Abstract
Hypericum is a large genus that includes more than 500 species of pharmacological, ecological and conservation value. Although latest advances in sequencing technologies were extremely exploited for generating and assembling genomes of many living organisms, annotated whole genome sequence data is not publicly available for any of the Hypericum species so far. Bioavailability of secondary metabolites varies for different tissues and the data derived from different cultures will be a valuable tool for comparative studies. Here, we report the single molecule real-time sequencing (SMRT) data sets of Hypericum perforatum L. plantlets and cell suspension cultures for the first time. Sequencing data from cell suspension cultures yielded more than 33,000 high-quality transcripts from 20 Gb of raw data, while more than 55,000 high-quality transcripts were obtained from 35 Gb of raw data from plantlets. This dataset is a valuable tool for comparative transcriptomic analysis and will help to understand the unknown biosynthetic pathways of high medicinal value in the Hypericum genus.
Similar content being viewed by others
Background & Summary
Hypericum perforatum L. is an herbaceous perennial plant native to Europe, Asia, and North Africa, which is used in traditional medicine worldwide since ancient times1,2. Extracts of H. perforatum are used in the treatment of various stages of depression3. H. perforatum contains unique secondary metabolites, namely hypericin and hyperforin4,5, which are the two most studied extensively for their pharmacological properties6,7. Although the medicinal application of H. perforatum in the pharmaceutical industry has already reached a multi-billion dollar market, the pathways leading to the biosynthesis of bioactive metabolites are not understood adequately8. On the other hand, this species is considered as a toxic and invasive weed in some parts of the world9,10.
Comparative transcriptome analyzes using next generation sequencing techniques were utilized widely to understand biosynthetic pathways and other aspects of plant biology. Several attempts have been made to sequence and analyze the transcriptome of H. perforatum de novo to predict the unigenes involved in the biosynthesis of secondary metabolites11,12. However, genes implicated in biosynthesis pathway of hypericin and hyperforin are not characterized so far. Although whole genome data of H. perforatum was reported recently13, a complete annotated genome is not yet available in public for any of the Hypericum species.
The advent of single-molecule real-time sequencing (SMRT) has the potential to decipher the complex transcriptomes of plants, animals and microbes14,15,16,17,18,19. The advantage of SMRT technology lies in the ultra-long sequences, which in principle correspond to the full length of transcript sequences subjected to sequencing. In recent days, SMRT sequencing has been widely used to enrich the reference genome in expression studies of plant species or cultivars without a complete reference genome20,21,22,23. SMRT sequencing is also widely used in studies of alternative splicing, sequence repeats, long noncoding RNA, fusion genes, and gene discovery14,20,24. Genes involved in flavonoid and terpenoid biosynthesis were identified using SMRT sequencing in Ginkgo biloba14. Using SMRT technology, the complete sequences of unique genes involved in the synthesis of triterpene saponins were identified in Panax ginseng25. Similarly, full-length sequences of genes representing the enzymes for the berberine biosynthesis pathway in Berberis koreana were identified using SMRT sequencing26.
In the present study, we constructed a reference library for H. perforatum of PacBio Iso-Seq long reads using the SMRT sequencing approach. By aligning Illumina-based RNAseq data from plantlets and cell suspension cultures, we validated the library by obtaining the expression levels of several genes involved in secondary metabolism as these cultures differ greatly in the accumulation of secondary metabolites27. The transcriptome reference library for H. perforatum generated in this study will be a valuable tool as a reference library for comparative transcriptomic analyzes in H. perforatum
Methods
Plant material
In the present study, the H. perforatum cultivar ‘Helos’ (Richters Seeds, Ontario, Canada) was used, which is tetraploid (DNA ploidy, 2n~4x~32 chromosomes) and its reproduction mode is facultative apomixis, as revealed by flow cytometric analysis28,29,30.
