Abstract
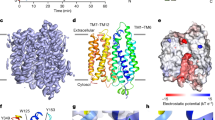
The Ca2+-activated TRPM5 channel plays essential roles in taste perception and insulin secretion. However, the mechanism by which Ca2+ regulates TRPM5 activity remains elusive. We report cryo-EM structures of the zebrafish TRPM5 in an apo closed state, a Ca2+-bound open state, and an antagonist-bound inhibited state. We define two novel ligand binding sites: a Ca2+ site (CaICD) in the intracellular domain and an antagonist site in the transmembrane domain (TMD). The CaICD site is unique to TRPM5 and has two roles: modulating the voltage dependence and promoting Ca2+ binding to the CaTMD site, which is conserved throughout TRPM channels. Conformational changes initialized from both Ca2+ sites cooperatively open the ion-conducting pore. The antagonist NDNA wedges into the space between the S1–S4 domain and pore domain, stabilizing the transmembrane domain in an apo-like closed state. Our results lay the foundation for understanding the voltage-dependent TRPM channels and developing new therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The cryo-EM density map and coordinates and atomic models have been deposited in the EMDB (Electron Microscopy Data Bank) and the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCS-PDB), respectively, under accession numbers EMD-23740 and PDB 7MBP (apo–TRPM5), EMD-23741 and PDB 7MBQ (Ca2+–TRPM5), EMD-23742 (apo–TRPM5(nanodisc)), EMD-23743 (Ca2+–TRPM5(nanodisc)), EMD-23744 and PDB 7MBR (apo–TRPM5(6 μM Ca2+)), EMD-23745 and PDB 7MBS (Ca2+–TRPM5(6 μM Ca2+)), EMD-23746 and PDB 7MBT (apo–TRPM5(E337A) single subunit and tetramer), EMD-23747 and PDB 7MBU (Ca2+–TRPM5(E337A) single subunit and tetramer) and EMD-23748 and PDB 7MBV ((Ca2+,NDNA)–TRPM5). Source data are provided with this paper.
Change history
20 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41594-021-00641-2
References
Pérez, C. A. et al. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 5, 1169–1176 (2002).
Zhang, Y. et al. Coding of sweet, bitter and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003).
Liu, D. & Liman, E. R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl Acad. Sci. USA (2003).
Prawitt, D. et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc. Natl Acad. Sci. USA 100, 15166–15171 (2003).
Roper, S. D. Signal transduction and information processing in mammalian taste buds. Pflug. Arch. 454, 759–776 (2007).
Taruno, A. et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495, 223–226 (2013).
Brixel, L. R. et al. TRPM5 regulates glucose-stimulated insulin secretion. Pflug. Arch. 460, 69–76 (2010).
Colsoul, B. et al. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc. Natl Acad. Sci. USA 107, 5208–5213 (2010).
Howitt, M. R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
Vennekens, R., Mesuere, M. & Philippaert, K. TRPM5 in the battle against diabetes and obesity. Acta Physiol. 222, e12949 (2018).
Nilius, B. & Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 12, 218 (2011).
Owsianik, G., Talavera, K., Voets, T. & Nilius, B. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 68, 685–717 (2006).
Hofmann, T. et al. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr. Biol. 100, 15166–15171 (2003).
Liman, E. R. in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades (eds Liedtke, W. B. & Heller, S.) Ch. 15 (CRC Press, 2007).
Ullrich, N. D. et al. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37, 267–278 (2005).
Nilius, B., Prenen, J., Janssens, A., Voets, T. & Droogmans, G. Decavanadate modulates gating of TRPM4 cation channels. J. Physiol. 560, 753–765 (2004).
Philippaert, K. et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 8, 14733 (2017).
Bryant, R. et al. Use of a TRPM5 inhibitor to regulate insulin and GLP-1 release. US patent 8,193,168 (2008).
Shigeto, M. et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Invest. 125, 4714–4728 (2015).
Guo, J. et al. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205–209 (2017).
Winkler, P. A., Huang, Y., Sun, W., Du, J. & Lü, W. Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552, 200–204 (2017).
Autzen, H. E. et al. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359, 228–232 (2018).
Duan, J. et al. Structure of full-length human TRPM4. Proc. Natl Acad. Sci. USA 115, 2377–2382 (2018).
Diver, M. M., Cheng, Y. & Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 365, 1434–1440 (2019).
Yin, Y. et al. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363, eaav9334 (2019).
