Abstract
Across the brain sciences, institutions and individuals have begun to actively acknowledge and address the presence of racism, bias, and associated barriers to inclusivity within our community. However, even with these recent calls to action, limited attention has been directed to inequities in the research methods and analytic approaches we use. The very process of science, including how we recruit, the methodologies we utilize and the analyses we conduct, can have marked downstream effects on the equity and generalizability of scientific discoveries across the global population. Despite our best intentions, the use of field-standard approaches can inadvertently exclude participants from engaging in research and yield biased brain–behavior relationships. To address these pressing issues, we discuss actionable ways and important questions to move the fields of neuroscience and psychology forward in designing better studies to address the history of exclusionary practices in human brain mapping.
Similar content being viewed by others
Main
The fields of neuroscience and psychology have advanced our understanding of the mechanisms of cognition and behavior through the varied thoughts, ideas, and perspectives of our fellow scientists. However, there is also a long history of both explicit and unrecognized racism in neuroscience and psychology. As a result, despite a groundswell of support to further diversify the neurosciences through the inclusion of traditionally minoritized and marginalized populations of scientists1,2,3,4, there is much work yet to be done. Moreover, the recent societal awakening in response to the murders of Black individuals in our community (while also recognizing centuries of Black suffering and pain) has drawn attention to the impact of racism in society and the corresponding need for profound changes across academia. While this attention has been placed on recruitment and training through hiring, promotion, and retention, much less thought has been paid to the process of scientific discovery. Here, the brain sciences face a complex and multifaceted problem, in that the existing research is primarily collected in ‘Western’ countries and focused on majority populations from relatively homogeneous demographic and environmental circumstances5. A primary goal of neuroscience is to discover the fundamental rules that link the brain to behavior. However, the participants we study, how we advertise and recruit, the data collection methods we use, and the statistical analyses we conduct can have a marked impact on the reliability, equity, and generalizability of our scientific discoveries.
Historically, beliefs concerning one group’s racial and ethnic superiority have been masked beneath the guise of scientific rigor and objectivity6. For instance, the physiognomic theories, popular among many 18th- and 19th-century European scientists, pathologized deviations from the idealized facial features of Western Europeans, and the use of craniometry (skull measurements) wrongfully proposed causal explanations for race and sex differences as they relate to intelligence7. This scientifically couched racism was carried through the end of the 19th and beginning of the 20th century in the work of Francis Galton and others, who infamously advocated for the improvement of populations through eugenics and selective breeding8. Within the United States, this ideology was reflected in the forced sterilization of tens of thousands of Americans, largely working-class women of color, and Better Baby contests, which featured explicitly racist ‘biological’ explanations to judge infant health9. Here, non-white babies were deemed ‘defective and heritability inferior,’ and thus more susceptible to dysfunctional neurological development. This work has been echoed in more recent claims of genetic factors underpinning different levels of intellectual functioning and social mobility in Black and European Americans10. Although the generational trauma and pernicious effects of these theories are still evident in our society, such approaches are now generally viewed as repugnant to modern scientists. However, despite a field-wide rejection of explicitly racist theory and the associated misuse of scientific methods to justify racial discrimination, less attention has been paid to a related and systemic issue in the field. Here, we point out that discriminatory practices and beliefs exist in neuroscience and psychology, specifically in the unintended yet systematic methodological exclusion of minoritized groups as evidenced in our research methods and analytic approaches.
Recruitment
Biased sampling in neuroimaging research can fundamentally distort our understanding of brain–behavior relationships. In this Perspective, we highlight issues pertaining to data collection in ‘Western’ countries, with a particular focus on the United States of America and the United Kingdom as the associated datasets are widely used to make inferences in a global context. Additionally, we primarily focus on the exclusion and marginalization of Black populations (see Box 1 for a discussion of associated impacts across other communities). Despite broad acknowledgment of issues associated with the recruitment of demographically restricted populations, the students, staff, and families affiliated with prestigious research institutions in America and Europe continue to be overrepresented in human research5. This biased approach to data collection is evident across basic, applied and clinical research11. The US Food and Drug Administration, as one example, has recently released plans to increase clinical trial participation from underrepresented racial and ethnic populations12. Here, Black and Hispanic/Latino Americans make up just 5% and 1% of participants, respectively. Moreover, according to the 2018–2021 National Institutes of Health (NIH) Research, Condition and Diseases Categorization (RCDC) Inclusion Statistics Reports, which record the percentage representation of participants in human studies across America, white participants are still overrepresented in human research, as the median percentage across all NIH neuroscience studies was ~70% (Fig. 1)13. According to the 2021 US Census, 59.3% of the population is white and not Hispanic or Latino. Conversely, 18.9% of the population is Hispanic. This reflects a substantial bias in study recruitment and participation relative to the reported NIH demographics. This under-recruitment is evident across minoritized communities. As reported in the census, 13.6% of the population is Black or African American, 1.3% is American Indian and Alaska Native, 6.1% is Asian and 2.9% is multiracial. Critically, this demographic snapshot masks sweeping and ongoing societal changes. For instance, in the United States, since 2018, white non-Hispanic residents have made up less than half (49.9%) of the nation’s population under the age of 15. This is reflected broadly at the generational level, where the percentage of white non-Hispanic members of each generation has steadily declined, from baby boomers (71.6%) through Generation X (59.7%), Millennials (55.1%), and Generation Z (50.9%). Millennials and Generation Z provide the bulk of participants for most cognitive neuroscience research, as they reflect the individuals who are often accessible at academic institutions and in the surrounding communities.
