n = 24). Different letters indicate significant differences in length of crown roots and number of secondary roots at P ≤ 0.05 (Tukey’s test).
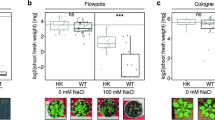
In case of dry weight, we found a significant increase, i.e. 1.6-fold, in 30-day-old chickpea plants colonized with P. indica as compared to the plants grown without fungus (P < 0.05) (Fig. 3). We observed a 0.22-fold decrease in the dry weight of 30-day-old chickpea plants infected with B. cinerea (P < 0.05) as compared with non-colonized plants (control). An improved biomass yield, i.e. a 1.8-fold increase in dry weight was observed in the plants first inoculated with B. cinerea and later inoculated with the P. indica as compared to the plants inoculated with B. cinerea only (Fig. 3) (P < 0.05). Further, we found, a 1.25-fold increase in dry weight in case of chickpea plants inoculated together with P. indica and B. cinerea at day 0 in comparison with chickpea plants grown without any fungus (Fig. 3 ). We have not observed any harmful effects of B. cinerea on the overall plant health in case of plants first inoculated with the P. indica and later infected with B. cinerea (Figs 2, 3).
Impact of colonization of P. indica on biomass yield. Impact of simultaneous and alternate inoculation of P. indica and B. cinerea on biomass yield (dry weight) relative to plants exposed to P. indica or B. cinerea alone or not exposed to any fungus at 30 days. Different letters indicate significant differences in biomass yield at P ≤ 0.05 (Tukey’s test).
A gradual increase in the band intensity of the tef gene was observed in case of P. indica- inoculated plants at 5, 15 and 30 days (Fig. 4a). We have obtained similar results for cpr1 gene in the case of plants inoculated with B. cinerea alone (Fig. 4b). In case of plants first inoculated with P. indica and later infected with the B. cinerea, we have observed a gradual increase in the band intensity of tef gene till 30 days, however in case of cpr1 gene only till day 15 band intensity was slightly increased. No band of cpr1 gene was observed at day 30 (Fig. 4c,d). Further we found an increase in the band intensity of tef gene till 30 days in case of plants first inoculated with B. cinerea and later inoculated with P. indica (Fig. 4e). Importantly, we observed a gradual decrease in the band intensity of the cpr1 gene in the same samples and at the end of the 30 days a very faint band was observed (Fig. 4f).
Interaction of P. indica and B. cinerea with chickpea plants. Amplification of DNA from chickpea roots after 5, 15 and 30 days of alternate inoculation of P. indica and B. cinerea. (a) Amplification of the P. indica tef gene from plants inoculated with P. indica alone. (b) Amplification of the B. cinerea cpr1 gene from plants inoculated with B. cinerea alone. (c) Amplification of the P. indica tef gene from P. indica inoculated plants later infected with B. cinerea. (d) Amplification of the B. cinerea cpr1 gene from P. indica inoculated plants later infected with B. cinerea. (e) Amplification of the P. indica tef gene from B. cinerea infected plants later inoculated with P. indica. (f) Amplification of the B. cinerea cpr1 gene from B. cinerea infected plants later inoculated with P. indica. (g) Amplification of the P. indica tef gene from P. indica inoculated and B. cinerea infected plants simultaneously. (h) Amplification of the B. cinerea cpr1 gene from P. indica inoculated and B. cinerea infected plants simultaneously. (i) Amplification of chickpea elongation factor 1-alpha (EF1-α) gene (control). M- 100 bp DNA ladder.
In case of plants inoculated simultaneously with P. indica and B. cinerea, we have observed a gradual increase in the band intensity of tef gene till 30 days, but in case of cpr1 a marked decrease in the band intensity was found and at the day 30, a very light band was observed (Fig. 4g,h).
Antibiosis assay of P. indica and B. cinerea
This assay was performed in order to check whether the increased resistance to B. cinerea is mediated by antibiotics/chemicals secreted by P. indica or not. We have not observed any growth inhibition of the two fungi when grown together which suggests that both fungi do not secret antibiotics or chemicals (Fig. 5).