Zhang, Z., Zhao, Z., Margolskee, R. & Liman, E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 27, 5777–5786 (2007).
Mähler, J. & Persson, I. A study of the hydration of the alkali metal ions in aqueous solution. Inorg. Chem. 51, 425–438 (2012).
Huffer, K. E., Aleksandrova, A. A., Jara-Oseguera, A., Forrest, L. R. & Swartz, K. J. Global alignment and assessment of TRP channel transmembrane domain structures to explore functional mechanisms. Elife 9, e58660 (2020).
Nilius, B. et al. The selectivity filter of the cation channel TRPM4. J. Biol. Chem. 280, 22899–22906 (2005).
Tang, L. et al. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505, 56–61 (2014).
Gao, Y., Cao, E., Julius, D. & Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351 (2016).
Liu, C. et al. A non-covalent ligand reveals biased agonism of the TRPA1 ion channel. Neuron 109, 273–284 (2021).
Nadezhdin, K. D. et al. Extracellular cap domain is an essential component of the TRPV1 gating mechanism. Nat. Commun. 12, 2154 (2021).
Yamaguchi, S., Tanimoto, A., Iwasa, S. & Otsuguro, K.-I. TRPM4 and TRPM5 channels share crucial amino acid residues for Ca2+ sensitivity but not significance of PI(4,5)P2. Int. J. Mol. Sci. 20, 2012 (2019).
Zhang, Z., Tóth, B., Szollosi, A., Chen, J. & Csanády, L. Structure of a TRPM2 channel in complex with Ca2+ explains unique gating regulation. Elife 7, e36409 (2018).
Hughes, T. E. T. et al. Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nat. Struct. Mol. Biol. 25, 53–60 (2018).
Nilius, B. et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 25, 467–478 (2006).
Tabur, S. et al. Role of the transient receptor potential (TRP) channel gene expressions and TRP melastatin (TRPM) channel gene polymorphisms in obesity-related metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 19, 1388–1397 (2015).
Valente, P. et al. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J. 22, 3298–3309 (2008).
Gregorio-Teruel, L., Valente, P., González-Ros, J. M., Fernández-Ballester, G. & Ferrer-Montiel, A. Mutation of I696 and W697 in the TRP box of vanilloid receptor subtype I modulates allosteric channel activation. J. Gen. Physiol. 143, 361–375 (2014).
Teng, J., Loukin, S. H., Anishkin, A. & Kung, C. L596–W733 bond between the start of the S4–S5 linker and the TRP box stabilizes the closed state of TRPV4 channel. Proc. Natl Acad. Sci. USA 112, 3386–3391 (2015).
Huang, Y., Winkler, P. A., Sun, W., Lü, W. & Du, J. Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149 (2018).
Huang, Y., Roth, B., Lu, W. & Du, J. Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel. Elife 8, e50175 (2019).
Wang, L. et al. Structures and gating mechanism of human TRPM2. Science 362, eaav4809 (2018).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Bai, X., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. W. Sampling the conformational space of the catalytic subunit of human γ-secretase. Elife 4, e11182 (2015).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
PyMOL Molecular Graphics System v2.3 (Schrödinger, 2015).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 74, 814–840 (2018).
Acknowledgements
We thank G. Zhao and X. Meng for support with data collection at the David Van Andel Advanced Cryo-Electron Microscopy Suite. We appreciate the high-performance computing team of VAI for computational support. We thank D. Nadziejka and M. Martin for technical editing. W.L. is supported by National Institutes of Health (NIH) grants (R56HL144929, R01HL153219 and R01NS112363). J.D. is supported by a McKnight Scholar Award, a Klingenstein–Simon Scholar Award, a Sloan Research Fellowship in neuroscience, a Pew Scholar in the Biomedical Sciences award and an NIH grant (R01NS111031). Z.R. is supported by an American Heart Association postdoctoral fellowship (20POST35120556).
Author information
Authors and Affiliations
Contributions
W.L. and J.D. supervised the project. E.H., Z.R. and R.R. generated TRPM5 mutants. Z.R. and E.H. carried out the purification, cryo-EM data collection and processing. I.J.O. performed electrophysiological experiments. Z.R., J.D. and W.L. analyzed the structures. M.S. and R.M. synthesized the NDNA. Z.R., E.H., I.J.O., M.S., R.M., J.D. and W.L. contributed to manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Patch-clamp analysis of CaICD mutants.
