Abstract
Probing non-equilibrium dynamics with single-molecule spectroscopy is important for dissecting biomolecular mechanisms. However, existing microfluidic rapid-mixing systems for this purpose are incompatible with surface-adhesive biomolecules, exhibit undesirable flow dispersion and are often demanding to fabricate. Here we introduce droplet-based microfluidic mixing for single-molecule spectroscopy to overcome these limitations in a wide range of applications. We demonstrate its robust functionality with binding kinetics of even very surface-adhesive proteins on the millisecond timescale.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Source data are provided with this paper.
Code availability
Fretica, a custom add-on package for Mathematica v.12.3 (Wolfram Research), was used for the analysis of single-molecule fluorescence data and is available at https://github.com/SchulerLab.
References
Lerner, E. et al. Toward dynamic structural biology: two decades of single-molecule Förster resonance energy transfer. Science 359, eaan1133 (2018).
Schuler, B. Single-molecule FRET of protein structure and dynamics—a primer. J. Nanobiotechnology 11, S2 (2013).
Capretto, L., Cheng, W., Hill, M. & Zhang, X. Micromixing within microfluidic devices. Top. Curr. Chem. 304, 27–68 (2011).
Knight, J. B., Vishwanath, A., Brody, J. P. & Austin, R. H. Hydrodynamic focusing on a silicon chip: mixing nanoliters in microseconds. Phys. Rev. Lett. 80, 3863–3866 (1998).
Lipman, E. A., Schuler, B., Bakajin, O. & Eaton, W. A. Single-molecule measurement of protein folding kinetics. Science 301, 1233–1235 (2003).
Pfeil, S. H., Wickersham, C. E., Hoffmann, A. & Lipman, E. A. A microfluidic mixing system for single-molecule measurements. Rev. Sci. Instrum. 80, 055105 (2009).
Gambin, Y. et al. Visualizing a one-way protein encounter complex by ultrafast single-molecule mixing. Nat. Methods 8, 239–241 (2011).
Wunderlich, B. et al. Microfluidic mixer designed for performing single-molecule kinetics with confocal detection on timescales from milliseconds to minutes. Nat. Protoc. 8, 1459–1474 (2013).
Zijlstra, N. et al. Rapid microfluidic dilution for single-molecule spectroscopy of low-affinity biomolecular complexes. Angew. Chem. Int. Ed. 56, 7126–7129 (2017).
Dingfelder, F. et al. Rapid microfluidic double-jump mixing device for single-molecule spectroscopy. J. Am. Chem. Soc. 139, 6062–6065 (2017).
Vogler, E. A. Protein adsorption in three dimensions. Biomaterials 33, 1201–1237 (2012).
Zhang, H. & Chiao, M. Anti-fouling coatings of poly(dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 35, 143–155 (2015).
Taylor, G. I. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. Lond. A 219, 186–203 (1953).
Wunderlich, B., Nettels, D. & Schuler, B. Taylor dispersion and the position-to-time conversion in microfluidic mixing devices. Lab Chip 14, 219–228 (2014).
Anna, S. L., Bontoux, N. & Stone, H. A. Formation of dispersions using ‘flow focusing’ in microchannels. Appl. Phys. Lett. 82, 364–366 (2003).
Song, H., Bringer, M. R., Tice, J. D., Gerdts, C. J. & Ismagilov, R. F. Experimental test of scaling of mixing by chaotic advection in droplets moving through microfluidic channels. Appl. Phys. Lett. 83, 4664–4666 (2003).
Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 12, 422–433 (2012).
Qin, D., Xia, Y. & Whitesides, G. M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Harinarayana, V. & Shin, Y. C. Two-photon lithography for three-dimensional fabrication in micro/nanoscale regime: a comprehensive review. Opt. Laser Technol. 142, 107180 (2021).
Dogan, J., Schmidt, T., Mu, X., Engström, Å. & Jemth, P. Fast association and slow transitions in the interaction between two intrinsically disordered protein domains. J. Biol. Chem. 287, 34316–34324 (2012).
Dyson, H. J. & Wright, P. E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 291, 6714–6722 (2016).