Bar graphs reflect the inclusion of median percentage statistics by race and ethnicity. Data were acquired from the NIH RCDC Inclusion Statistics Reports from fiscal years 2018–2021 (ref. 13).
The demographic pattern noted above is evident across large-scale neuroimaging collection efforts. The UK Biobank, the largest neuroimaging database collection in the world, includes ~95% white participants14,15. Another large US-based neuroimaging consortium, the Human Connectome Project (HCP), comprises 76% white individuals16,17. However, even in instances where these samples are broadly representative of the demographics within a given country, such as the HCP and the UK Biobank18, sampling generalizability issues become amplified when utilizing datasets in a global context where white individuals represent just 12% of the world’s population19. The NIH is one of the largest scientific funders, but it is important to note that the associated initiatives are often and explicitly not available to fund research projects and scientists outside America. As a consequence, it is primarily research groups within wealthier countries that possess the necessary resources to conduct in vivo imaging work. One of the most critical issues in diverse recruitment is the overreliance on convenience-based recruitment practices; even demographically balanced recruitment within a catchment area can reflect sampling bias when contrasted with other regions. Moreover, these high-throughput studies provide the primary opportunity for well-powered genome-wide association studies in the context of brain imaging. Here, a key concern relates to population stratification, or the presence of undetected population structure whereby individuals may systematically differ in both genetic ancestry and cultural or environmental factors that can influence the phenotype under investigation. The most direct way to avoid biases associated with population stratification is through the use of genetically homogeneous populations. As such, ancestrally mixed and non-European populations are almost universally excluded from associated genetic analyses. This lack of diversity in existing, well-powered, imaging genetic datasets probably arose as a result of both scientific and logistical challenges, such as difficulty recruiting participants from minoritized populations as well as the unequal distribution of research funding across institutions and countries. Regardless of how these barriers may have emerged, the exclusion of minoritized groups from human genetic research has the potential to exacerbate existing healthcare disparities, as discoveries in genetics and/or neuroimaging are increasingly being used to improve illness treatment and prediction. Where possible, researchers should consider recruitment designs that more broadly generalize to national and/or global populations. Much of the research on cognitive and psychological processes and associated brain functions is derived from mostly white, college-educated, young adult participants. This limits the extent to which discoveries can be generalized beyond a very specific set of demographic and environmental circumstances5. Expanding the scope of our recruitment efforts is critical for uncovering shared, or unique, brain–behavior relationships across populations, understanding how biological processes may be modulated by environmental experiences to influence phenotypic expression, and fostering diversity and inclusion in the brain sciences.
To address the overrepresentation of white males in biomedical research, the NIH implemented a policy in 2015 urging American scientists to include sex as a biological variable and to diversify the human participant pool20. Although the recruitment of female participants was neglected in the past, biomedical research has made considerable progress since the NIH mandated the enrollment of women in human clinical trials in 1993. For example, in large-scale collection efforts such as the HCP and Adolescent Brain Cognitive Development (ABCD) Study, considerable effort has been put into balancing associated sex distributions. This change in recruitment priorities is observable across countries; for instance, there is a 52% female and 48% male sex distribution in participants with available anatomical and/or functional imaging data in the UK Biobank dataset21. Yet despite this progress, the study of sex or gender differences in neurobiology is far from settled. For example, most research in this area neglects the potential influence of the menstrual cycle on both brain and behavior, and sex-linked or gender-linked biases in self-report and task performance are often not considered. Unfortunately, sex-based analyses remain limited, as many research studies fail to quantify their samples by sex20.
