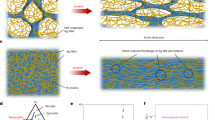
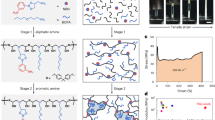
Abstract
Replacement of liquid electrolytes with polymer gel electrolytes is recognized as a general and effective way of solving safety problems and achieving high flexibility in wearable batteries1,2,3,4,5,6. However, the poor interface between polymer gel electrolyte and electrode, caused by insufficient wetting, produces much poorer electrochemical properties, especially during the deformation of the battery7,8,9. Here we report a strategy for designing channel structures in electrodes to incorporate polymer gel electrolytes and to form intimate and stable interfaces for high-performance wearable batteries. As a demonstration, multiple electrode fibres were rotated together to form aligned channels, while the surface of each electrode fibre was designed with networked channels. The monomer solution was effectively infiltrated first along the aligned channels and then into the networked channels. The monomers were then polymerized to produce a gel electrolyte and form intimate and stable interfaces with the electrodes. The resulting fibre lithium-ion battery (FLB) showed high electrochemical performances (for example, an energy density of about 128 Wh kg−1). This strategy also enabled the production of FLBs with a high rate of 3,600 m h−1 per winding unit. The continuous FLBs were woven into a 50 cm × 30 cm textile to provide an output capacity of 2,975 mAh. The FLB textiles worked safely under extreme conditions, such as temperatures of −40 °C and 80 °C and a vacuum of −0.08 MPa. The FLBs show promise for applications in firefighting and space exploration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Fan, X. et al. Opportunities of flexible and portable electrochemical devices for energy storage: expanding the spotlight onto semi-solid/solid Electrolytes. Chem. Rev. 122, 17155–17239 (2022).
Sumboja, A. et al. Electrochemical energy storage devices for wearable technology: a rationale for materials selection and cell design. Chem. Soc. Rev. 47, 5919–5945 (2018).
Zhu, Y.-H., Yang, X.-Y., Liu, T. & Zhang, X.-B. Flexible 1D batteries: recent progress and prospects. Adv. Mater. 32, 1901961 (2020).
Chang, J., Huang, Q., Gao, Y. & Zheng, Z. Pathways of developing high-energy-density flexible lithium batteries. Adv. Mater. 33, 2004419 (2021).
Mackanic, D. G., Chang, T.-H., Huang, Z., Cui, Y. & Bao, Z. Stretchable electrochemical energy storage devices. Chem. Soc. Rev. 49, 4466–4495 (2020).
Zhao, Q., Stalin, S., Zhao, C.-Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Vijayakumar, V., Anothumakkool, B., Kurungot, S., Winter, M. & Nair, J. R. In situ polymerization process: an essential design tool for lithium polymer batteries. Energy Environ. Sci. 14, 2708–2788 (2021).
Chen, S. et al. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 120, 2811–2878 (2020).
Zhao, Q., Liu, X., Stalin, S., Khan, K. & Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019).
Ling, S. et al. Densifiable ink extrusion for roll-to-roll fiber lithium-ion batteries with ultra-high linear and volumetric energy densities. Adv. Mater. 35, 2211201 (2023).
Wang, Y. et al. 3D-printed all-fiber Li-ion battery toward wearable energy storage. Adv. Funct. Mater. 27, 1703140 (2017).
Rao, J. et al. All-fiber-based quasi-solid-state lithium-ion battery towards wearable electronic devices with outstanding flexibility and self-healing ability. Nano Energy 51, 425–433 (2018).
Ren, J. et al. Elastic and wearable wire-shaped lithium-ion battery with high electrochemical performance. Angew. Chem. Int. Ed. 53, 7864–7869 (2014).
Khudiyev, T. et al. Thermally drawn rechargeable battery fiber enables pervasive power. Mater. Today 52, 80–89 (2022).
Wang, Y., Song, Y., & Xia, Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 45, 5925–5950 (2016).
Zapata-Benabithe, Z., Carrasco-Marín, F. & Moreno-Castilla, C. Preparation, surface characteristics, and electrochemical double-layer capacitance of KOH-activated carbon aerogels and their O- and N-doped derivatives. J. Power Sources 219, 80–88 (2012).
El-Kady, M. F. et al. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl Acad. Sci. USA 112, 4233–4238 (2015).
Nishi, T., Nakai, H. & Kita, A. Visualization of the state-of-charge distribution in a LiCoO2 cathode by in situ Raman imaging. J. Electrochem. Soc. 160, A1785 (2013).
Maher, K., & Yazami, R. Effect of overcharge on entropy and enthalpy of lithium-ion batteries. Electrochim. Acta 101, 71–78 (2013).
Chen, Q. et al. Yolk–shell NiS2 nanoparticle-embedded carbon fibers for flexible fiber-shaped sodium battery. Adv. Energy Mater. 8, 1800054 (2018).
Guan, Q. et al. Dendrite-free flexible fiber-shaped Zn battery with long cycle life in water and air. Adv. Energy Mater. 9, 1901434 (2019).