Altman, R. B. et al. Cyanine fluorophore derivatives with enhanced photostability. Nat. Methods 9, 68–71 (2011).
Dogan, J., Jonasson, J., Andersson, E. & Jemth, P. Binding rate constants reveal distinct features of disordered protein domains. Biochemistry 54, 4741–4750 (2015).
Borgia, A. et al. Extreme disorder in an ultrahigh-affinity protein complex. Nature 555, 61–66 (2018).
Sottini, A. et al. Polyelectrolyte interactions enable rapid association and dissociation in high-affinity disordered protein complexes. Nat. Commun. 11, 5736 (2020).
Charmet, J., Arosio, P. & Knowles, T. P. J. Microfluidics for protein biophysics. J. Mol. Biol. 430, 565–580 (2018).
Yang, T. et al. Droplet-based microfluidic temperature-jump platform for the rapid assessment of biomolecular kinetics. Anal. Chem. 94, 16675–16684 (2022).
Abate, A. R., Hung, T., Mary, P., Agresti, J. J. & Weitz, D. A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl Acad. Sci. USA 107, 19163–19166 (2010).
Hofmann, H. et al. Single-molecule spectroscopy of protein folding in a chaperonin cage. Proc. Natl Acad. Sci. USA 107, 11793–11798 (2010).
Dunlop, P. J. & Stokes, R. H. The diffusion coefficients of sodium and potassium iodides in aqueous solution at 25°1. J. Am. Chem. Soc. 73, 5456–5457 (1951).
Zosel, F., Soranno, A., Buholzer, K. J., Nettels, D. & Schuler, B. Depletion interactions modulate the binding between disordered proteins in crowded environments. Proc. Natl Acad. Sci. USA 117, 13480–13489 (2020).
Schilling, J., Schöppe, J. & Plückthun, A. From DARPins to LoopDARPins: novel LoopDARPin design allows the selection of low picomolar binders in a single round of ribosome display. J. Mol. Biol. 426, 691–721 (2014).
Cull, M. G. & Schatz, P. J. in Methods in Enzymology Vol. 326, 430–440 (Academic Press, 2000).
Soranno, A. et al. Single-molecule spectroscopy reveals polymer effects of disordered proteins in crowded environments. Proc. Natl Acad. Sci. USA 111, 4874–4879 (2014).
Müller-Späth, S. et al. Charge interactions can dominate the dimensions of intrinsically disordered proteins. Proc. Natl Acad. Sci. USA 107, 14609–14614 (2010).
Holmstrom, E. D. et al. in Methods in Enzymology Vol. 611 (ed. Rhoades, E.) 287–325 (Academic Press, 2018).
Hellenkamp, B. et al. Precision and accuracy of single-molecule FRET measurements—a multi-laboratory benchmark study. Nat. Methods 15, 669–676 (2018).
Hess, D. et al. Exploring mechanism of enzyme catalysis by on-chip transient kinetics coupled with global data analysis and molecular modeling. Chem 7, 1066–1079 (2021).
Acknowledgements
We thank the FIRST-CLA Cleanroom at ETH Zurich for their support in microfabrication and the Functional Genomics Center Zurich for performing mass spectrometry. This work was supported by the Swiss National Science Foundation (B.S., A.dM.), the Forschungskredit of the University of Zurich (T.Y.), the Novo Nordisk Foundation Challenge program REPIN (no. NNF18OC0033926, B.S.) and the Human Frontier Science Program (B.S.).
Author information
Authors and Affiliations
Contributions
T.Y. and B.S. conceived the study. T.Y. performed microfluidic system design, microfabrication and system characterization. T.Y. performed single-molecule experiments with input from K.B. and A.S. X.C. performed mask-free two-photon 3D printing. A.dM. provided microfabrication instrumentation. K.B. and A.S. performed protein sample preparation. D.N. developed single-molecule instrumentation and data analysis software. T.Y. and D.N. developed analysis tools for experimental data. T.Y. analyzed the experimental data, with input from K.B., A.S., B.S. and D.N. B.S. supervised the project. T.Y., D.N. and B.S. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
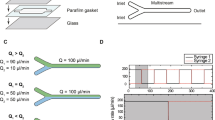
Extended Data Fig. 1 Designs of the droplet-based microfluidic mixers.