Surface sterilized seeds were aseptically germinated and grown seedlings were transferred to Murashige and Skoog’s (MS - Duchefa Biochemie, Haarlem, The Netherlands) liquid medium supplemented with 0.1 mg/L α-naphthaleneacetic acid (NAA - Sigma-Aldrich, USA) and 1 mg/L 6-benzyladenine (BA - Sigma-Aldrich, USA) to establish shoot cultures containing plantlets (Fig. 1A,B). H. perforatum cell suspension cultures were established as reported before1. Cell suspension cultures were maintained in Erlenmeyer flasks (250 ml) containing MS and 0.5 mg/L NAA on an Orbi shaker (Benchmark Scientific, USA) at 110 rpm in a growth chamber with a photoperiod of 16/8 (day/night), an irradiance of 80 µmol m−2 s−1, 70% relative humidity, and a temperature of 25 °C. To keep the cells in the growth phase, 10 ml of the grown culture was subcultured into 70 ml of fresh medium once in 7 days (Fig. 1C,D).
RNA extraction
The biomass of the cell suspension culture and plantlets was freshly harvested and ground into a fine powder in a sterile mortar and pestle with liquid nitrogen. Total RNA was isolated from 100 mg of biomass using the Sigma Spectrum RNA extraction kit (Sigma-Aldrich, USA). To remove any DNA contamination on column DNase treatment (Sigma-Aldrich, USA) was performed to purify the RNA according to the manufacturer’s instructions. RNA quantity was measured using the NanoDrop™ OneC, and RNA integrity was measured using the Agilent Bioanalyzer 2100. RNA samples with an RNA integrity number (RIN) greater than 7 were used for Illumina sequencing, and samples used for Iso-Seq library preparation had an RIN value of at least 8.
High-throughput sequencing
Iso-Seq library preparation and sequencing were performed using the full-length PacBio cDNA libraries and sequencing kit according to the manufacturer’s protocol (Pacific Biosciences of California, Inc., Menlo Park, CA, USA) by the sequencing service provider (Novogene, Beijing, China) on the Sequel system. For RNA sequencing, the mRNA was randomly fragmented and the cDNA was synthesized using random hexamers primers. The second strand was synthesized using custom second-strand synthesis buffer (Illumina), dNTPs, RNase H, and DNA polymerase I. The double-stranded cDNA was enriched by PCR after size selection, and Illumina sequencing was performed in 150 bp paired-read mode (Novogene, Beijing, China).
Construction of a reference library
Processing of raw sequencing data was performed by a standard Iso-seq protocol in SMRT® Link v. 9.0 adhering to the guidelines provided by the manufacturer (https://www.pacb.com/wp-content/uploads/SMRT_Tools_Reference_Guide_v90.pdf), consisting of generating consensus sequences from raw data, removing primers, removing noise, clustering, polishing, and filtering high-quality transcripts. In processing, the parameter “minPasses 1” was set, and only sequences with both 5′ and 3′ adapters and a Poly(A) tail were selected to obtain the set of full-length transcripts. Annotation of transcripts was done in OmicsBox ver. 2.0.10 (https://www.biobam.com/omicsbox) by blast against nr protein database restricted to the data from taxon Magnoliopsida (e-value cut-off 1e-10) and other tools from the functional analysis suite in that software, e.g., Gene Ontology terms and KEGG pathway assignment. Completeness of the transcriptome was assessed in Busco v. 3.0 using lineage data for Eudicotyledons, with default parameters, at www.cyverse.org31.
Data analysis
To validate the library, transcripts expression was assessed based on mRNA Illumina sequencing of 4 samples from cell suspension cultures and 3 samples from plantlets. Quantifiication was done using Salmon v. 0.12.032 in mapping mode with the set of PacBio-based transcripts as reference sequences. Repeat sequences in transcripts were identified using RepeatMasker ver. 4.0.9 (http://www.repeatmasker.org) with RepeatMasker-RepBase33 Sequence Database (species maize, file RMRBSeqs.embl rel. 20181026, www.girinst.org), at public server usegalaxy.org34. Prediction of coding/noncoding sequences was done using the lncFinder ver. 1.1.4 package in R (ver 4.1.0) (using the “wheat” model) and PLEK35 (with parameter minlength = 1). Alternative splicing (AS) was investigated by gapped mapping of Illumina reads in sets of PacBio transcripts using TopHat ver. 2.1.1 (parameters: number of mismatches 1,–no-discordant), and analysis of coordinates of discovered exon junctions (corresponding to introns retained in PacBio transcripts); only AS events supported by data from all biological replications and by coverage of more than 50 Illumina reads in each replication were selected. Statistical characteristics and tests (χ2 test for comparison of frequencies, Mann-Whitney test for comparison of distributions) were computed in Genstat ver. 1936. Statistical graphs were made using GraphPad Prism software version 9 (GraphPad, La Jolla, CA, USA).