Perhaps even more concerning, if we make a concerted effort to increase recruitment of participants from marginalized communities, there may still be a reluctance for Black, Indigenous and minoritized populations to participate in research. Broadly, this reluctance follows generations of unethical and at times gruesome treatment22,23,24,25. While some of the sources of medical and scientific mistrust in minoritized communities are common knowledge in academic circles, such as the Tuskegee experiment in which syphilis was left untreated in African American men, leading to nearly 100 deaths26,27, other historical events are less well known. From the involuntary surgeries of James Marion Sims28 to the intentional infection of thousands of uninformed and non-consenting Guatemalan people with sexually transmitted diseases29,30 and the cervical tissue samples of Henrietta Lacks, taken and distributed without her consent31, these previous actions by some members of the scientific community have left many individuals understandably wary of the entire medical and scientific establishment32. Critically, these abuses are often explicitly referenced when people from minoritized groups are asked to detail their reasons for their current and ongoing mistrust of the medical and academic research communities33. The presence of distrust of the scientific and medical establishment has been extensively studied and well documented, and continues to present a formidable barrier to research participation among marginalized populations32,34,35,36. In part, this also emerges from recent experiences of disrespect, devaluation, and discrimination toward minoritized communities, contributing to skepticism and distrust of academic and medical research25,37,38. These concerns, and their associated societal impacts, were particularly evident recently in hesitancy in COVID-19 vaccine adoption in minoritized groups39. Given this history, a complementary step must be the conscious acknowledgment of these past wrongdoings and the associated trauma as we take steps toward diversifying participant recruitment (Box 2).
Methodology
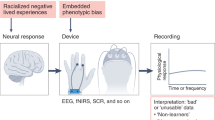
There has been a recent focus on methodological exclusion and phenotypic biases against dark skin tone and coarser hair in electroencephalography (EEG) or functional near-infrared spectroscopy (fNIRS) research40,41,42,43. Phenotypic biases against dark skin tone and curly hair are present in optical imaging, which requires adequate contact with the scalp and is influenced by melanin44. As a result, participants with darker skin pigmentation and coarser hair (for instance, a large subset of the Black and Hispanic/Latino populations) are often excluded from EEG and fNIRS studies40,45. Excitingly, Etienne and colleagues have recently designed methods for obtaining high-quality EEG readings from individuals with coarse and curly hair, to address this systemic collection bias in EEG studies41. However, these approaches have yet to be widely adopted, with nearly half of EEG researchers reporting having recorded data from fewer than five Black-identified or African American-identified participants45, and similar attention has been lacking in other imaging approaches, such as functional magnetic resonance imaging (fMRI).
Barriers to participation, poor data quality, and biased signals can affect the data acquired and conclusions drawn from MRI studies as well. We need to deepen our understanding of how cultural biases and racism are entangled with the methodology we use. Consistent with the issues noted above, something seemingly as innocuous as an individual’s hairstyle can present a considerable barrier to recruitment. In MRI studies, there can be difficulties in fitting a natural hairstyle (that is, afro-textured hair, dreadlocks and/or braids) into the constricted space of the head coil. In addition, sew-ins and hair extensions, common styles worn by Black women, can contain metal tracks that line the scalp or have metallic threads woven in as a decorative element46,47. These tracks can pose a risk in the magnetic field of the scanner environment and make it difficult for researchers to collect usable study data. Magnetic susceptibility artifacts can also emerge from products commonly used in Black hairstyles48,49. Unfortunately, while the systemic consequences of these data collection nuances are often overlooked by researchers and neglected as a part of operator training, these exclusionary barriers have considerable downstream consequences on the demographics of the datasets we collect.
Lastly, current MRI methodologies create signal bias. Functional MRI detects a blood-oxygen-level-dependent (BOLD) signal when imaging the brain. The BOLD signal is dependent on changes in deoxyhemoglobin levels, driven by changes in blood flow. In the United States for instance, risk of chronic medical issues varies by population. For example, Wang and colleagues have found differences in heart rate variability, a measure of cardiac health, between African American and white American youth50. Study results may be influenced by the presence of increased illness rates in Black Americans51, for instance respiratory illness, which can impact measures of both peripheral and cerebral vascular physiology52, as well as diabetes, where elevated blood sugar over time damages the blood vessels in the brain that carry oxygen-rich blood53,54,55. Additionally, the prevalence of hypertension in Black and Hispanic/Latino Americans is greater than in white non-Hispanic Americans56. Previous work also demonstrates that a proportion of BOLD signal time courses can result from neurovascular changes in blood pressure52,57. Considering that there are racial and ethnic differences in blood pressure profiles56,58, it can be postulated that there are variations in the BOLD signal related to race and ethnicity.