a) Layout of the standard-scale microfluidic mixer, scale bar 1 mm. Oil is used as the carrier phase for the droplets. Three inlets labeled as sample 1, buffer, and sample 2 are used to deliver the aqueous phase to the microfluidic mixer. Right after entering the mixer, all liquids pass microfabricated filters composed of small pillars to avoid dust particles or aggregates entering the channels, as shown in the zooms 1 and 2. The buffer solution sheathing sample 1 allows on-chip dilution and prevents premature mixing with sample 2. Droplets are formed using a flow-focusing geometry (zoom 3), where part of the oil is branched off just before droplet formation and recombined afterwards, which accelerates the droplets directly after formation38. A serpentine channel (zoom 3) follows, which ensures sub-millisecond mixing inside each droplet by chaotic advection. After mixing, most of the oil is drained off via two side channels (zoom 3) to slow down the droplets by two orders of magnitude and allow sufficiently long passage times through the confocal detection volume that enable single-molecule fluorescence detection in the observation channel. A groove at the top of the observation channel (zooms 3, 4) stabilizes the droplets in the channel center during signal detection to avoid droplet motion perpendicular to the flow direction, especially in the case of varying droplet sizes in different measurements. Markers (zoom 4) were introduced along the observation channel with known spacing to be able to determine the detection position for the position-to-time conversion. The height of the microchannels is 20 μm, the height of the groove is 10 μm, the width of the flow focusing channels is 14 µm, of the observation channel 55 μm, and of the serpentine channel 20 μm. Scale bars in zoom 1, 2, 3, 4: 100 μm. b) Layout of the small-scale microfluidic mixer, scale bar: 1 mm. The device follows the same working principle as the standard-scale microfluidic mixer in a), but with scaled-down dimensions of the height (12 μm), the width of the flow focusing channels (6 µm), the observation channel (20 μm), and the serpentine channel (12 μm) to achieve shorter dead times. Double filter arrays are introduced right after the inlets to ensure that no dust particles or aggregates block the small channels. There is no groove structure on top of the observation channel since the droplets fully occupy the channel in this device. More details of the designs can be found in the CAD files provided as supplementary material.
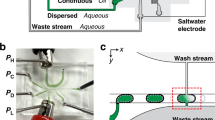
Extended Data Fig. 2 Fluid control system for the droplet-based microfluidic mixer.
Four precision pressure-based flow controllers connected to compressed air were used to provide accurate control of the fluids. Three of the flow controllers were each coupled to an XS flow sensor to accurately monitor the delivery of aqueous phase to sample inlet 1, buffer inlet, and sample inlet 2 with known flow rates for achieving the target concentrations. The three flow controllers delivering aqueous phase were connected via 5/32’’ OD tubing to a P-CAP sealed on 2-ml Eppendorf tubes containing double-distilled water as reservoirs. 1/32’’ OD PEEK tubing was used to connect the water reservoirs to the inlets and outlets of the flow sensors with filters inbetween preventing dust particles from clogging the XS flow sensors. Due to a high risk of clogging given the small internal channel diameter (20 µm) of the flow sensor by dust particles, salt precipitates or biomolecular aggregates, we avoided running sample and buffer solutions through the flow sensor and instead stored sample and buffer solutions in the 1/16’’ OD PTFE tubing between the flow sensors and the inlets of the microfluidic device. The fourth flow controller coupled to an M-size flow sensor was connected via 5/32’’ OD tubing to the air-tight P-CAP sealed on a 15-ml Falcon tube containing the oil phase delivered to the microfluidic chip. 1/32’’ OD PEEK tubing was used to connect the oil-phase reservoir to the inlet and outlet of the flow sensors. 1/16’’ OD PTFE tubing was used to connect the outlet of the flow sensor to the microfluidic oil inlet via an adaptor. The outlets of the microfluidic chip were connected to the waste reservoir via 1/16’’ OD PTFE tubing. Pressures and flow rates of the four inlets were controlled and recorded during measurements using the Fluigent A-i-O software.