Quantitative real-time PCR
To further validate the library, specific fragments of genes putatively involved in hypericin biosynthesis such as polyketide synthase1 (PKS1), polyketide synthase 2 (PKS2), isochorismate synthase 2 (ICS2), chorismate synthase (CHOSYN), polyketide cyclase (PKC), berberine bridge enzymes (BBE7, BBE13, BBE15 and BBE17)5,9 were analyzed with the SYBR Green-based Q-RT-PCR assay using a Lightcycler 480 real-time PCR system (Roche, Switzerland). The assay was performed as in a previous study37. Briefly, each 10 μL Q- RT-PCR reaction consisted of 4.2 μL diluted cDNA template (0.1 μg), 5 μL SensiFAST SYBR No- ROX (Bioline, UK), 0.4 μL forward primer (10 μM), and 0.4 μL reverse primer (10 μM). Amplification was performed under the following conditions: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, and extension at 72 °C for 25 s. Relative expression was calculated using the 2−ΔΔCt method38.
Data Records
Sequence data were deposited into the functional genomics data collection (ArrayExpress) of European Bioinformatics Institute and are available with accession numbers E-MTAB-1142339 and E-MTAB-1132540. Annotation data, protein representation data and Q-RT-PCR assay data were deposited into figshare and made available to public41.
Technical Validation
Transcript characteristics
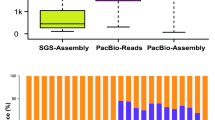
Long cDNA sequence reads of RNA isolated from cell suspension cultures and plantlets of H. perforatum were obtained using SMRT sequencing technology39,42. The length of the transcripts varied from 72 bp to 9041 bp in the cell suspension culture and 268 bp to 7986 bp in the plantlets (Table 1). In terms of a mutual blast (identity >95%), 83.19% of the transcripts from the cells and 68.21% of the transcripts from the plantlets matched. Thus, 5585 (16.81%) and 17606 (31.79%) of the transcripts were specific to cells and plantlets, respectively.
Transcript annotation
From the cell suspension culture and plantlet PacBio data, 97.22 and 98.17% of the transcripts were matched to the nonredundant (nr) protein database, respectively (Table S1). Of all transcripts, 89.37% of transcripts from cell suspension culture and 90.9% of transcripts from plantlets were annotated with known proteins (Table 2). A total of 78297 unigenes from both data sets were annotated with 17254 known proteins41,43.
Proteins were represented by 1 to 320 transcripts. Of all proteins, 3536 (20.49%) were found only in cells and 7465 (43.27%) were found only in plantlets, whereas 6253 (36.24%) proteins were observed in both data sets. The transcripts were annotated with protein sequences from 24 plant species, including Hevea brasiliensis (12,244 annotations), Jatropha curcas (10,494 annotations), Manihot esculenta (8422 annotations), Ricinus communis (7733 annotations), H. perforatum (1736 annotations), etc. (Table 3).
In total, the transcripts were assigned to 6103 GO terms and 1320 enzymes (Table S2). The annotations mainly related to the data sets are shown in Table S3 and Table S4. The BUSCO tool identified 48.7% and 65.2% of the genes in single copy (for the eudicotyledons) in the transcriptomes of the cell suspension cultures and plantlets, respectively (Table S2).
Repeat Sequences in H. perforatum Transcript
The frequency of repeat sequences in transcripts was determined. Sequence data from cell suspension cultures contained 2.3 Mb of repeat sequences in 14619 transcripts. Similarly, plantlet data had 2.1 Mb of repeat sequences in 25729 transcripts (Table 4).
A total of 15833 trinucleotide sequence repeats (SSRs) and 11848 dinucleotide SSRs were identified. TCT, TCC, GAA, CTT, CTC, CCT, and AAG were the most frequently observed trinucleotide SSRs, and four simple repeats, namely TC, GA, CT, and AG, represented 92% dinucleotide SSRs (Fig. 2).
