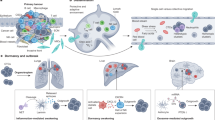
Abstract
Cervical squamous cell carcinoma (CSCC) exhibits a limited response to immune-checkpoint blockade. Here we conducted a multiomic analysis encompassing single-cell RNA sequencing, spatial transcriptomics and spatial proteomics, combined with genetic and pharmacological perturbations to systematically develop a high-resolution and spatially resolved map of intratumoral expression heterogeneity in CSCC. Three tumor states (epithelial-cytokeratin, epithelial-immune (Epi-Imm) and epithelial senescence), recapitulating different stages of squamous differentiation, showed distinct tumor immune microenvironments. Bidirectional interactions between epithelial-cytokeratin malignant cells and immunosuppressive cancer-associated fibroblasts form an immune exclusionary microenvironment through transforming growth factor β pathway signaling mediated by FABP5. In Epi-Imm tumors, malignant cells interact with natural killer and T cells through interferon signaling. Preliminary analysis of samples from a cervical cancer clinical trial (NCT04516616) demonstrated neoadjuvant chemotherapy induces a state transition to Epi-Imm, which correlates with pathological complete remission following treatment with immune-checkpoint blockade. These findings deepen the understanding of cellular state diversity in CSCC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Raw sequencing data generated in this study are deposited in Genome Sequence Archive for Human (https://ngdc.cncb.ac.cn/gsa-human/) with accession number HRA004971. Processed sequencing data are available at Science Data Bank (https://doi.org/10.57760/sciencedb.11624). Existing RNA sequencing datasets of NSCLC (OAK and POPLAR) and TCGA CESC cohorts are downloaded from European Genome-phenome Archive (EGA; EGAS00001005013) and UCSC Xena (https://xenabrowser.net/datapages/?cohort=GDC%20TCGA%20Cervical%20Cancer%20(CESC)). Reference genome files for alignment of single-cell data can be downloaded from https://support.10xgenomics.com/single-cell-gene-expression/software/release-notes/build. Human protein sequences from Swiss-Prot for searching of MS/MS data can be downloaded from https://ftp.uniprot.org/pub/databases/uniprot/previous_releases/release-2020_06/knowledgebase/uniprot_sprot-only2020_06.tar.gz. Source data are provided with this paper.
Code availability
Code required to reproduce the analyses in this paper is available through GitHub (https://github.com/FlyPythons/CSCC_heterogeneity).
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Pfaendler, K. S. & Tewari, K. S. Changing paradigms in the systemic treatment of advanced cervical cancer. Am. J. Obstet. Gynecol. 214, 22–30 (2016).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Frenel, J.-S. et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J. Clin. Oncol. 35, 4035–4041 (2017).
Hornburg, M. et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 39, 928–944 (2021).
Yuan, S., Norgard, R. J. & Stanger, B. Z. Cellular plasticity in cancer. Cancer Discov. 9, 837–851 (2019).
Barkley, D. et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 54, 1192–1201 (2022).
The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 543, 378–384 (2017).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624 (2017).
Bedard, M. C. et al. Single cell transcriptomic analysis of HPV16-infected epithelium identifies a keratinocyte subpopulation implicated in cancer. Nat. Commun. 14, 1975 (2023).
Puram, S. V. et al. Cellular states are coupled to genomic and viral heterogeneity in HPV-related oropharyngeal carcinoma. Nat. Genet. 55, 640–650 (2023).
Kinker, G. S. et al. Pan-cancer single-cell RNA-seq identifies recurring programs of cellular heterogeneity. Nat. Genet. 52, 1208–1218 (2020).
Gruosso, T. et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Invest. 129, 1785–1800 (2019).
Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 185, 1777–1792 (2022).
Vahid, M. R. et al. High-resolution alignment of single-cell and spatial transcriptomes with CytoSPACE. Nat. Biotechnol. (2023). https://doi.org/10.1038/s41587-023-01697-9
Wang, H. et al. Tumor immunological phenotype signature-based high-throughput screening for the discovery of combination immunotherapy compounds. Sci. Adv. 7, eabd7851 (2021).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Schubert, M. et al. Perturbation-response genes reveal signaling footprints in cancer gene expression. Nat. Commun. 9, 20 (2018).
Derynck, R., Turley, S. J. & Akhurst, R. J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 18, 9–34 (2021).