Biased signals can also emerge through our measurements of behavior, cognition, and peripheral physiology. Pulse oximeters and eye-trackers are often used in conjunction with neuroimaging technology, but they can result in poor data quality for a subset of participants. Pulse oximeters rely on measuring the scattering and absorption of light to assess blood oxygenation, by which the recoverable signal monotonically decreases as melanin increases44. Further, the use of acrylic nails, which are more popular among women, can also make it difficult for the light to penetrate and receive an accurate signal with pulse oximeters59. The societal-level structural components of racism can bleed into the study scales, tasks, and measures we collect. This is evident across a host of measures, such as participant responses to outgroup faces through familiarity with seemingly innocuous images, which can impact both immediate responses and potentially perpetuate associated biases in long-term memory60. Even income inequality, which is often stratified across societies by race, culture, and ethnicity, can bias participants’ responses to study manipulations61 as well as their expectations and valuation of their monetary compensation for participation62,63. Thus, current experimental designs and statistical models, focusing on a one-size-fits-all approach, may skew understanding of our increasingly diverse population64,65, while many research groups have not collected the necessary health and demographic data to account for these biasing factors. Critically, this can extend beyond factors associated with race and ethnicity, influencing observed results across individuals who might defy sample stereotypes65.
Analysis and beyond
Racial biases permeate our analyses as a direct result of the issues plaguing recruitment and data collection. Perceived race, on the part of participants, researchers and funding agencies, can be viewed in many ways as a socially constructed category encompassing geographic origins, ancestry, cultural norms and traditions, as well as other historical factors. Traditionally, neuroimaging analyses have largely sought to regress out the effects of race to simplify analyses and subsequent interpretations. However, when one regresses out these effects, it can remove crucial information capturing race-specific variability and, in some instances, introduce omitted variable bias, which could mask and/or spuriously generate effects of interest. As an alternative, analyses considering how race interacts with the study variables would have the potential to reveal any race-related differences between groups, providing additional information on how relationships may differ across populations and associated demographic and environmental features. Calls for sex-specific reporting of data and sex-aggregated analyses have been made to address sex bias and sex omission66. Similarly, race-specific reporting of data and race-aggregated analyses must also be implemented to address racial bias and racial omission.
In recent years, large-scale open-access neuroimaging datasets have served as a key resource to uncover neurobiological correlates of behavior. As mentioned above, these datasets are largely homogeneous, in part reflecting local demographics, and present inherent racial biases in the available data67. As a result, brain–behavior associations that are identified in these high-throughput datasets continue to be primarily driven by the dominant, or most represented, population, and findings are prone to interpretation bias, particularly when applied across demographic groups. Recent work from Li and colleagues evaluated racial bias in predictions of behavior based on functional connectivity and found that statistical models were able to more accurately predict behaviors in white participants than in Black participants, highlighting the need for caution in generalizing applicable findings16. Other work investigating biases across races and sexes has revealed unfairness in the extent to which models are accurately able to predict behaviors in one population relative to another (that is, in a particular race or sex). Therefore, brain–behavior associations captured by these models are representative of the specific populations they were trained on as opposed to the general relationships across the populations16,68. Even the use of field-standard covariates, such as the proportional intracranial volume correction for anatomical analyses, can differentially bias observed brain–behavior relationships across the sexes69. Given these findings, it is not unreasonable to expect that issues in recruitment and methodology will continue to impact our analyses and findings until they are addressed.
While accounting for race in our analyses is a start, it is not enough, and efforts must still be made to diversify our recruitment. Recent work from Coley and colleagues has revealed how suicide prediction models based on health records data (which tend to exhibit similar representation issues to neuroimaging datasets) are likely to perpetuate inequalities in access to healthcare and treatment70. The models accurately predicted suicide risk in white, Hispanic/Latino, and Asian patients, but not in Black and American Indian or Alaskan Native patients; the implementation of such models would benefit certain races but not others, amplifying existing healthcare disparities70. This study highlights the importance of including a diverse population in our analyses so our results are more generalizable across all individuals. For the sake of scientific reproducibility and generalizability, we must dismantle racial and ethnic barriers to participation and representation in research studies.