Among the identified transposon elements, long interspersed nuclear elements (LINE) and long terminal repeat (LTR) retrotransposons were predominantly represented, with frequencies higher in cell sequences (Table 5). LTR/copia and LTR/gypsy were the highly represented LTR in H. perforatum sequence data. Additionally, other TEs, including hAT, Mutator-like elements (MULE), P instability factor (PIF), and helitron, also were abundant in the data.
Coding prediction
The selection of coding/non-coding RNAs was done by two tools: lncFinder and PLEK. Their predictions agreed for 77.08% of transcripts (70.06% as “coding”, 7.02 as “non-coding”) (Table 6).
Functional Annotation of transcripts
Functional classification of transcripts was carried out using Gene Ontology (GO) terms analysis. In total, 27516 transcripts from cells and 46902 transcripts from plantlets were assigned to GO terms (Fig. 3)41,42. Most dominant subclasses in biological process were metabolic process and cellular process.
In addition, Kegg’s pathway mapping was performed to identify genes involved in secondary metabolism41,42. Using the enzyme codes, 30900 transcripts from plantlets and 18953 transcripts from cells linked to 150 pathways were predicted after manual curation out of which, 6147 transcripts from plantlets and 3964 transcripts from cells were associated with biosynthesis of secondary metabolites (phenylpropanoid, anthocyanin, flavonoid, isoflavonoid, alkaloid, polyketide, stilbenoid, terpenoid, brassinosteroid etc) pathways (Fig. 4).
Identification of Alternative Splicing Isoforms
In the current study, 494 alternative splicing events in 376 clusters (transcripts) were identified based on gapped mapping of Illumina reads in transcripts. In this, 130 clusters were in cell suspension culture sequence data, and 245 were in sequence data of plantlets. Insertions (intron retentions) of the size of 100–200 bp were predominantly observed (Fig. 5A). Most of the transcripts with alternative splicing events had one isoform, while a maximum of 7 isoforms was observed in one transcript (Fig. 5B).
Expression data
Illumina sequencing of 7 samples yielded data sets with 45.0–52.4 million and 42.7–48.3 million read pairs for cells and plantlets, respectively. Expression levels (mean across all biological replicates) were calculated and transcripts were classified as “expressed” if the mean number of mapped reads was greater than 10 (Table S5).
A similar expression analysis was performed at the level of groups of transcripts assigned to the same protein, called “protein groups” (Table S5). The expression level for each protein group (in samples representing cells and plantlets) was determined as mean expression over all transcripts in the group. Protein groups were classified as “expressed” if the mean expression was greater than 10
To confirm the quality of the expression data, 9 genes were validated for their differential expression via quantitative real-time PCR (Q-RT-PCR) analysis44 in plantlets and cells. (Fig. 6).
Code availability
All software used in this paper have been described in the Methods section with the respective version number. For this study, no custom code was generated.
References
Selvakesavan, R. K. & Franklin, G. Robust in vitro culture tools suitable for sustainable bioprospecting of the genus Hypericum. Ind Crops Prod. 170, 113715 (2021).
Milutinović, M., Miladinović, M., Gašić, U., Dimitrijević-Branković, S. & Rajilić-Stojanović, M. Recovery of bioactive molecules from Hypericum perforatum L. dust using microwave-assisted extraction. Biomass Convers Biorefin, https://doi.org/10.1007/s13399-022-02717-5 (2022).
Wurglics, M. & Schubert-Zsilavecz, M. Hypericum Perforatum: A ‘Modern’ Herbal Antidepressant. Clin Pharmacokinet. 45, 449–468 (2006).
Zeliou, K. et al. Metabolomic fingerprinting and genetic discrimination of four Hypericum taxa from Greece. Phytochemistry 174, 112290 (2020).
Pradeep, M. & Franklin, G. Understanding the hypericin biosynthesis via reversible inhibition of dark gland development in Hypericum perforatum L. Ind Crops Prod. 182, 114876 (2022).
Jafarirad, S., Kosari-Nasab, M., Mohammadpour Tavana, R., Mahjouri, S. & Ebadollahi, R. Impacts of manganese bio-based nanocomposites on phytochemical classification, growth and physiological responses of Hypericum perforatum L. shoot cultures. Ecotoxicol Environ Saf. 209, 111841 (2021).