Antioxidant enzyme activities
In case of roots, antioxidant enzyme activities were found to be significantly increased i,e. 34, 71, 26 and 19 fold for GST, GR, CAT and SOD respectively in chickpea plants colonized with P. indica as compared to non-colonized plant (Fig. 6A–D) (P < 0.05). GST and GR activity were found to be significantly increased i,e. 6 and 50 folds respectively in case of plant infected with B. cinerea as compared to non-infected plant (P < 0.05), (Fig. 6A,B). Under similar conditions, in case of CAT and SOD no significant difference was found, though we observed an increase in the activity in case of both the enzymes (Fig. 6C,D). In case of simultaneous and alternate inoculation of P. indica and infection of B. cinerea, we found significant increase (P < 0.05) in the GST activity as compared to plant infected with B. cinerea only (Fig. 6A) while significantly (P < 0.05) decreased activity of CAT was observed (Fig. 6C). However, no significant differences were found in the activity of GR and SOD enzymes (Fig. 6B,D).
Antioxidant enzyme activities in chickpea roots. Activity of antioxidant enzymes in root of plants with alternate (P→B) and (B→P) and simultaneous (P+B) colonization/infection of P. indica (P) and B. cinerea (B). (A) GST, (B) GR, (C) CAT and (D) SOD activities. Specific activities compared with those of control plants not exposed to any fungus. Different letters indicate significant differences in different antioxidant enzymes at P ≤ 0.05 (Tukey’s test).
In case of shoot, the antioxidant enzyme activities were found to be significantly increased i,e. 49, 13, 27 and 18 fold for GST, GR, CAT and SOD respectively in chickpea plants colonized with P. indica as compared to non-colonized plant (P < 0.05) (Fig. 7A–D). In case of simultaneous and alternate inoculation and infection of P. indica and B. cinerea, a decreased activity of GST, GR, CAT and SOD were found as compared to plant infected with B. cinerea, however, significant decreased activity was found in case of plants inoculated first with the P. indica and later infected with the B. cinerea as compared to the plants infected with the B. cinerea only (P < 0.05) (Fig. 7A–D ). In case of plants, infected with B. cinerea only, SOD activity was found to be significantly increased i,e. 110 fold as compared to plants not infected with B. cinerea (P < 0.05) (Fig. 7D).
Antioxidant enzyme activities in chickpea shoots. Activity of antioxidant enzymes in shoot of plants with alternate (P→B and B→P) and simultaneous (P+B) colonization/infection of P. indica (P) and B. cinerea (B). (A) GST, (B) GR, (C) CAT and (D) SOD activities. Specific activities compared with those of control plants not exposed to any fungus. Different letters indicate significant differences in different antioxidant enzymes at P ≤ 0.05 (Tukey’s test).
Role of P. indica in disease resistance
We observed a significant enhancement of disease resistance in P. indica-colonized chickpea plants towards BGM disease caused by B. cinerea. We found plants colonized with the P. indica healthier and developed as compared to the B. cinerea infected plants. As shown in Fig. (8) plants infected with B. cinerea showed severe symptoms of BGM disease. Stems and leaves of the infected plants were found to be yellowish, curled and dried as compared to the plants inoculated with the P. indica.
Bioprotection role of P. indica against Botrytis Grey Mould (BGM) disease. Chickpea plant infected with B. cinerea showing symptom of Botrytis grey mould (BGM) disease (A) Control plant not exposed to any fungus, (B) B. cinerea-infected chickpea plant is showing severe disease symptoms. (C) P. indica colonized chickpea plant infected with B. cinerea, showing decreased BGM disease symptoms.
Discussion
Symbiotic relation is very important for plants as well as for the microorganism which helps them to cope up with the environmental adverse conditions. Microbes help host plant from both biotic and abiotic stresses. AMF association enhances growth of the host plant and provides tolerance against biotic and abiotic stresses22,23. P. indica mimics AMF and has strong association with a large number of medicinal and economically important plants. Since P. indica colonizes a variety of unrelated hosts and due to its beneficial nature, it is a putative bio-control agent and biofertilizer19,24.