Moving forward
Despite the issues noted above, there have been positive movements toward actionable change. Organizations such as Black in Neuro, Black in Psych, LatinX in Psych, Queer in Neuro, Vanguard STEM, Black in the Ivory, Next-Gen Psych Scholars, and others have been actively working against the underrepresentation of minoritized people within academia and research practices71,72,73. Yet, although we have seen increased efforts to amplify the voices of marginalized groups, recruitment and retention bias remains in the scientific process.
Recent exploration and technological advancements in neuroimaging offer potential immediate solutions to the biases in research practices we address in this paper. The development of more inclusive EEG technology, as one example, supports diverse coarse and curly hairtypes41 and expands the recruitment opportunities in EEG studies. Still, the vast majority of large-scale population studies have excluded minoritized populations. There are some notable efforts underway to increase the diversification of large datasets in neuroimaging, for instance, the Chinese Color Nest Project generated a longitudinal neurodevelopmental curve composed of a culturally diverse Chinese cohort across the lifespan74.
Unfortunately, there are also examples of the downsides to this progress, including the overreliance on minority scientists to solve scientific and historical barriers. According to the National Science Foundation, of the 974 doctoral recipients in neuroscience or neurobiology in 2020, only 21 were Black or African American75. From this report, we can estimate that, assuming a 50.3% female/49.7% male sex balance that is reflective of the general Black population, approximately 11 Black or African American women graduated with neuroscience or neurobiology PhDs in 2020 (ref. 75). Importantly, intersectionalism interacts with structural exclusion to further marginalize individuals, eroding trust in the academic establishment. We cannot rely on the few Black neuroscientists to solve the entirety of exclusion problems in neuroimaging. Fair and balanced data collection efforts from the entire neuroimaging community can facilitate more representative brain mapping, allowing researchers and clinicians to better generalize their discoveries across populations, and/or tailor care to individuals throughout the population.
Given the issues noted above and with sensitivity and respect to researchers across many cultures, we call for the expansion of imaging technology to embrace diversity and inclusion of individuals beyond the overrepresented white, non-Hispanic/Latino population. Dismantling racially exclusionary practices and phenotypic bias in our research requires a coordinated effort from neuroscientists, psychologists, engineers, and academic administrators (Box 2). We have demonstrated how current methodological practices inadvertently exclude individuals who do not fit the phenotypic societal standard, contributing to the widespread presence of bias and racially exclusionary practices within neuroimaging. We insist that scientists actively combat the presence of structural inequalities within the neuroimaging community through improvements in recruitment, methodological, and analysis practices.
We also recognize that our call to action can provoke lingering questions about the impacts of exclusion in neuroscience and psychology research, to which the field does not yet have answers (Box 3). Nevertheless, all neuroscientists, psychologists, and biomedical engineers should work collectively to address these questions. Diversity and active anti-racism efforts will allow for the identification and dissemination of generalizable brain–behavior relationships across all populations. We hope that, by providing historical context and actionable items to organize resources and create executable solutions, we move forward as a field in addressing these systemic issues in human neuroscience and psychology research.
Change history
09 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41593-023-01516-z
References
Caicedo, H. H. Surfacing anti-Black science and building antiracist teams. Nat. Biotechnol. 38, 923–925 (2020).
Gilpin, N. W. & Taffe, M. A. Toward an anti-racist approach to biomedical and neuroscience research. J. Neurosci. 41, 8669–8672 (2021).
Wheaton, L. A. Racial equity and inclusion still lacking in neuroscience meetings. Nat. Neurosci. 24, 1645–1647 (2021).
De Los Reyes, A. & Uddin, L. Q. Revising evaluation metrics for graduate admissions and faculty advancement to dismantle privilege. Nat. Neurosci. 24, 755–758 (2021).
Henrich, J., Heine, S. J. & Norenzayan, A. Most people are not WEIRD. Nature 466, 29–29 (2010).
Halpin, Z. T. Scientific objectivity and the concept of ‘the other’. Women’s Stud. Intl Forum 12, 285–294 (1989).
Allchin, D. Pseudohistory and pseudoscience. Sci. Educ. 13, 179–195 (2004).
Challis, D. The Archaeology of Race: the Eugenic Ideas of Francis Galton and Flinders Petrie (A&C Black, 2013).
Selden, S. Transforming better babies into fitter families: archival resources and the history of the American eugenics movement, 1908–1930. Proc. Am. Philos. Soc. 149, 199–225 (2005).
Ma, C. & Schapira, M. An Analysis of Richard J. Herrnstein and Charles Murray’s: The Bell Curve: Intelligence and Class Structure in American Life (Macat Library, 2017).