Guedes, A. P., Amorim, L. R., Vicente, A. M. S., Ramos, G. & Fernandes-Ferreira, M. Essential Oils from Plants and in Vitro Shoots of Hypericum androsaemum L. J Agric Food Chem. 51, 1399–1404 (2003).
Rizzo, P., Altschmied, L., Ravindran, B. M., Rutten, T. & D’Auria, J. C. The Biochemical and Genetic Basis for the Biosynthesis of Bioactive Compounds in Hypericum perforatum L., One of the Largest Medicinal Crops in Europe. Genes 11, 1210 (2020).
Rey, J. M. & Walter, G. Hypericum perforatum (St John’s wort) in depression: pest or blessing? Med J Aust. 169, 583–586 (1998).
Buckley, Y. M., Briese, D. T. & Rees, M. Demography and management of the invasive plant species Hypericum perforatum. II. Construction and use of an individual-based model to predict population dynamics and the effects of management strategies. J Appl Ecol. 40, 494–507 (2003).
He, M., Wang, Y., Hua, W., Zhang, Y. & Wang, Z. De Novo Sequencing of Hypericum perforatum Transcriptome to Identify Potential Genes Involved in the Biosynthesis of Active Metabolites. PLoS One 7, e42081 (2012).
Su, H. et al. Physiological and Transcriptomic Analysis Provide Insight into Low Temperature Enhancing Hypericin Biosynthesis in Hypericum perforatum. Molecules 26, 2294 (2021).
Zhou, W. et al. Whole-genome sequence data of Hypericum perforatum and functional characterization of melatonin biosynthesis by N-acetylserotonin O-methyltransferase. J Pineal Res. 70, e12709 (2021).
Sun, S. et al. Full-length sequencing of ginkgo transcriptomes for an in-depth understanding of flavonoid and terpenoid trilactone biosynthesis. Gene 758, 144961 (2020).
Gao, S. et al. The growth and photosynthetic responses of white LEDs with supplemental blue light in green onion (Allium fistulosum L.) unveiled by Illumina and single-molecule real-time (SMRT) RNA-sequencing. Environ Exp Bot. 197, 104835 (2022).
Gomes-dos-Santos, A. et al. PacBio Hi-Fi genome assembly of the Iberian dolphin freshwater mussel Unio delphinus Spengler, 1793. Sci Data 10, 340 (2023).
Xu, F. et al. A chromosome-scale reference genome for Spironucleus salmonicida. Sci Data 9, 585 (2022).
Ma, F. et al. Gap-free genome assembly of anadromous Coilia nasus. Sci Data 10, 360 (2023).
Zhang, Y. et al. Chromosome-level genome assembly and annotation of the prickly nightshade Solanum rostratum Dunal. Sci Data 10, 341 (2023).
Schaarschmidt, S. et al. Utilizing PacBio Iso-Seq for Novel Transcript and Gene Discovery of Abiotic Stress Responses in Oryza sativa L. Int J Mol Sci. 21, 8148 (2020).
Hou, C., Deng, N. & Su, Y. PacBio Long-Read Sequencing Reveals the Transcriptomic Complexity and Aux/IAA Gene Evolution in Gnetum (Gnetales). Forests 10, 1043 (2019).
Sen, S. et al. De novo transcriptome assembly from the nodal root growth zone of hydrated and water-deficit stressed maize inbred line FR697. Sci Rep. 13, 1960 (2023).
Luo, W. et al. A chromosome-level reference genome of the wax gourd (Benincasa hispida). Sci Data 10, 78 (2023).
Zhao, Z., Elsik, C. G., Hibbard, B. E. & Shelby, K. S. Detection of alternative splicing in western corn rootworm (Diabrotica virgifera virgifera LeConte) in association with eCry3.1Ab resistance using RNA-seq and PacBio Iso-Seq. Insect Mol Biol. 30, 436–445 (2021).
Jo, I.-H. et al. Isoform Sequencing Provides a More Comprehensive View of the Panax ginseng Transcriptome. Genes (Basel) 8, 228 (2017).
Roy, N. S. et al. Gene Expression and Isoform Identification of PacBio Full-Length cDNA Sequences for Berberine Biosynthesis in Berberis koreana. Plants 10, 1314 (2021).