We showed that colonization of the P. indica promotes the growth, biomass production and lowers the disease development caused by the pathogen B. cinerea in the economically important chickpea plant. Similar results have also been reported in case of P. indica colonized maize plant and later challenged by the Fusarium, thus support our data19. Similarly, it has been reported that P. indica protects Arabidopsis plant infected by soil-borne pathogen Verticillium dahliae by down regulation of the plant defence response25. Bio-protection role of P. indica against the leaf parasite B. graminis has also been shown by Waller and co-workers3. Authors have concluded that P. indica colonization with the barley plants helps in the development of resistance to B. graminis infection thus these findings support our data.
It has been shown that P. indica protects colonized maize and rice plants against the root pathogen F. verticillioides and parasitic fungus B. graminis respectively3,19. As we have observed that plants recovered their biomass when first infected with B. cinerea and later inoculated with P. indica. This suggests that P. indica acts as a bioprotective agent. We found that presence of the B. cinerea does not affect overall plant growth and development in the presence of P. indica. Our observations suggest the role of P. indica in bioprotection and disease resistance. In order to support our observation, with respect to whether inoculation with P. indica inhibits the colonization by B. cinerea, we have performed PCR analyses using P. indica- and B. cinerea-specific primers. We observed that in case of alternate as well as simultaneous interaction of both the fungi, an increased band intensity was noticed for tef gene of P. indica till day 30 and a decreased band intensity was observed in case of cpr1 gene of B. cinerea. We conclude that gradual increase in the colonization by P. indica, results in the gradual decrease in the colonization of B. cinerea in the plants, which suggests that P. indica helps the plants to resist B. cinerea and helps the colonized plant to recover biomass as well as overall health.
Antibiosis assay demonstrates that there is no antibiotic/chemical secretion by P. indica to inhibit the growth of B. cinerea. This suggests that plant has developed the resistance towards the B. cinerea due to the colonization of the P. indica. Similar finding have also been reported by Kumar and co-workers19. Further authors have also found that inhibition in the growth of the pathogen Fusarium in the maize plant occurred due to the colonization of the P. indica and not by any antibiotic secretion. In another study by Deshmukh and Kogel26, it was reported that P. indica did not have direct inhibitory effect on the growth of the pathogenic fungus Fusarium. Recently, Rabiey and co-workers27 have also reported the similar findings during the interaction of P. indica with wheat plants infected with the Fusarium. Thus these studies support our hypothesis that resistance conferred to the chickpea plants by P. indica against pathogenic fungus B. cinerea is not due to any antibiotics or chemicals secreted by the P. indica.
To know whether root infestation by P. indica protects chickpea shoots from B. cinerea infections. Since P. indica does not infest shoot, protection against shoot pathogens would require a systemic response originate from the root, however, this phenomenon has not been reported for P. indica in case of chickpea plants. Therefore in the present study, we have also determined the antioxidant enzyme activity in root as well as in the case of shoot in chickpea plants colonized by the P. indica and infected with the B. cinerea. Inoculation of chickpea plants with P. indica strongly stimulates the activities of the antioxidative enzymes both in roots and shoots. In case of root and shoot we found 34 and 48 folds increase respectively in the GST activity during the colonization of the chickpea plants by P. indica. GST was found to be increased during the infection of Tobacco plants with Colletotrichum orbiculare 28. Similarly, Gullner and co-workers29 concluded that most likely role of GST is to reduce necrosis by detoxifying lipid hydroperoxides which are produced during the peroxidation of membrane. A consistent increase in GST level in our findings may also apply to the role of GST in limiting B. cinerea infection in chickpea plants, but this need warrant investigation.
It has been reported that CAT attenuate the elicitation of plant defence response by scavenging H2O2 and promotes intra-radical fungal growth30. In our study we found induction of CAT activities in case of chickpea plants infected with the B. cinerea. However, we observed a decrease in the CAT activities when plants first colonized by P. indica and later infected with the B. cinerea and vice versa. We suggest that less CAT activities results in the increase activity of H2O2 level which may lead to more production of the ROS that result in enhanced plant defence response against B. cinerea. In case of F. culmorum infected barley roots, a significant reduction in the activities of antioxidative enzymes like GR, DHAR, SOD, MDHAR and APX were observed20. Contrasting to this report, we found an increased activity of GR and SOD antioxidant enzymes in case of chickpea plants infected with B. cinerea. Similar results were also reported in case of maize plants infected with the Fusarium, thus support our data19. Elevated activities of antioxidant enzymes can be explained as this increased antioxidant activity minimizes the chances of oxidative burst and therefore B. cinerea might be protected from the oxidative defence system during infection.