Representative traces of inside-out voltage-clamp measurements (+200 mV to –200 mV) from tsA201 cells overexpressing human TRPM4 (hsTRPM4), human TRPM5 (hsTRPM5), and zebrafish TRPM5 (drTRPM5) channels. Patches were stimulated with 1, 30, 100, or 1000 μM Ca2+. The number of patches analyzed: a, hsTRPM4(WT): 1 µM Ca2+ [n = 4], 100 µM [n = 4], 1000 µM [n = 4] from 3 transfections; b, drTRPM5(E337A): 1 µM Ca2+ [n = 6], 30 µM [n = 6], 100 µM [n = 5], 1000 µM [n = 5] from 8 transfections; c, drTRPM5(C324A): 1 µM Ca2+ [n = 6], 30 µM [n = 6], 100 µM [n = 6], 1000 µM [n = 6] from 4 transfections; d, drTRPM5(D333A): 1 µM Ca2+ [n = 5], 30 µM [n = 4], 100 µM [n = 5], 1000 µM [n = 3] from 3 transfections; e, drTRPM5(E212A): 1 µM Ca2+ [n = 4], 30 µM [n = 3], 100 µM [n = 4], 1000 µM [n = 3] from 3 transfections; f, drTRPM5(D336A): 1 µM Ca2+ [n = 3], 30 µM [n = 3], 100 µM [n = 3], 1000 µM [n = 3] from 2 transfections; g, hsTRPM5(WT): 1 µM Ca2+ [n = 5], 30 µM [n = 5] from 3 transfections; h, hsTRPM5(E351A): 1 µM Ca2+ [n = 3], 30 µM [n = 3] from 1 transfection; and i, hsTRPM5(E560A): 1 µM Ca2+ [n = 5], 30 µM [n = 3], 100 µM [n = 3], 1000 µM [n = 3] from 5 transfections. j–r, Mean current (50 ms) of experiments were plotted versus indicated voltage. The +200 mV clamp was chosen for normalization. Horizontal bars represent SEM. The current-voltage relation plots of drTRPM5(E337A) are identical to those presented in Fig. 3e. s–u, Individual patch clamp measurements I–200mV / I+200mV, I+200mV, I–200mV, of experiments are shown as individual points, where bars represent mean values. v, w, Currents of drTRPM5 WT and CaICD mutants plotted as a function of Ca2+ concentration for voltage clamps of –200 mV and +200 mV. Symbols represent mean current and horizontal bar is SEM. Source data for j-r are available online.
Extended Data Fig. 2 Cyro-EM analysis of apo–TRPM5.
a, The representative 2D class average of apo–TRPM5. The experiment was repeated twice with similar result. b, The Fourier shell correlation (FSC) curves for the apo–TRPM5. The cryo-EM map FSC is shown in black and the model vs. map FSC is shown in red. The map resolution was determined by the gold-standard FSC at 0.143 criterion, whereas the model vs. map resolution was determined by a threshold of 0.5. c, The angular distribution of particles that gave rise to the apo–TRPM5 cryo-EM map reconstruction. d, A schematic domain organization of a single TRPM5 subunit. Secondary structures and important domains are labeled. e, The atomic model of a single TRPM5 subunit in cartoon representation. The domains are colored as in (d). The left and right panels are two different views of the same subunit rotated 180° along the central axis.
Extended Data Fig. 3 Cyro-EM analysis of TRPM5 in the presence of different concentrations of Ca2+, or in the presence of Ca2+ and NDNA.
a and d, The representative 2D class average the 5 mM Ca2+ dataset (a) and 6 μM Ca2+ dataset (d), respectively. Two independent replicates of the experiment were carried out with similar result. b and e, The Fourier shell correlation (FSC) curves for the 5 mM Ca2+ dataset (b) and 6 μM Ca2+ dataset (e). The cryo-EM map FSC is shown in black and the model vs. map FSC is shown in red. The map resolution is determined by the gold-standard FSC at 0.143 criterion, whereas the model vs. map resolution is determined by a threshold of 0.5. c and f, The angular distribution of particles that give rise to the cryo-EM map reconstruction for 5 mM Ca2+ dataset (c) and 6 μM Ca2+ dataset (f). g, The close-up view of the CaTMD and CaICD of the 6 μM Ca2+ dataset. From left to right, CaTMD of apo–TRPM5(6 μM Ca2+), CaTMD of Ca2+–TRPM5(6 μM Ca2+), CaICD of apo–TRPM5(6 μM Ca2+), and CaICD of Ca2+–TRPM5(6 μM Ca2+). The cryo-EM densities are shown in mesh representation. The expected Ca2+ density is indicated by a circle. h, The representative 2D class average the (Ca2+, NDNA)–TRPM5 dataset. The experiment was repeated twice with similar result. i, The Fourier shell correlation (FSC) curves for the (Ca2+, NDNA)–TRPM5. The cryo-EM map FSC is shown in black and the model vs. map FSC is shown in red. The map resolution was determined by the gold-standard FSC at 0.143 criterion, whereas the model vs. map resolution was determined by a threshold of 0.5. j, The angular distribution of particles that gave rise to the (Ca2+, NDNA)–TRPM5 cryo-EM map reconstruction.