Pasqua, G., Avato, P., Monacelli, B., Santamaria, A. R. & Argentieri, M. P. Metabolites in cell suspension cultures, calli, and in vitro regenerated organs of Hypericum perforatum cv. Topas. Plant Sci. 165, 977–982 (2003).
Matzk, F., Meister, A., Brutovská, R. & Schubert, I. Reconstruction of reproductive diversity in Hypericum perforatum L. opens novel strategies to manage apomixis. Plant J. 26, 275–282 (2001).
Bruňáková, K. et al. Phytochemical profiling of several Hypericum species identified using genetic markers. Phytochem. 187, 112742 (2021).
Selvakesavan, R. K., Nuc, M., Kolarčik, V., Krajewski, P. & Franklin, G. Flow cytometric determination of ploidy level and reproduction modes in Hypericum perforatum cv. Helos. Figshare, https://doi.org/10.6084/m9.figshare.24782763 (2023).
Merchant, N. et al. The iPlant Collaborative: Cyberinfrastructure for Enabling Data to Discovery for the Life Sciences. PLoS Biol. 14, e1002342 (2016).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14, 417–419 (2017).
Bao, W., Kojima, K. K. & Kohany, O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mobile. DNA 6, 11 (2015).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 44, W3–W10 (2016).
Li, A., Zhang, J. & Zhou, Z. PLEK: a tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 15, 311 (2014).
VSN International. Genstat for Windows 19th Edition. VSN International, Hemel Hempstead, UK. Genstat.co.uk (2017).
Selvakesavan, R. K. & Franklin, G. Nanoparticles affect the expression stability of housekeeping genes in plant cells. Nanotechnol Sci Appl. 13, 77–88 (2020).
Kubista, M. et al. The real-time polymerase chain reaction. Mol Aspects Med. 27, 95–125 (2006).
ArrayExpress https://identifiers.org/arrayexpress:E-MTAB-11423 (2022).
ArrayExpress https://identifiers.org/arrayexpress:E-MTAB-11325 (2022).
Selvakesavan, R. K., Nuc, M., Krajewski, P. & Franklin, G. Single molecule real-time sequencing data sets of Hypericum perforatum L. cell suspension and shoot cultures. Figshare https://doi.org/10.6084/m9.figshare.c.6744552.v1 (2023).
Selvakesavan, R. K., Nuc, M., Krajewski, P. & Franklin, G. Hypericum perforatum transcripts obtained using PacBio Iso-seq protocol, with annotation and characteristics. Figshare https://doi.org/10.6084/m9.figshare.23691846 (2023).
Selvakesavan, R. K., Nuc, M., Krajewski, P. & Franklin, G. Proteins represented by Hypericum perforatum transcripts obtained using PacBio Iso-seq protocol, with characteristics. Figshare https://doi.org/10.6084/m9.figshare.23691840 (2023).
Selvakesavan, R. K., Nuc, M., Krajewski, P. & Franklin, G. Quantitative Real-time PCR analysis of the Hypericum perforatum plantlets compared to cell suspension culture. Figshare https://doi.org/10.6084/m9.figshare.23691843 (2023).
Acknowledgements
This work was supported by the National Science Centre (NCN), OPUS projects Reg. No 2016/23/b/NZ9/02677 and 2017/25/B/NZ9/00720. M.N. is recipient of PhD fellowship from the project 2016/23/b/NZ9/02677 and R.K.S. is recipient of postdoctoral fellowship from the project 2017/25/B/NZ9/00720. Part of the computations were done at Poznań Supercomputing and Networking Center (www.psnc.pl).
Author information
Authors and Affiliations
Contributions
R.K.S. performed the isolation and purification of RNA. M.N. and P.K. performed the sequence analysis. V.K. performed the flow cytometry analysis to determine the ploidy level and reproduction mode of H. perforatum genotype used in this study. G.F. conceived the idea of this work and obtained funding for the research. R.K.S., P.K., V.K. and G.F. were involved in drafting the manuscript and all authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selvakesavan, R.K., Nuc, M., Kolarčik, V. et al. Single molecule real-time sequencing data sets of Hypericum perforatum L. plantlets and cell suspension cultures. Sci Data 11, 42 (2024). https://doi.org/10.1038/s41597-023-02878-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02878-6