It has been reported that pathogenic fungus F. culmorum infection with barley plants severely reduced growth. However, when plants pre-inoculated with P. indica and challenged with Fusarium, were found to be improved in the total biomass19,20. Interestingly, we have found the same pattern of reduced biomass in case of plant infected by B. cinerea. However, plants colonized with P. indica and later infected with B. cinerea recovered the loss in biomass caused due to B. cinerea infection as compared to control plant. This suggests P. indica colonizes roots of host plants and increases biomass and provide resistance against pathogenic fungus and, thus can be considered a bio-control agent. Under greenhouse conditions, it has been reported by Serfling and co-workers31 that due to the colonization of P. indica in winter wheat, symptom severity was found to be decreased significantly in case of infection by B. graminis f. sp. tritici, Pseudocercosporella and Fusarium and thus support our data.
Enhanced activity of SOD was found in case of plants colonized with the P. indica which may results in the accumulation of more H2O2, due to which less infection of Fusarium was observed in maize plants, therefore plants recover their biomass19. In the present study also, total activity of SOD was found to be increased in P. indica colonized plants as compared to the control plant, whereas when P. indica colonized plants were later infected with B. cinerea, the pathogen induced effects in SOD activity were attenuated as compared to plants infected with B. cinerea alone. This suggests, regulation of the SOD activity was much systemically repressed by P. indica in shoot and not repressed in case of roots in the presence of both fungi, however it needs further investigation.
It has been reported that P. indica and other fungal endophytes may enable plants to more efficiently scavenge ROS or prevent ROS production under stress conditions20,32,33,34,35. Our data suggest that antioxidant defence was maintained at a high level in plants colonized by the P. indica in response to B. cinerea infection. It is well-established that B. cinerea utilize production of ROS to accelerate cell death and facilitate subsequent infection36,37. We have observed a decreased antioxidant enzyme activity in the shoot in all the cases of alternate and simultaneous inoculations of P. indica and B. cinerea as compared to the plants only infected with the B. cinerea. We hypothesize that this decrease in the antioxidant enzyme activity would result in the induction of the oxidative stress exerted by ROS which may impart adverse effect in spread of B. cinerea infection. Therefore in all the cases we found an increase in the biomass of the P. indica inoculated plants as compared to the B. cinerea infected plants.
We have shown that colonization of P. indica with chickpea plants have strong growth promoting effects, systemic resistance to biotic stress and also results in the enhanced antioxidant capacity. Our study offers evidences that colonization of P. indica with chickpea plants might stimulate antioxidant enzymes which destroy ROS in plant cells to trigger subsequent defence reactions which in turn helps the chickpea plants to develop resistance against the infection of B. cinerea. As P. indica can be grown axenically, therefore, we suggest the use of P. indica as a bio-control agent in agriculture field to control pathogens and to overcome the use of pesticides and chemical fertilizers.