Girolamo, T., Parker, T. C. & Eigsti, I. M. Incorporating dis/ability studies and critical race theory to combat systematic exclusion of Black, Indigenous, and People of Color in clinical neuroscience. Front Neurosci. 8, 988092 (2022).
US Food & Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry; availability. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations (2022).
US Department of Health and Human Services. NIH RCDC Inclusion Statistics Report (National Institutes of Health, 2018–2021).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Li, J. et al. Cross-ethnicity/race generalization failure of behavioral prediction from resting-state functional connectivity. Sci. Adv. 8, eabj1812 (2022).
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Henrich, J., Heine, S. J. & Norenzayan, A. The weirdest people in the world? Behav. Brain Sci. 33, 61–83 (2010).
Woitowich, N. C., Beery, A. & Woodruff, T. Meta-research: a 10-year follow-up study of sex inclusion in the biological sciences. Elife 9, e56344 (2020).
Littlejohns, T. J. et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat. Commun. 11, 2624 (2020).
George, S., Duran, N. & Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 104, e16–e31 (2014).
Habibi, A., Sarkissian, A. D., Gomez, M. & Ilari, B. Developmental brain research with participants from underprivileged communities: strategies for recruitment, participation and retention. Mind Brain Educ. 9, 179–186 (2015).
Hodge, F. S. No meaningful apology for American Indian unethical research abuses. Ethics Behav. 22, 431–444 (2012).
Scharff, D. P. et al. More than Tuskegee: understanding mistrust about research participation. J. Health Care Poor Underserved 21, 879–897 (2010).
Gamble, V. N. Under the shadow of Tuskegee: African Americans and healthcare. Am. J. Public Health 87, 1773–1778 (1997).
Brandt, A. M. Racism and research: the case of the Tuskegee Syphilis Study. Hastings Cent. Rep. 8, 21–29 (1978).
Khabele, D., Holcomb, K., Connors, N. K. & Bradley, L. A perspective on James Marion Sims, MD, and anti-black racism in obstetrics and gynecology. J. Minim. Invasive Gynecol. 28, 153–155 (2021).
Rodriguez, M. A. & García, R. First, do no harm: the US sexually transmitted disease experiments in Guatemala. Am. J. Public Health 103, 2122–2126 (2013).
Reverby, S. M. Ethical failures and history lessons: the US Public Health Service research studies in Tuskegee and Guatemala. Public Health Rev. 34, 189206 (2012).
Skloot, R. The Immortal Life of Henrietta Lacks (Broadway Paperbacks, 2017).
Corbie-Smith, G., Thomas, S. B. & George, D. M. M. S. Distrust, race, and research. Arch. Intern. Med. 162, 2458–2463 (2002).
Momplaisir, F. et al. Understanding drivers of coronavirus disease 2019 vaccine hesitancy among blacks. Clin. Infect. Dis. 73, 1784–1789 (2021).
Hussain‐Gambles, M., Atkin, K. & Leese, B. Why ethnic minority groups are under‐represented in clinical trials: a review of the literature. Health Soc. Care Community 12, 382–388 (2004).
Dula, A. African American suspicion of the healthcare system is justified: what do we do about it? Camb. Q. Healthc. Ethics 3, 347–357 (1994).
Brandon, D. T., Isaac, L. A. & LaVeist, T. A. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J. Natl Med. Assoc. 97, 951 (2005).
Jaiswal, J. & Halkitis, P. N. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav. Med. 45, 79–85 (2019).
Mehra, R. et al. Black pregnant women ‘Get the Most Judgment’: a qualitative study of the experiences of Black women at the intersection of race, gender, and pregnancy. Women’s Health Issues 30, 484–492 (2020).
Razai, M. S., Osama, T., McKechnie, D. G. & Majeed, A. COVID-19 vaccine hesitancy among ethnic minority groups. BMJ 372, n513 (2021).
Choy, T., Baker, E. & Stavropoulos, K. Systemic racism in EEG research: considerations and potential solutions. Affec. Sci. https://doi.org/10.1007/s42761-021-00050-0 (2021).
Etienne, A. et al. Novel electrodes for reliable EEG recordings on coarse and curly hair. 42nd Annual Intl Conf. of the IEEE Engineering in Medicine & Biology Society (EMBC) 6151–6154 (IEEE, 2020).
Webb, E. K., Etter, J. A. & Kwasa, J. A. Addressing racial and phenotypic bias in human neuroscience methods. Nat. Neurosci. 25, 410–414 (2022).