Chong, W. G. et al. Lithium–sulfur battery cable made from ultralight, flexible graphene/carbon nanotube/sulfur composite fibers. Adv. Funct. Mater. 27, 1604815 (2017).
Lin, H. et al. Twisted aligned carbon nanotube/silicon composite fiber anode for flexible wire-shaped lithium-ion battery. Adv. Mater. 26, 1217–1222 (2014).
Xia, Z. et al. Manipulating hierarchical orientation of wet-spun hybrid fibers via rheological engineering for Zn-ion fiber batteries. Adv. Mater. 34, 2203905 (2022).
Zeng, Y. et al. An ultrastable and high-performance flexible fiber-shaped Ni–Zn battery based on a Ni–NiO heterostructured nanosheet cathode. Adv. Mater. 29, 1702698 (2017).
Zhang, Y. et al. Flexible and stretchable lithium-ion batteries and supercapacitors based on electrically conducting carbon nanotube fiber springs. Angew. Chem. Int. Ed. 53, 14564–14568 (2014).
Hoshide, T. et al. Flexible lithium-ion fiber battery by the regular stacking of two-dimensional titanium oxide nanosheets hybridized with reduced graphene oxide. Nano Lett. 17, 3543–3549 (2017).
Weng, W. et al. Winding aligned carbon nanotube composite yarns into coaxial fiber full batteries with high performances. Nano Lett. 14, 3432–3438 (2014).
Fang, X., Weng, W., Ren, J. & Peng, H. A cable-shaped lithium sulfur battery. Adv. Mater. 28, 491–496 (2016).
He, J. et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 597, 57–63 (2021).
Zhang, Y. et al. Super-stretchy lithium-ion battery based on carbon nanotube fiber. J. Mater. Chem. A 2, 11054–11059 (2014).
Wang, C., He, T., Cheng, J., Guan, Q. & Wang, B. Bioinspired interface design of sewable, weavable, and washable fiber zinc batteries for wearable power textiles. Adv. Funct. Mater. 30, 2004430 (2020).
Acknowledgements
This work was supported by MOST (2022YFA1203001 and 2022YFA1203002), NSFC (T2321003, 22335003, 52122310, 22075050, 52222310, T2222005 and 22175042), STCSM (21511104900) and China Postdoctoral Science Foundation (2023M740651). We thank XPLORER PRIZE for its support in fabricating and studying FLBs.
Author information
Authors and Affiliations
Contributions
H.P. conceived and designed the project. C.L., H.J. and X.C. performed the design of the FLB with channels, gel electrolytes and FLB textiles. J.H., Y.L., Y.C. and X.G. performed the fabrication of the FLB. K.Z., J.L., Z.Z., X. Shi, L.Y. and M.L. analysed the data. J. Wu, J. Wang and Y.Z. performed the fabrication of weaving textiles. X. Sun, B.W., P.C. and Y.W. contributed to the discussion on the data, figures and manuscript. C.L., X.C. and H.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Electrochemical performances of FLBs deposited with different particle layers.
a,b, Electrochemical impedance spectra and discharge profiles of the FLBs, respectively. The FLBs were discharged at a rate of 0.2C.
Extended Data Fig. 2 Architecture and wetting capability of a fibre electrode with aligned and networked channels.
a, SEM image of a fibre electrode with a rotating architecture. b,c, Magnified SEM images of the aligned fibres and the channel between them, respectively. d,e, Images reconstructed from X-ray computed tomography data of the networked channels (blue) among particles (grey), respectively. The particles were highly interconnected with each other, forming channels among them. The small channels showed sizes of several micrometres, while the large channels showed sizes of tens of micrometres. f, Channel size distribution tested by the mercury intrusion method. g–j, Fluorescence microscopy images showing the monomer solution infiltrating along the aligned channels. k, Monomer solution wetting into the networked channels of an electrode. Scale bars, 1 mm (a); 300 μm (b); 100 μm (c); 20 μm (d,e); and 200 μm (g–j).
Extended Data Fig. 3 Electrochemical characterization of FLBs with and without aligned channels.
a,b, Cyclic voltammograms of the FLBs with and without aligned channels at increasing scan rates, respectively. FLBs without aligned channels showed obvious polarizations at high scan rates. The voltage ranges were set within 50 mV near the open-circuit voltage to obtain capacitance currents. c, Specific capacitances of FLBs with and without aligned channels at increasing scan rates. d, Charge–discharge profiles of FLBs with and without aligned channels. The FLB with aligned channels showed much higher specific capacity because of sufficient wetting by the electrolyte through the aligned channels. The electrolyte amounts were around 8 g/Ah.