Materials and Methods
Plant and fungal culture and growth conditions
Chickpea seeds (Cicer arietinum, PUSA 1105) were surface-sterilized for 30 minutes in 2.5% sodium hypochlorite solution containing Tween-20. Seeds were finally washed in sterile distilled water few times and then imbibed in sterile distilled water overnight. A sterile filter paper was used in order to air-dry the seeds. Further, seeds were germinated by placing them (10 seeds) on Petri plate containing wet germination paper. For germination purpose, Petri plate contacting seeds was kept in the incubation at 23 ± 2 °C38. Seedlings were placed in pots (9 cm height and 10 cm diameter). P. indica (Varma, Kost, Rexer & Franken, 1997, European patent office, Muenchen, Germany. Patent No. 97121440.8-2105, Nov 1998) was a gift from Prof. Ajit Varma. P. indica was cultured in the laboratory routinely on solidified Aspergillus modified medium39 and were incubated for 7–10 days at 30 ± 2 °C. Growth of P. indica was studied in 250 ml culture flasks (containing KF media) with constant shaking at 100 rpm and 30 ± 2 °C in a metabolic shaker (Multitron Incubator Shaker, HT-Infors, Switzerland). B. cinerea was cultured on solidified potato dextrose agar (PDA) solid media or potato dextrose broth (1.5%) liquid media at 20–22 °C for 15–20 days. Surface sterilized pre-germinated chickpea seedlings were transferred to pots filled with a mixture of sterile sand and soil (3:1; garden soil and acid-washed riverbed sand). P. indica inoculation was performed as described previously19. Initially the plants were inoculated with P. indica through direct mixing of culture in sterile soil and by spraying B. cinerea grown culture suspension on the areal part of the plant. Ten millilitres of spore suspension (1 × 105 conidia/mL) of B. cinerea was spray inoculated on chickpea plants. As control, one set of plants were grown without any fungus and mock treatment was given using autoclaved double distilled water. Chickpea plants were grown in a controlled environment in a green house at 23 ± 2 °C with a 12-hour light/dark photoperiod, a relative humidity of 60–70% and a light intensity of 1000 μmol/m2s. Twice in a week plants were given water and fertilized with Hoagland solution40. Plant samples were harvested at different time intervals after inoculation of the fungus (or mock treatment). These collected samples were carefully washed with running tap water, rinsed in deionised autoclaved water and weighed. Further, samples were frozen in liquid nitrogen for enzyme assay and root colonization analysis.
Histochemical analysis
To study colonization, 10 randomly selected root samples were heated at 90 °C in 10% KOH for 15 min, followed by a treatment with 1 N HCl. They were stained with 0.05% trypan blue overnight or 60 °C for 1 h and destined in lactophenol41,42. Observation was done under the light microscope (Nikon Eclipse Ti). Percentage of colonization by the P. indica in the roots of plants was measured according to the method described previously19,43.
Role of P. indica in bioprotection
All plants were initially grown for 10 days (day 0) and further, following sets were made: (1) chickpea plants grown for additional 30 days without any fungus (control); (2) plants were inoculated with P. indica at day 0 and then grown for 30 days; (3) plants were infected with B. cinerea at day 0 and grown for 30 days; (4) plants were first infected with B. cinerea at day 0 and then inoculated with P. indica at day 10 and grown for additional 20 days; (5) plants were first inoculated with P. indica at day 0 and later infected with B. cinerea at day 10 and grown for additional 20 days; (6) plants were simultaneously exposed to both fungi at day 0 and then grown for 30 days. Six pots each having 4 plantlets were used for each experiment.
For the analysis of root colonization or plant growth, the same experimental setup was used and the roots were harvested at different time points. The growth promoting effect of the two fungi was measured as fresh and dry weight. After the removal of plants from the soil, they were washed properly with water and surface moisture was evaporated on soft tissue paper. For the determination of the dry weight, plants were dried in an oven at 80 °C for 72 h.
The antibiosis activity of P. indica and B. cinerea was assayed by inoculating both fungi on the same plate. The appearance of margins of the growing fungi is the sign of antibiotic activity.