Parker, T. C. & Ricard, J. A. Structural racism in neuroimaging: perspectives and solutions. Lancet Psychiatry 9, e22 (2022).
Sjoding, M. W., Dickson, R. P., Iwashyna, T. J., Gay, S. E. & Valley, T. S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 383, 2477–2478 (2020).
Bradford, D. E. et al. Whose signals are we amplifying? Towards a more equitable clinical psychophysiology. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/c2naf (2022).
Thompson, C. Black women, beauty, and hair as a matter of being. Women’s Studies 38, 831–856 (2009).
Kapoor, R., Wang, J., Zavala, A. M., Truong, A. T. & Truong, D.-T. Metallic microbeads for hair extensions: hidden dangers for magnetic resonance imaging. Radiol. Case Rep. 17, 3274–3276 (2022).
Duncan, I. C. The ‘aura’ sign: an unusual cultural variant affecting MR imaging. AJR Am. J. Roentgenol. 177, 1487 (2001).
McKinstry, R. C. & Jarrett, D. Y. Magnetic susceptibility artifacts on MRI: a hairy situation. Am. J. Roentgenol. 182, 532 (2004).
Wang, X., Thayer, J. F., Treiber, F. & Snieder, H. Ethnic differences and heritability of heart rate variability in African and European American youth. Am. J. Cardiol. 96, 1166–1172 (2005).
Celedón, J. C. et al. Respiratory health equality in the United States. The American Thoracic Society perspective. Ann. Am. Thorac. Soc. 11, 473–479 (2014).
Whittaker, J. R., Driver, I. D., Venzi, M., Bright, M. G. & Murphy, K. Cerebral autoregulation evidenced by synchronized low frequency oscillations in blood pressure and resting-state fMRI. Front. Neurosci. 13, 433 (2019).
Van Harten, B., de Leeuw, F. -E., Weinstein, H. C., Scheltens, P. & Biessels, G. J. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 29, 2539–2548 (2006).
Kalaria, R. N. Diabetes, microvascular pathology and Alzheimer disease. Nat. Rev. Neurol. 5, 305–306 (2009).
Black, J. L. & Macinko, J. Neighborhoods and obesity. Nutr. Rev. 66, 2–20 (2008).
Bennett, A., Parto, P. & Krim, S. R. Hypertension and ethnicity. Curr. Opin. Cardiol. 31, 381–386 (2016).
Murphy, K., Birn, R. M. & Bandettini, P. A. Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–359 (2013).
Calvin, R. et al. Racism and cardiovascular disease in African Americans. Am. J. Med. Sci. 325, 315–331 (2003).
Hinkelbein, J., Koehler, H., Genzwuerker, H. V. & Fiedler, F. Artificial acrylic finger nails may alter pulse oximetry measurement. Resuscitation 74, 75–82 (2007).
Stolier, R. M. & Freeman, J. B. Neural pattern similarity reveals the inherent intersection of social categories. Nat. Neurosci. 19, 795–797 (2016).
Sturge-Apple, M. L. et al. Vagal tone and children’s delay of gratification: differential sensitivity in resource-poor and resource-rich environments. Psychol. Sci. 27, 885–893 (2016).
Miller, G. E., White, S. F., Chen, E. & Nusslock, R. Association of inflammatory activity with larger neural responses to threat and reward among children living in poverty. Am. J. Psychiatry 178, 313–320 (2021).
White, S. F., Nusslock, R. & Miller, G. E. Low socioeconomic status is associated with a greater neural response to both rewards and losses. J. Cogn. Neurosci. 34, 1939–1951 (2022).
Handwerker, D. A., Ollinger, J. M. & D’Esposito, M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage 21, 1639–1651 (2004).
Greene, A. S. et al. Brain–phenotype models fail for individuals who defy sample stereotypes. Nature 609, 109–118 (2022).
Will, T. R. et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 4, ENEURO.0278-17.2017 (2017).
Goldfarb, M. G. & Brown, D. R. Diversifying participation: the rarity of reporting racial demographics in neuroimaging research. NeuroImage 254, 119122 (2022).
Dhamala, E., Jamison, K. W., Jaywant, A. & Kuceyeski, A. Shared functional connections within and between cortical networks predict cognitive abilities in adult males and females. Hum. Brain Mapp. 43, 1087–1102 (2022).
Dhamala, E. et al. Proportional intracranial volume correction differentially biases behavioral predictions across neuroanatomical features and populations. NeuroImage 260, 119485 (2022).