Song, J. et al. The role of FABP5 in radiation-induced human skin fibrosis. Radiat. Res. 189, 177–186 (2017).
Krishnamurty, A. T. et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 611, 148–154 (2022).
Dominguez, C. X. et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10, 232–253 (2020).
Zhu, K., Cai, L., Cui, C., de los Toyos, J. R. & Anastassiou, D. Single-cell analysis reveals the pan-cancer invasiveness-associated transition of adipose-derived stromal cells into COL11A1-expressing cancer-associated fibroblasts. PLoS Comput. Biol. 17, e1009228 (2021).
Chu, T., Wang, Z., Pe’er, D. & Danko, C. G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 3, 505–517 (2022).
Fan, J. et al. Multi-omics characterization of silent and productive HPV integration in cervical cancer. Cell Genom. 3, 100211 (2023).
Sengupta, S. et al. Mesenchymal and adrenergic cell lineage states in neuroblastoma possess distinct immunogenic phenotypes. Nat. Cancer 3, 1228–1246 (2022).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Chen, J. et al. Neoadjuvant camrelizumab plus chemotherapy for locally advanced cervical cancer (NACI Study): a study protocol of a prospective, single-arm, phase II trial. BMJ Open 13, e067767 (2023).
Rittmeyer, A. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265 (2017).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Patil, N. S. et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 40, 289–300 (2022).
Zha, H. et al. S100A9 promotes the proliferation and migration of cervical cancer cells by inducing epithelial–mesenchymal transition and activating the Wnt/β‑catenin pathway. Int. J. Oncol. 55, 35–44 (2019).
Wang, S. et al. SERPINB3 (SCCA1) inhibits cathepsin L and lysoptosis, protecting cervical cancer cells from chemoradiation. Commun. Biol. 5, 46 (2022).
Seo, J. et al. Fatty-acid-induced FABP5/HIF-1 reprograms lipid metabolism and enhances the proliferation of liver cancer cells. Commun. Biol. 3, 638 (2020).
Yan, S. et al. SAR studies on truxillic acid mono esters as a new class of antinociceptive agents targeting fatty acid binding proteins. Eur. J. Med. Chem. 154, 233–252 (2018).
Vitale, I., Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 27, 212–224 (2021).
Lin, F. et al. Stanniocalcin 1 promotes metastasis, lipid metabolism and cisplatin chemoresistance via the FOXC2/ITGB6 signaling axis in ovarian cancer. J. Exp. Clin. Cancer Res. 41, 129 (2022).
Chen, K. et al. Single cell RNA-seq reveals the CCL5/SDC1 receptor–ligand interaction between T cells and tumor cells in pancreatic cancer. Cancer Lett. 545, 215834 (2022).
Yang, H. et al. Therapeutic potential of targeting membrane-spanning proteoglycan SDC4 in hepatocellular carcinoma. Cell Death Dis. 12, 492 (2021).
Kurozumi, A. et al. Tumor‐suppressive microRNA‐223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 107, 84–94 (2016).
Morgan, M. R., Humphries, M. J. & Bass, M. D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969 (2007).
Wang, W. et al. Identification of biomarkers for lymph node metastasis in early-stage cervical cancer by tissue-based proteomics. Br. J. Cancer 110, 1748–1758 (2014).
Liu, F. et al. Identification of FABP5 as an immunometabolic marker in human hepatocellular carcinoma. J. Immunother. Cancer 8, e000501 (2020).
O’Sullivan, S. E. & Kaczocha, M. FABP5 as a novel molecular target in prostate cancer. Drug Discov. Today 25, 2056–2061 (2020).
Adamson, J. et al. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene 22, 2739–2749 (2003).
Al-Jameel, W. et al. Inactivated FABP5 suppresses malignant progression of prostate cancer cells by inhibiting the activation of nuclear fatty acid receptor PPARγ. Genes Cancer 10, 80–96 (2019).
Farrell, M. et al. Fatty acid binding proteins as a novel therapeutic target in multiple myeloma. Blood 138, 1569 (2021).
Levi, L. et al. Genetic ablation of the fatty acid–binding protein FABP5 suppresses HER2-induced mammary tumorigenesis. Cancer Res. 73, 4770–4780 (2013).
Tokunaga, R. et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – a target for novel cancer therapy. Cancer Treat. Rev. 63, 40–47 (2018).