Extended Data Fig. 4 Local resolution estimation of TRPM5 structures and representative densities.
a-d, The local resolution estimation for apo–TRPM5(GDN) (a), Ca2+–TRPM5(GDN) (b), Ca2+–TRPM5(E337A)(GDN) consensus (c), (Ca2+, NDNA)–TRPM5(GDN) (d). For each map, a side view, a top-down view of the TMD from the extracellular side, and a focused side view of the S6 and pore helix are shown. The color bar unit is in angstroms. e, Representative densities from Ca2+–TRPM5(GDN) map. For the GDN density, one maltose group of the molecule is not resolved in the cryo-EM density map.
Extended Data Fig. 5 The gate and the selectivity filter of TRPM5.
a, Cryo-EM densities of the CaICD site, contoured at 0.018. b, Cryo-EM densities of the CaTMD site, contoured at 0.022. c, Cryo-EM densities of the water molecule and residues in the selectivity filter, contoured at 0.023. Hydrogen bonds are shown as solid yellow lines. The ‘lower’ water molecule is surrounded by the sidechain of Q906 and the backbone oxygen atoms of F904 and G905, forming three hydrogen bonds. d, Cryo-EM densities of I966, which forms the channel gate, contoured at 0.03. e, The selectivity filter formed by two layers of ordered water molecules (blue spheres) and backbone oxygen atoms (pink spheres) of G905. f and g, The two hydration layers of the selectivity filter viewed from the extracellular side. Upper layer in (f) and lower layer in (g).
Extended Data Fig. 6 Comparison of TRPM5 with other TRPM channels.
a, A structural comparison between Ca2+–TRPM5 and TRPM4 (PDBID: 6BQV)22. A single subunit is in color and shown as a cartoon. The TRPM5 channel is more compact, but wider than, the TRPM4 channel. b, An overlap of the selectivity filter of Ca2+–TRPM5 (red) and TRPM4 (yellow). c, A comparison of the CaTMD site for the available TRPM members. From left to right, drTRPM5, hsTRPM4 (6BQV), drTRPM2 (6DRJ), nvTRPM2 (6CO7), hsTRPM2 (6PUS), and pmTRPM8 (6O77)22,24,35,42,43; Shown in parentheses are the PDBIDs. d, Comparison of the ‘square’ helices in TRPM2 (6PUO), TRPM4 (6BQR), TRPM5, TRPM7 (5ZX5), TRPM8 (6O6A); Shown in parentheses are the PDBIDs22,24,43,45. Only TRPM5 has a broken square helix. e, A sequence alignment of the square helix across different TRPM5 orthologs and TRPM family members. Red indicates that the α-helix is observed in structures. For TRPM1, TRPM3, and TRPM6, in which no structures are currently available, the helical annotation is based on the secondary structure prediction from PSIPRED server46.
Extended Data Fig. 7 Comparison of (Ca2+, NDNA)–TRPM5 with apo–TRPM5 and Ca2+–TRPM5.