Extended Data Fig. 3 Device stability during smFRET measurements.
a) and b) droplet velocities at various reaction times (corresponding to different positions along the observation channel) for measurements with ACTR/NCBD and ProTα/H1, respectively. Signal acquisition at each point was for 10 min, corresponding to between 21,000 and 26000 droplets. Mean values and standard deviations are shown. The total recording time was about three hours. c) Droplet frequency at various reaction times from the same data set as in b). The mean and standard deviation are shown as generated from 600 seconds of measurement at each point. The stable droplet velocities and droplet generation frequencies in a), b), and c) demonstrate the long-term stability of the microfluidic mixing device during measurements lasting for hours. d) Droplet frequency over time at one representative reaction time (16.2 ms; see arrow in panel c) showing the droplet frequency every 20 seconds.
Extended Data Fig. 4 Quantifying the mixing time via diffusion-limited quenching of fluorescein with potassium iodide (KI).
a) Schematic of the microfluidic channels with the solutions applied to the inlets indicated. b) Brightfield images taken from a movie recorded at 3000 frames/s for measuring the droplet velocity, U, in the mixing channel, yielding U= 40.3 cm/s (droplet manually colored for clarity). Scale bar: 40 µm. c) Fluorescence image taken with a 3-second exposure, averaged over 10 consecutive frames showing the decrease of the intensity along the channel due to quenching. At least 1200 droplets were sampled. d) The fluorescence intensities at different positions along the mixing channel were normalized between 0 and 1 and converted to the time after the droplets enter the mixing channel. We performed the measurements at a fixed droplet size (90 µm in the mixing channel) but different velocities from 19.3 to 62.2 cm/s (see legend) and fitted the mixing profiles with Eq. (2). e) The mixing times (90% of intensity change) were obtained from the fits and plotted as a function of (w/U)×log(Pe)16, where w is the width of the channel, U is the velocity of the droplet, and Pe=w U/D is the Péclet number, which indicates the ratio of the rate of advection to the rate of diffusion of the fluid. The linear fit of the plot is used as a calibration curve to determine the mixing time based on the velocities of the droplets and the diffusion coefficients of the components in the single-molecule FRET measurements.
Extended Data Fig. 5 Microfluidic device fabrication workflow.
In the lithography process for master fabrication, SU8-2015 photoresist was deposited on a silicon wafer by spin coating at 2200 rpm to achieve a film thickness of 20 µm. After soft baking (not shown), the wafer was exposed to UV light (energy density: 140 mJ/cm2 at 365 nm) with the main channel mask aligned above. Then the wafer was placed on a hot plate for 3 minutes at 95 °C for the post-exposure bake (not shown). Another layer of SU8-2007 was spin-coated on the previous layer at 1500 rpm to achieve a thickness of 10 µm, and was soft-baked for 1 minute at 65 °C, followed by 2 minutes at 95 °C (not shown). Utilizing the mask aligner, the groove channel photomask was aligned with the exposed structure on the wafer, and another UV exposure (energy density: 120 mJ/cm2 at 365 nm) was used to create the groove channel. After post-exposure baking for 3 minutes at 95 °C (not shown), non-crosslinked photoresist was removed by gently shaking the wafer in SU8 developer. Prior to replica molding, the masters were silanized with TMCS (trimethylchlorosilane) in a desiccator to prevent PDMS adhesion. Microfluidic devices were manufactured using replica molding. A 10:1 wt/wt mixture of PDMS base and curing agent was poured onto the master structure, degassed, and cured in the oven at 70 °C for 4 h. The cured PDMS structure was peeled off the wafer, cut, and inlet and outlet ports were introduced. A 23 mm×30 mm glass cover slide was first cleaned and then spin coated with a thin layer of PDMS (spin coating at 4000 rpm, ~20 μm thickness) as the bottom layer. The structured PDMS device was bonded to the bottom layer after plasma activation. To prevent wetting of the microchannel surfaces by the water phase, and ensure stable and uniformly sized droplets, microfluidic channels were filled with a 5% (by volume) PFOCTS isopropanol solution for 3 min at room temperature right after plasma bonding, then the solution was removed with pressured air, and the device was then placed on a hot plate at 120 °C for two hours.