Coley, R. Y., Johnson, E., Simon, G. E., Cruz, M. & Shortreed, S. M. Racial/ethnic disparities in the performance of prediction models for death by suicide after mental health visits. JAMA Psychiatry 78, 726–734 (2021).
Singleton, K. S., Tesfaye, R., Dominguez, E. N. & Dukes, A. J. An open letter to past, current and future mentors of Black neuroscientists. Nat. Rev. Neurosci. 22, 71–72 (2021).
Subbaraman, N. How #BlackInTheIvory put a spotlight on racism in academia. Nature 582, 327–328 (2020).
Murray, D.-S. et al. Black in neuro, beyond one week. J. Neurosci. 41, 2314–2317 (2021).
Liu, S. et al. Chinese Color Nest Project: an accelerated longitudinal brain-mind cohort. Dev. Cogn. Neurosci. 52, 101020 (2021).
National Science Foundation. Doctorate Recipients from US Universities: 2020. https://ncses.nsf.gov/pubs/nsf22300/report (2020).
Blignaut, P. & Wium, D. Eye-tracking data quality as affected by ethnicity and experimental design. Behav. Res. Methods 46, 67–80 (2014).
United States Census Bureau: American Community Survey, S1601 Language Spoken at Home. https://data.census.gov/cedsci/table?q=United%20States%20Spanish%20speaking&g=0100000US (2020).
Flores, A. 2015, Hispanic Population in the United States Statistical Portrait. Pew Research Center’s Hispanic Trends Project. https://www.pewresearch.org/hispanic/2017/09/18/2015-statistical-information-on-hispanics-in-united-states/ (2020).
Ramírez-Castañeda, V. Disadvantages in preparing and publishing scientific papers caused by the dominance of the English language in science: the case of Colombian researchers in biological sciences. PLoS ONE 15, e0238372 (2020).
Blasi, D. E., Henrich, J., Adamou, E., Kemmerer, D. & Majid, A. Over-reliance on English hinders cognitive science. Trends Cogn. Sci. 14, S1364–S6613 (2022).
Krueger, P. M. & Reither, E. N. Mind the gap: race/ethnic and socioeconomic disparities in obesity. Curr. Diab. Rep. 15, 95 (2015).
Ocloo, J. & Matthews, R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual. Saf. 25, 626–632 (2016).
Ginde, A. A., Foianini, A., Renner, D. M., Valley, M. & Camargo, C. A. Jr The challenge of CT and MRI imaging of obese individuals who present to the emergency department: a national survey. Obesity 16, 2549–2551 (2008).
Roberts, S. O., Bareket-Shavit, C., Dollins, F. A., Goldie, P. D. & Mortenson, E. Racial inequality in psychological research: Trends of the past and recommendations for the future. Perspect. Psychol. Sci. 15, 1295–1309 (2020).
DeJesus, J. M., Callanan, M. A., Solis, G. & Gelman, S. A. Generic language in scientific communication. Proc. Natl Acad. Sci. USA 116, 18370–18377 (2019).
Dotson, V. M. & Duarte, A. The importance of diversity in cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 181–191 (2020).
Conley, M. I. et al. The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Res. 270, 1059–1067 (2018).
Nichols, T. E. et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 20, 299–303 (2017).
Harnett, N. G. & Ressler, K. J. Structural racism as a proximal cause for race-related differences in psychiatric disorders. Am. J. Psychiatry 178 579–581 (2021).
Harrell, C. J. P. et al. Multiple pathways linking racism to health outcomes. Du Bois Rev. 8, 143–157 (2011).
Acknowledgements
This work was supported by the National Institute of Mental Health (R01MH120080 and R01MH123245 to A.J.H.) and the Kavli Institute for Neuroscience at Yale University (Postdoctoral Fellowship for Academic Diversity to E.D.). J.K. was supported by NIH K00NS115331 and the Burroughs Wellcome Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Deanna Barch, Karla Miller, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ricard, J.A., Parker, T.C., Dhamala, E. et al. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nat Neurosci 26, 4–11 (2023). https://doi.org/10.1038/s41593-022-01218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01218-y
This article is cited by
-
Remembering the null hypothesis when searching for brain sex differences
Biology of Sex Differences (2024)
-
A research agenda for understanding how social inequality is linked to brain structure and function
Nature Human Behaviour (2024)
-
Accessible computing platforms democratize neuroimaging data analysis
Nature Methods (2024)
-
Recommendations for the responsible use and communication of race and ethnicity in neuroimaging research
Nature Neuroscience (2024)
-
Let’s talk about diversity in human neuroscience
Nature Methods (2023)