a, the chemical structure of N’-(3,4-dimethoxybenzylidene)-2-(naphthalen-1-yl)acetohydrazide (NDNA). b, Two close-up views of the cryo-EM densities of NDNA molecule. The surrounding protein structural element is shown in cartoon representation. c, Comparison of the NDNA binding site between (Ca2+, NDNA)–TRPM5 and apo–TRPM5 structures. The W869 is flipped in the (Ca2+, NDNA)–TRPM5 structure (cyan) compared to that in apo–TRPM5 structure (blue). d, Overlay of (Ca2+, NDNA)–TRPM5 (cyan) with apo–TRPM5 (blue) and Ca2+–TRPM5 (red) structures view from the intracellular side. One subunit is shown in cartoon representation and the other three subunits are in surface representation. The ICD of (Ca2+, NDNA)–TRPM5 adopts an intermediate state compared to the apo–TRPM5 and Ca2+–TRPM5 structures. e, The superimposition of the S1-S4 domain between (Ca2+, NDNA)–TRPM5 (cyan) and apo–TRPM5 (blue). f, The superimposition of the S1-S4 domain between (Ca2+, NDNA)–TRPM5 (cyan) and Ca2+–TRPM5 (red). g, A close-up view of the CaTMD site in (Ca2+, NDNA)–TRPM5 structure. The Q771 moved away from CaTMD. h and i, An overlay of the pore domain between (Ca2+, NDNA)–TRPM5 (cyan) with apo–TRPM5 (blue) (h) and Ca2+–TRPM5 (red) (i) structures viewed from the extracellular side.
Extended Data Fig. 8 Patch-clamp electrophysiology experiments of CaTMD mutants of zebrafish TRPM5.
a, Representative whole-cell current traces of tsA overexpressing WT and CaTMD mutant TRPM5 channels. Clamps were imposed from +200 mV to –200 mV. The number of cells measured were tsA201 [n = 4], TRPM5(WT) [n = 5], TRPM5(E768A) [n = 5], TRPM5(Q771A) [n = 4], TRPM5(N794A) [n = 4], TRPM5(D797A) [n = 4], and TRPM5(E994A) [n = 4] from 2–3 transfections. b, Mean current amplitudes of experiments in (a) were measured at 50 ms and plotted as a function of clamp voltage. Horizontal bars represent SEM. c, Individual measurements at clamps of +200 mV (I+200mV) and –200 mV (I–200mV) of experiments in (a) are shown as individual points, with bars representing mean values. Source data for b are available online.
Extended Data Fig. 9 Patch-clamp electrophysiology experiments on the NDNA binding site mutants.
a–j, Current voltage relations of whole-cell measurements in tsA cells over-expressing WT and mutant TRPM5 channels. Symbols represents mean current and horizontal bars are SEM. k–n, Individual measurements (of experiments in a–j) for clamps of +100 mV and –100 mV. Each point represents an individual cell and bars represent mean current. Cells were first measured (clamps from –100 mV to +100 mV) in bath solution and then re-measured following bath perfusion of 10 µM NDNA. See the legend of Fig. 4 for the number of cells used. Source data for k-n are available online.
Extended Data Fig. 10 Ca2+–TRPM5(E337A) in GDN detergent.
a, The representative 2D class average of Ca2+–TRPM5(E337A). The experiment was repeated twice with similar result. b, The FSC curve for the consensus map of Ca2+–TRPM5(E337A). The map resolution was determined by the gold-standard FSC at 0.143 criterion. c, The angular distribution of particles that give rise to the consensus map of Ca2+–TRPM5(E337A). d, The FSC curve for apo–TRPM5(E337A) (left) and Ca2+–TRPM5(E337A) (right). For each panel, the cryo-EM map FSC curve is shown in black and the model vs. map FSC is shown in red. The map resolution was determined by the gold-standard FSC at 0.143 criterion, whereas the model vs. map resolution was determined by a threshold of 0.5.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Supplementary Data 1
Source data of current from individual cells for Supplementary Fig. 6.
Source data
Source Data Fig. 1
Current from individual cells.
Source Data Fig. 3
Current from individual cells.
Source Data Fig. 4
Current ratio from individual cells.
Source Data Extended Data Fig. 1
Current from individual cells.
Source Data Extended Data Fig. 8
Current from individual cells.
Source Data Extended Data Fig. 9
Current from individual cells.
Rights and permissions
About this article
Cite this article
Ruan, Z., Haley, E., Orozco, I.J. et al. Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition. Nat Struct Mol Biol 28, 604–613 (2021). https://doi.org/10.1038/s41594-021-00607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00607-4
This article is cited by
-
Thermal gradient ring for analysis of temperature-dependent behaviors involving TRP channels in mice
The Journal of Physiological Sciences (2024)
-
TRPM5 activation depends on a synergistic effect of calcium and PKC phosphorylation
Communications Biology (2024)
-
TRPM channels in health and disease
Nature Reviews Nephrology (2024)
-
Genetic lineage tracing identifies adaptive mechanisms of pancreatic islet β cells in various mouse models of diabetes with distinct age of initiation
Science China Life Sciences (2024)
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases
Signal Transduction and Targeted Therapy (2023)