Extended Data Fig. 6 Fluorescence intensity images of static droplets in the microfluidic channel to monitor surface adhesion.
a) With too low a PEG-PFPE2 concentration in the oil phase of 0.25%, surface passivation of the water/oil interface was insufficient, and Cy3B/LD650-labeled ACTR adsorbed to the interface (excitation at 532 nm, detection at 550–610 nm for donor, >647 nm for aceptor). b) With the PEG-PFPE2 concentration increased to 1%, Cy3B/LD650-labeled ACTR did not accumulate at the interfaces (indicated by white dased lines), but was homogeneously distributed inside the droplets. c) Under the same conditions, also no adsorption of Alexa488/594-labeled Protα-H1 to the water/oil interface was observed (excitation at 488 nm, detection at 500–550 nm for donor, 600–700 nm for acceptor emission). d) The oil phase containing 1% PEG-PFPE2 showed increased fluorescence intensity compared to the droplets containing buffer without sample (excitation at 488 nm). Scale bars: 20 µm. e) Measurement performed in droplets containing buffer but no labeled protein (excitation at 488 nm, detection at 500–550 nm for donor, 600–700 nm for acceptor emission, measured for 15 min, corresponding to 37,185 droplets). The small number of bursts illustrates the low background contribution from PDMS and buffer. Images a), b), c), d), and plot e) are representative experiments from at least 3 independent repeats with similar results.
Extended Data Fig. 7 Time trace of signals from donor and acceptor channels of droplets containing 50 pM Alexa488/594-labeled ProTα-H1 complex formed in oil containing 1% (w/w) PEG-PFPE2.
The fluorescence intensity in the oil phase on average exhibits a photon rate of ~150 to 200 ms−1, which is higher than in the droplet phase containing Alexa488/594-labeled ProTα-H1 complex (488 nm excitation). The two phases can be identified directly from the donor and acceptor time traces as indicated.
Extended Data Fig. 8 Series of transfer efficiency histograms of donor- and acceptor-labeled ACTR measured at different times after mixing with unlabeled NCBD.
Donor- and acceptor-labeled ACTR (50 pM) was mixed with an excess of unlabeled NCBD (50 nM). a) Colored circles indicate the positions along the observation channel at which the measurements were performed. b) Histograms measured after mixing, corresponding to the data set shown in Fig. 2c, analyzed with a global fit of all histograms. The low transfer efficiency peak (〈E〉 ≈0.51) corresponds to unbound ACTR, and the high transfer efficiency peak (〈E〉 ≈ 0.74) to ACTR bound to NCBD. Over time, the fraction of bound ACTR increases and reaches equilibrium. The peak close to 〈E〉 = 0 originates from molecules without active acceptor dye. Each transfer efficiency histogram was built up from at least 3000 bursts. c) Representative histograms of the number of photons per burst and the burst duration from the measurement at 30 ms in b). The mean number of photons per burst is 79, and the mean burst duration is 0.19 ms, as indicated by the vertical lines. The total number of bursts is ~7,300, corresponding to ~0.3 bursts per droplet.
Extended Data Fig. 9 Series of transfer efficiency histograms of donor- and acceptor-labeled ProTα measured at different times after mixing with unlabeled H1.
Donor- and acceptor-labeled ProTα (50 pM) was mixed with an excess of unlabeled H1 (7 nM). a) Colored circles indicate the positions along the observation channel at which the measurements were performed. b) Histograms measured after mixing, corresponding to the data set shown in Fig. 2h, analyzed with a global fit of all histograms. The low transfer efficiency peak (〈E〉 ≈0.33) corresponds to unbound ProTα, and the high transfer efficiency peak (〈E〉 ≈ 0.51) to ProTα bound to H1. Over time, the fraction of bound ProTα increases and reaches equilibrium. The peak close to 〈E〉 = 0 originates from molecules without active acceptor dye. Each transfer efficiency histogram was built up from at least 3000 bursts. The 〈E〉 values are within ± 0.03 of the values obtained from equilibrium measurements25. The broadening of the peaks compared to equilibrium measurements is due to the shorter residence time of the molecules as they flow through the confocal volume and the correspondingly lower thresholds for the number of photons used for burst identification. c) Representative histograms of the number of photons per burst and the burst duration from the measurement at 81 ms in b). The mean number of photons per burst is 102, and the mean burst duration is 0.25 ms, as indicated by the vertical lines. The total number of bursts is ~11,200, corresponding to ~0.9 bursts per droplet.