PCR analyses
Bioprotection role of P. indica against the shoot pathogen fungus B. cinerea was checked by PCR as described previously19. For this purpose, following sets of plants were used (1) Chickpea plants inoculated with P. indica alone; (2) plants inoculated with B. cinerea alone; (3) Plants inoculated first with P. indica and later infected (at day 10) with B. cinerea; (4) Plants inoculated first with B. cinerea and later (at day 10) with P. indica; (5) Plants inoculated simultaneously with P. indica and B. cinerea. After 5, 15 and 30 days of inoculation, 10 plants were sampled from each set and examined for the presence of P. indica in plant roots tissues and B. cinerea within the shoot tissues. For PCR evaluation, total genomic DNA was isolated from the root and shoot tissues by the cetyltrimethylammonium bromide (CTAB) method as described previously44. PCR reactions were carried out in a final volume of 50 μl, containing 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 1.5 mM MgCl2; 200 μM of dNTPs; 3 μM of each primer; 3 units of Phusion High-Fidelity DNA polymerase (Thermo fisher Scientific)) and 50 ng of DNA as template. To quantify the presence of P. indica and B. cinerea in chickpea plants, we analysed the expression of EF-1-alpha (tef) gene (AJ249912.1) of P. indica, the cpr1 (cpr1) gene (AJ609393.1) of B. cinerea and the elongation factor 1-alpha (EF1-α) (LOC101488243) of the chickpea plants by using the following primer pairs, for P. indica, Pitef-F (5′TCGTCGCTGTCAACAAGATG3′) and Pitef-R (5′GAGGGCTCGAGCATGTTGT3′) for B. cinerea, BCcpr1-F (5′GCTCGGCGCTACAAGAATTG3′) and BCcpr1-R(5′CAGCTTCACGTTCCTGGAGT3′) and for chickpea, CaEF1-α-F (5′TCACCATGGTTGCTGCTGAA3′) and CaEF1-α-R (5′ATATGACCACCGCCGATCAA3′). PCR reactions were performed at the following reaction conditions: denaturation at 95 °C for 2 min, one cycle; for 35 cycles, denaturation at 95 °C for 30 s, annealing at 58 °C, 59 °C and 58 °C for 30 s for tef, cpr1 and the EF1-α gene, respectively; and extension at 72 °C for 30 s.
Antioxidant enzyme activities in chickpea plants
Antioxidant activities were checked in presence or absence of the parasitic fungus B. cinerea and P. indica. The roots and shoots were separately frozen in liquid nitrogen and homogenized with an ice-chilled mortar and liquid nitrogen in QB buffer without 1,4-dithiothreitol [for SOD, CAT and GST assays]. For the GR assay, 50 mg polyvinyl pyrrolidone per gram of tissue was added. The crude homogenates were centrifuged at 15000 g for 15 min at 4 °C and the supernatants were frozen at −20 °C. The protein concentrations were determined by the method of Bradford using bovine serum albumin as standard45.
The SOD activity was determined as described by Roth and Gilbert46. One millilitre of reaction mixture contained 50 mM sodium phosphate buffer (pH 7.8), 100 µM EDTA, an appropriate amount of the prepared extract and 10 mM of pyrogallol. The enzyme activity was calculated by measuring the absorbance change at 420 nm for 120 seconds against a blank sample without extract.
CAT activity was assayed by measuring H2O2 disappearance using the method described previously by Beers and Sizer47. One millilitre of the CAT assay reaction mixture contained 0.05 mM sodium phosphate, pH 7.0, an appropriate amount of prepared extract and 1 mM H2O2. The decrease in H2O2 concentration was followed at 240 nm and the activity was calculated using the extinction coefficient of 40 mM cm−1 for H2O2. The blank sample contained no plant extract.
GST activity was determined as per the method described by Habig and co-workers48. One millilitre of reaction mixture contained 0.1 M sodium phosphate pH 6.5, an appropriate amount of prepared extract, 2% 1-chloro-2,4-dinitrobenzene and 1 mM reduced glutathione. The enzyme activity was measured at 340 nm for 180 seconds against a blank without the plant extract.
GR activity was determined by the oxidation of NADPH at 340 nm at 25 °C with the extinction coefficient of 6.2 mM cm−1 as mentioned by Nordhoff and co-workers49. The reaction mixture was composed of 100 mM potassium phosphate, pH 7.8, 2 mM EDTA, 0.2 mM NADPH and 0.5 mM glutathione (oxidized form) and an appropriate volume of the prepared extract in a 1 ml volume. The reaction was initiated by the addition of NADPH.
Statistical Analysis
Statistical calculations were performed using Microsoft office excel 2007. ANOVA and Tukey’s test was used to test the significance of the data. For this purpose, Graph Pad Prism version 6 was used.
References
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis. Cambridge: Academic press. (2010).