Shahbandi, A. et al. Breast cancer cells survive chemotherapy by activating targetable immune-modulatory programs characterized by PD-L1 or CD80. Nat. Cancer 3, 1513–1533 (2022).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41, e108 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Liu, C. et al. Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci. Adv. 9, eadd8977 (2023).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Kaufmann, M. et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat. Neurosci. 25, 944–955 (2022).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 82141106 to D.M., grant no. 82073259 to G.C. and grant no. 81974408 to C.S.), the Key R&D Program of Hubei Province (grant no. 2023BCB023 to C.S.) and Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province (grant no. CXPJJH122006-1006 to B.Y.).
Author information
Authors and Affiliations
Contributions
C.S. and J.F. conceived the study idea. X.Z., E.G. and B.Y. collected the samples and performed the IHC staining. J.F. and W.P. performed the bioinformatics analyses. F.L., T.Q., Y.L. and X.H. performed the experiments. C.S., J.F., W.P., T.Q. and F.L. wrote the manuscript. Z.F., Y.Y., E.G., B.Y., X.L., Y.F., X.K. and Z.W. collected the patients’ clinical information and provided support with the analysis. X.M., K.L., P.W., L.H. and G.B.M. provided expertise and feedback. C.S., G.C., D.M., P.W., K.L. and X.M. supervised the project and provided valuable critical discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Ajit Johnson Nirmal and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The cell atlas revealed by scRNA-seq offered a fundamental perspective to interrogate CSCC heterogeneity.
(a) Distributions of count (top) and mitochondrial fraction (bottom) per cell in each sample. The inner boxplots are represented with the middle dot indicating the median, while the upper and lower hinges indicate the 25% and 75% quartiles, respectively. The upper or lower whiskers indicate the extremum values no larger or smaller than 1.5 × interquartile range. (b) Expression of selected cell markers in UMAP space. (c) Preference of each stromal cell types in CSCC and HD estimated by the ratio of observed to expected cell numbers (Ro/e). (d) Heatmap of CNV inferred from scRNA-seq and WES. The bottom panel displays the short (grey) and long (black) arm of each chromosome. For each tumor, CNV from scRNA-seq is inferred by inferCNV using normal epithelial cells as control and expressed as median CNV score of tumor cells, while CNV from WES is inferred by CNVkit using paired PBMC as control. (e) Profile of somatic mutations of each tumor ordered by gene. Gene mutation are colored by mutational type.
Extended Data Fig. 2 The robustness of meta-programs and comparison to pan-cancer and HNSCC programs.
(a) The NMF programs were clustered into MPs using the algorithm described by Gavish. Heatmap showing the clustering of MPs and Jaccard similarity among NMF programs. (b) Heatmap showing Jaccard similarity between MPs identified in (a) with the clustering method proposed by Gavish et al. and MPs identified through UPGMA method (Methods). mp7 (RP high) featured by ribosomal protein genes from Gavish et al. was excluded from analysis. (c) Scatter plot shows Jaccard similarity (y axis) and Pearson correlation of single-cell scores (x axis) between MPs and previously identified HNSCC and pan-cancer programs. MP1 was excluded from downstream analysis due to enrichment of mitochondrial genes.
Extended Data Fig. 3 Correlations between MPs and tumor immune microenvironment cells and differences between MP cells and normal epithelial cells.
(a) Dot plot displays the expression of squamous and glandular epithelial markers. (b) Heatmap displays the Pearson correlation coefficients calculated among signature scores of distinct tumor MPs of this study. (c) Scatter plot shows the correlation between MP7 signature scores of tumor cells and the fractions of CAF and CD8_Tex-GZMK cells in each patient. The MP6 signature score for each sample is displayed as the median score of tumor cells. P values are derived from the spearmanr function in scipy. (d) UMAP plots of 2,942 epithelial cells colored by epithelial subtype and the expression of epithelial markers in HDs. (e) Expression of representative MP6, MP7 signature genes and glandular marker genes in selected cell types. (f) Box plot shows the expression of selected MP6 signature genes between selected cell types (CSCC-MP6 (n = 3666), CSCC-MP7 (n = 2491), CSCC-MP8 (n = 1285), HD-Basal (n = 500), HD-Squamous (n = 173)). Data are represented as boxplots with the middle line indicating the median, while the upper and lower hinges indicate the 25% and 75% quartiles, respectively. The upper or lower whiskers indicate the extremum values no larger or smaller than 1.5 × interquartile range. P values are from two-sided Wilcoxon rank-sum tests.