Extended Data Fig. 10 Small-scale microfluidic mixer for reactions with observed rates above ~150 s−1.
a) Snapshots of a manually colored droplet in the small-scale microfluidic mixer indicating the changes of droplet velocity in different regions of the device. Scale bar: 100 µm. The width of the microfluidic channels is 12 µm in the mixing channel, 20 µm in the observation channel, and the channel height is 10 µm. The flow focusing produces smaller droplets than in the standard-scale device that are slightly squeezed as an ellipse with a major axis of ~22 µm. Under the flow conditions used (oil at 5.8 µL/min, ProTα, buffer, and H1 at 10 nL/min), the dead time before a droplet enters the observation channel is reduced to 1.7 ± 0.3 ms. The length of the observation channel is 8.7 mm, corresponding to an observation time window of 1.4 s. The representative snapshots are based on at least 10 independent repeats with similar results. b) Comparison of kinetics measured with the standard-scale microfluidic mixing device (triangles) and the small-scale mixing device (circles). ProTα/H1 association kinetics with high concentrations of H1 were measured on both devices. The concentration of ProTα was 50 pM, the concentration of H1 20 nM (blue) and 30 nM (orange) at 200 mM ionic strength. Note the shorter times accessible with the small-scale device. The agreement between the measurements in both devices supports the robustness of the results.
Supplementary information
Supplementary Video 1
Recording of the standard-scale droplet-based microfluidic device in operation, showing droplet formation and acceleration, passage through the serpentine-shaped mixing channel, deceleration and motion along the observation channel in the standard-scale microfluidic mixer (see Fig. 1 for annotation of the device features). The droplet takes ~8 ms from its formation to entering the observation channel. The width of the observation channel is 55 μm, and the width of the serpentine mixing channel 20 μm. The groove on top of the observation channel stabilizes the droplets passing through. The video was recorded at 3,000 frames s–1 and runs at 40 frames s–1.
Supplementary Video 2
Recording of the small-scale droplet-based microfluidic device in operation, showing droplet formation and acceleration, passage through the serpentine-shaped mixing channel, deceleration and motion along the observation channel in the standard-scale microfluidic mixer (see Fig. 1 for annotation of the device features). The droplet takes ~2 ms from its formation to entering the observation channel, resulting in a shorter dead time than in the standard-scale device (Supplementary Video 1). The width of the observation channel is 20 μm, the width of the serpentine mixing channel 12 μm. The video was recorded at 3,000 frames s–1 and runs at 40 frames s–1.
Supplementary Data 1
CAD design of the standard-scale and small-scale microfluidic mixers.
Source data
Source Data Fig. 1
Source data of Fig. 1f,h–k.
Source Data Fig. 2
Source data of Fig. 2d,h.
Source Data Extended Data Fig. 3
Source data of Extended Data Fig. 3a–d.
Source Data Extended Data Fig. 4
Source data of Extended Data Fig. 4d,e.
Source Data Extended Data Fig. 6
Source data of Extended Data Fig. 6e.
Source Data Extended Data Fig. 7
Source data of Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source data of Extended Data Fig. 8b,c.
Source Data Extended Data Fig. 9
Source data of Extended Data Fig. 9b,c.
Source Data Extended Data Fig. 10
Source data of Extended Data Fig. 10b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, T., Buholzer, K.J., Sottini, A. et al. Rapid droplet-based mixing for single-molecule spectroscopy. Nat Methods 20, 1479–1482 (2023). https://doi.org/10.1038/s41592-023-01995-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01995-9