Kogel, K., Franken, P. & Hu, R. Endophyte or parasite – what decides? 9, 358–363 (2006).
Waller, F. et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance and higher yield. Proc. Natl. Acad. Sci. USA 102, 13386–13391 (2005).
Jung, S. C. & Martinez-medina, A. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 38, 651–664 (2012).
Barna, B., Fodor, J., Harrach, B. D., Pogány, M. & Király, Z. Plant Physiology and Biochemistry The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 59, 37–43 (2012).
Salzer, P., Corbière, H. & Boller, T. Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta. 208, 319–325 (1999).
Alscher, R. G., Donahue, J. L. & Cramer, C. L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 100, 224–233 (1997).
Pande, S. et al. Integrated management of botrytis gray mold of chickpea. International Crops Research Institute for the Semi-Arid Tropics, 32 (2001).
Reddy, M., Singh, O., Bharati, M., Sah, R. & Joshi, S. Botrytis grey mold epiphytotic of chickpea in Nepal. Internat. Chickpea Newslett. 19, 15 (1988).
Williamson, B., Tudzynski, B., Tudzynski, P. & Van-Kan, J. A. L. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580 (2007).
Varma, A. et al. Piriformospora indica: a cultivable mycorrhiza-like endosymbiotic fungus. In TheMycota IX, ed. B. Hock (Berlin: Springer-Verlag), 125–150 (2001).
Fakhro, A. et al. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 20, 191–200 (2010).
Oelmüller, R. et al. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 49, 1–17 (2009).
Shahollari, B., Varma, A. & Oelmu, R. Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J. Plant Physiol. 162, (2005).
Das, A. et al. The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal. Behav 7, 1–10 (2012).
Verma, S. et al. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90, 896–903 (1998).
Yadav, V. et al. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J. Biol. Chem. 285, 26532–26544 (2010).
Jogawat, A., Vadassery, J., Verma, N. & Oelmüller, R. PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Nat. Publ. Gr. 6, 1–15 (2016).
Kumar, M., Yadav, V., Tuteja, N. & Johri, A. K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 155, 780–790 (2009).
Harrach, B. D., Baltruschat, H., Barna, B., Fodor, J. & Kogel, K. The Mutualistic Fungus Piriformospora indica Protects Barley Roots from a Loss of Antioxidant Capacity Caused by the Necrotrophic Pathogen. Fusarium culmorum. 26, 599–605 (2013).
Matsuo, M. et al. High redox responsive transcription factor1 levels result in accumulation of reactive oxygen species in arabidopsis thaliana shoots and roots. Mol. Plant. 8, 1253–1273 (2015).
Gosling, P., Hodge, A., Goodlass, G. & Bending, G. D. Arbuscular mycorrhizal fungi and organic farming. Agri. Ecosys.& Environ. 113, 17–35 (2006).
Harrier, L. A. & Watson, C. A. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest. Manag. Sci. 157, 149–157 (2004).
Varma, A. et al. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 65, 2741–2744 (1999).
Sun, C. et al. The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC. Plant Biol. 14, 1–16 (2014).
Deshmukh, S. D. & Kogel, K. H. Piriformospora indica protects barley from root rot caused by Fusarium graminearum. J. Plant Dis. Protect. 114, 263–268 (2007).
Rabiey, M., Ullah, I. & Shaw, M. W. The endophytic fungus Piriformospora indica protects wheat from fusarium crown rot disease in simulated UK autumn conditions. Plant Pathaol. 64, 1029–1040 (2015).
Dean, J. D., Goodwin, P. H. & Hsiang, T. Induction of glutathione S -transferase genes of Nicotiana benthamiana following infection by Colletotrichum destructivum and C. orbiculare and involvement of one in resistance. J. Exp. Bot. 56, 1525–1533 (2005).
Gullner, G. & Kömives, T. The role of glutathione and glutathione-related enzymes in plant-pathogen interactions. Netherlands:Springer, 207–239 (2001).
Wu, C. et al. Activation of Host Defense Mechanisms by Elevated, in Transgenic Plants Production of H. Plant Physiol. 115, 427–435 (1997).