Extended Data Fig. 4 MP7 associated tumor infiltrated phenotypes with abundant T cells skewed toward a cytotoxic or exhaustion state.
(a) Representative IHC of CD4 at stromal (str) and epithelial (epi) regions in CSCCs with infiltrated or excluded/desert TIPs. (b) Heatmap showing IHC score of CD4 or CD8 positive cells in tumor epithelial regions (epiCD4 or epiCD8). (c) UMAP plot of cells colored by TIP in CSCC. (d) Lollipop plots of p values based on comparing each cell cluster between infiltrated and excluded/desert TIPs. The lollipops are colored by enrichment in TIPs (red for infiltrated and blue for excluded/desert TIP, no significant (P > = 0.05) cell types are colored by grey). P values are calculated by two-sided t-tests.
Extended Data Fig. 5 Identification of MP6 and MP7 tumor spots on spatial transcriptomics.
(a) Workflow for identifying MP6 and MP7 tumor spots by integrating scRNA-Seq and spatial transcriptomic data. (b) Scatterplot of MP6 and MP7 scores in tumor spots of 15 slides. (c) Scatterplot of MP6 and MP7 scores in tumor spots of TJH34. Spots were defined as MP6 or MP7 spots by hierarchical clustering. Red and blue dots indicate MP6 and MP7 tumor spots, respectively. (d) Spatial distribution of tumor MP6 (blue) and MP7 (red) spots among 15 slides.
Extended Data Fig. 6 Spatial transcriptomics demonstrate inverse associations of MP6 and MP7 with immune cell infiltration.
(a) Composition of MP6 and MP7 spots in tumor spots of 15 slides. (b) Dot plot of average cell type score in MP6 and MP7 spots of TJH34. (c) scRNA-seq data mapped to the Stereo-seq profile by CytoSPACE, with annotated cell types from the scRNA-seq atlas distinguished by color. MP6 and MP7 region are circled by solid and dashed lines respectively. (d) Heat maps depicting CytoSPACE performance for aligning scRNA-seq data to spatial locations in Stereo-seq. CD4 T cells and CD8 T cells with distinct spatial distribution are shown. Bar plots show the proportion of CD4 and CD8 T cells per spatial spot type. (e) T cell exhaustion signatures enrichment in CD4 and CD8 T cell within MP7 tumor core when compared to T cells in tumor stroma. P values are derived from clusterProfiler package and adjusted with Benjamini-Hochberg procedure. NES: normalized enrichment score. (f) Spatial distribution of pan-cancer immune score in slide TJH34. (g) Spatial distribution of MP6 score, MP7 score, T cell inflamed GEP and Hot tumor-related genes score in slide TJH34. GEP: gene expression profile. (h) Representative area of MP6, MP7 tumor showing spatial distribution of MP6, MP7 tumor spots and stromal spots as well as spatial expression of MP7 score, T cell inflamed GEP and HOT tumor-related genes score.
Extended Data Fig. 7 Multimodal analyses of tumor states demonstrate TGFβ signaling in MP6 tumors.
(a) Bar plot shows enriched pathways from GO BP, REACTOME and HALLMARK using DEGs of tumor MP6, MP7, MP3 and MP4/5 cells profiled by scRNA-seq. FDR q values are derived from clusterProfiler package. (b) Dot plot shows the expression of immune inhibitory molecules in MP6 and MP7 tumor cells profiled by scRNA-seq. (c) Bar plot displays enriched pathways from GO BP using DEGs of immune-rich (left) and immune-poor (right) ROIs profiled by spatial proteomics. FDR q values are derived from clusterProfiler package.
Extended Data Fig. 8 The inverse relationship of MP6 tumor cell phenotype and immune infiltration is present in multiple CSCC datasets.
(a) Heatmap showing the significantly associated proteins or phosphorylation sites with MP6/7 scores in our in-house CSCC cohort. Proteins or phosphorylation sites are marked if they are MP6 or MP7 related genes. (b) Multiplex immunofluorescence of tumor (pCK), CD8T (CD8), Treg (FOXP3), Macrophage (CD68), fibroblasts (alpha-SMA) and nucleus (DAPI) in representative samples with high and low MP6/7 scores. (c) Heatmap of scaled values of genes in immune activation, immune evasion signaling and the cell composition inferred with xCell within CSCC samples in TCGA CESC datasets. Samples are ranked by increasing MP6/7 score. The top panel shows MP6, MP7 scores, and information about clinical stage, grade, age, histopathology and iCluster. (d, e) Kaplan-Meier curves comparing the probability of survival according to MP6/7 score in non-squamous cancers patients of OAK (d) and POPLAR (e) cohorts. Patients are dichotomized as high or low score using the median MP6/7 score as cutoff. P values were calculated with log-rank test.