Serfling, A., Wirsel, S. G. R., Lind, V. & Deising, H. B. Performance of the Biocontrol Fungus Piriformospora indica on Wheat Under Greenhouse and Field Conditions. Phytopath 97, 523–531 (2007).
Baltruschat, H. et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 180, 501–510 (2008).
Rodriguez, R. J. et al. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2, 404–416 (2008).
Sherameti, I. et al. The Root-Colonizing Endophyte Pirifomospora indica Confers Drought Tolerance in Arabidopsis by Stimulating the Expression of DroughtStress – Related Genes in Leaves. Mol. Plant-Microbe Interact. 21, 799–807 (2008).
Sun, C. et al. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 167, 1009–1017 (2010).
Torres, M. A., Jones, J. D. G. & Dangl, J. L. Reactive Oxygen Species Signaling in Response to Pathogens 1. 141, 373–378 (2006).
Govrin, E. M. & Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea.Curr. Biol. 10, 751–757
Akbulut, M., Yücel, M. & Öktem, H. A. Analysis and optimization of DNA delivery into chickpea (Cicer arietinum L.) seedlings by Agrobacterium tumefacience. Afr. J. Biotechnol. 7, 1011–1017 (2008).
Hill, T. W. & Kafer, E. Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet. Newslett. 48, (2001).
Arnon, D. I. & Hoagland, D. R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. soil Sci. 50, 463–483 (1940).
Dickson, S. & Smith, S. E. Evaluation of vesicular-arbuscular mycorrhizal colonisation by staining. Mycorrhiza Manual 77-83 (1998).
Phillips, J. M. & Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161 (1970).
Mcgonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L. & Swan, J. A. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol. 115, 495–501 (1990).
Bousquet, J., Simon, L. & Lalonde, M. DNA amplification from vegetative and sexual tissues of trees using polymerase chain reaction. Can J Res. 20, 254–257 (1990).
Bradford, M. M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 72, 248–254 (1976).
Roth, E. F. & Gilbert, H. S. The pyrogallol assay for superoxide dismutase: absence of a glutathione artifact. Anal. Biochem. 137, 50–53 (1984).
Beers, R. F. & Sizer, I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952).
Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Nordhoff, A., Bucheler, U. S., Werner, D. & Schirmer, R. H. Folding of the four domains and dimerization are impaired by the Gly446–> Glu exchange in human glutathione reductase. Implications for the design of antiparasitic drugs. Biochem. 32, 4060–4066 (1993).
Acknowledgements
AKJ and MD are thankful to Jawaharlal Nehru University for providing DST-PURSE-II, UPOE-II and UGC-Resource NET-working grant. OPN is grateful to the Indian Council of Medical Research (ICMR), Government of India for their financial support. NV and DP are thankful to Jawaharlal Nehru University for providing research fellowship. We are thankful to Prof. Rupam Kapoor, University of Delhi for providing B. cinerea culture.
Author information
Authors and Affiliations
Contributions
Project was initiated and supervised by A.K.J., M.D. and R.O. O.P.N., N.V., D.P. and A.K.S. have performed the experiments. M.K., A.K.J. and M.D. have designed the experiments. Chemicals were provided by A.K.J., R.K. and M.D. M.S. is written by A.K.J., O.P.N., R.K. and M.D.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Narayan, O.P., Verma, N., Singh, A.K. et al. Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea . Sci Rep 7, 13553 (2017). https://doi.org/10.1038/s41598-017-12944-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12944-w
This article is cited by
-
Harnessing phytomicrobiome signals for phytopathogenic stress management
Journal of Biosciences (2022)
-
The mechanism of sesame resistance against Macrophomina phaseolina was revealed via a comparison of transcriptomes of resistant and susceptible sesame genotypes
BMC Plant Biology (2021)
-
Plant mineral transport systems and the potential for crop improvement
Planta (2021)
-
Chitin Derived Bionanoparticles Evoke Defense Responses in Chickpea: a Cost-effective Strategy for Sustainable Chickpea Production
BioNanoScience (2021)
-
Phomopsis liquidambaris inoculation induces resistance in peanut to leaf spot and root rot
BioControl (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.