Extended Data Fig. 9 MP6/7 signature provides an additional and complementary signal independent of immune-hot dichotomy.
(a–b). Kaplan-Meier curves comparing the survival probability based on MP6/7 score, T cell inflamed GEP and hot tumor-related genes score in OAK squamous cancer patients with immunotherapy (a) or chemotherapy (b). Patients are dichotomized as high/low subgroup using the median value cutoff. Hazard ratios (HR) with 95% CI were derived from a cox proportional model fit. (c–d) Scatter plot comparing T cell inflamed GEP signature (c) and hot tumor-related genes (d) with MP6/7 score in OAK squamous cancer patients with immunotherapy. Dashed vertical and horizontal lines represent the optimal cut points using maximally selected rank statistics. MP6/7hi and MP6/7lo groups are defined by cutoffs greater than or equal to and less than 0.05. GEPhi and GEPlo groups are defined by cutoffs greater than or equal to and less than 0.42. HOThi and HOTlo groups are defined by cutoffs greater than or equal to and less than 0.5. GEP: T cell inflamed GEP score. HOT: hot tumor-related genes score. (e–f) Kaplan-Meier curves showing relationship of T cell inflamed GEP (e), hot tumor-related genes score (f) and MP6/7 score with OS in OAK squamous immunotherapy cohort as treated per GEP cutoff (e), HOT cutoff (f) and MP6/7 cutoff. HR with 95% CI were derived from a cox proportional model fit.
Extended Data Fig. 10 FABP5 is critical for MP6 state maintenance and contributed to the activated TGFβ and interaction with immunosuppressive CAF.
(a) Heatmap shows relative expression of MP6 signatures after knockdown of each MP6 signature gene by siRNA for 48 hours in HeLa and C33A cells. The relative expression of MP6 signatures as calculated by the mean value of MP6 genes (except the knockdown gene) relative to negative control (NC) and shown as log2 transformed. (b) Quantification of MP6 genes by qPCR in SCC25 and CAL27 cells after si-FABP5 for 48 hours. (c) Western blot of expression of MP6 proteins in SCC25 and CAL27 cells after si-FABP5 for 72 hours. (d) Morphological photography and HE staining of patient-derived organoids (PDO) P36 after treatment with or without 10 μM FABP5-IN-1 for 6 days. (e) Immunofluorescence of indicated proteins in PDO P36 after treatment with or without 10 μM FABP5-IN-1 for 6 days. (f) Quantifications of CSCC PDOs’ diameters after treatment with 10 μM FABP5-IN-1 for 6 days. (g) Quantifications of fluorescence intensity of S100A8 and SERPINB3 in PDOs after treatment with or without 10 μM FABP5-IN-1 for 6 days. (h) qPCR of genes from TGFβ pathway in HeLa, SCC25, CAL27 and A431 cells after si-FABP5. (i) Western blot of proteins from TGFβ pathway in C33A cells after treatment with increasing concentration of FABP5-IN-1 for 72 hours. (j) The weight curves of C57BL/6 mice during treatment with vehicle or FABP5-IN-1 (n = 6; two independent experiments). (k) The work flow procedure of flow cytometry analysis in TC-1 tumors at treatment termination. (l) Tumor growth curves (left) and tumor sizes (right) of parental WT and FABP5 knockout (ko-FABP5 sg#A13) tumors in C57BL/6 mice (n = 6). The circles on mouse indicate the location of tumor and are colored by source. (m) Representative images and quantification of CD4+ and CD8+ cells in WT and FABP5 knockout tumors in B1 mice. For b,f-h,j,l,m, data represent the mean ± s.e.m. with three independent replicates if not indicated otherwise. P values are calculated by two-side t-tests. n.s.: not significant.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Figs. 1–7 and Methods.
Source data
Source data
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, J., Lu, F., Qin, T. et al. Multiomic analysis of cervical squamous cell carcinoma identifies cellular ecosystems with biological and clinical relevance. Nat Genet 55, 2175–2188 (2023). https://doi.org/10.1038/s41588-023-01570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01570-0