Extended Data Fig. 4 Characterization of FLBs with and without an inner small particle layer.
a,b, Cross-sectional SEM images of cathode fibres with and without an inner small particle layer, respectively. c, Charge–discharge profiles of FLBs with and without an inner small particle layer. The FLBs were discharged at a rate of 2C. d,e, Raman shifts of LiCoO2 particles at 5 different positions in the deposition layer of cathode fibre with and without inner small particles, respectively. The cathode fibre with an inner small particle layer showed sufficient lithium ion intercalation after discharging, indicated by the characteristic peaks of the LiCoO2 at 486 cm−1 and 587 cm−1; while the cathode fibre without an inner small particle layer showed insufficient lithium ion intercalation. a.u., arbitrary units. Scale bars, 20 μm (a,b).
Extended Data Fig. 5 Stability of the gel electrolyte–graphite interface.
a–c, Raman mapping images of the gel electrolyte and graphite particles with the FLB in the uncharged state at 3.0 V, half charged state at 3.9 V and a fully charged state at 4.4 V. d,e, Raman mapping images of the gel electrolyte and graphite particles after the FLB was stored at −40 °C and 80 °C for 1 h, respectively. f, Magnified area of the gel electrolyte–graphite interface. g, Corresponding Raman shifts of the graphite particles at 3.0 V, 3.9 V, and 4.4 V. The characteristic peaks of graphite at 1,350 cm−1 and 1,580 cm−1 varied for the different charged states. a.u., arbitrary units. Scale bars, 3 μm (a–e); 1 μm (f).
Extended Data Fig. 6 Architectures and electrochemical properties of FLBs with increasing numbers of electrode fibres.
a–c, SEM images of electrodes with 4, 6, and 8 fibres. The anode fibres were wrapped with separators to prevent short circuits before rotated with the cathode fibres. d, Discharge profiles of FLBs made with increasing numbers of electrode fibres. e, Specific capacities of the FLBs made with increasing numbers of electrode fibres. Error bars show the standard deviations for the results from three samples. Scale bars, 1 mm (a–c).
Extended Data Fig. 7 Charge–discharge performances of different fibre electrodes and LiCoO2//graphite FLBs with different electrolytes.
Charge–discharge profiles of the fibre electrodes of the anode materials of mesocarbon microbeads (MCMB) (a), hard carbon (b), silicon carbon composites (SiC) (c), and Li4Ti5O12 (LTO) (d). Charge–discharge profiles of the fibre electrodes of the cathode materials of LiNi0.5Co0.2Mn0.3O2 (NCM523) (e) and LiMn2O4 (LMO) (f). From a to f, lithium metal was used as the counter electrode, and poly(ethylene glycol) dimethacrylate monomer was used to prepare the gel electrolyte. Charge–discharge profiles of LiCoO2//graphite FLBs were based on the gel electrolytes prepared from different monomers of tri(propylene glycol) diacrylate (TPGDA) (g), pentaerythritol tetraacrylate (PETEA) (h), 1,6-hexanediol dimethacrylate (HADMA) (i), poly(ethylene glycol) diacrylate (PEGDA) (j), and trimethylolpropane triacrylate (TPTEA) (k). l, Initial Coulombic efficiencies of FLBs with different gel electrolytes polymerized from monomers from g to k.
Extended Data Fig. 8 Electrochemical stabilities of the FLB at different temperatures and after immersion in water.
a, FLBs charged at 25 °C at 0.2C and discharged at 0.1C, 0.2C and 0.5C at 0 °C, 25 °C and 80 °C, respectively. b, The FLB charged at 25 °C at 0.2C and discharged at −40 °C at 0.1C with a cut-off voltage of 2 V. c, Capacity retention of the FLBs over the range from −40 °C to 80 °C. The error bars show the standard deviations for the results from three samples. d, Voltage (red) and temperature (blue) records of the FLB when immersed in ice water and heated to the boiling point.
Extended Data Fig. 9 Electrochemical performances of the FLB textile.
a, Charge–discharge profiles of the FLB textile at discharge rates of 0.08C, 0.2C and 0.5C. b, Bending durability of FLB textiles. The specific capacities and midpoint voltages remained stable after bending, with an average capacity retention of more than 93% after bending for 100,000 cycles. The error bars show the standard deviations for the results from three samples. c, Cycling performance of the FLB textile. Both high capacities and high Coulombic efficiencies were maintained after charging and discharging for 1,000 cycles. d–f, Voltage-time curves measured for the FLB textile at 22 °C and 51% relative humidity (RH), at 80 °C and in a vacuum of −0.08 MPa, respectively.
Extended Data Fig. 10 National standard safety tests of the FLB textile.
a, The FLB textile discharged to a low voltage of 0.15 V at 2,000 mA and charged to 4.4 V. b, After reaching the fully charged state at 4.4 V, the FLB textile continued to charge to a high voltage of 6 V at 1,000 mA and was kept at 6 V for 1 h. c, The FLB textile discharged at a high current of 4,500 mA. d, The FLB textile charged at a high current of 1,500 mA after discharged to 3 V. The FLB textile did not leak, burn or explode during the safety tests.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figs. 1–20 and Supplementary Tables 1–3.
Supplementary Video 1
FLB textile normally charges a cellphone when cut.
Supplementary Video 2
Thirty-metre-long FLB textile.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, C., Jiang, H., Cheng, X. et al. High-performance fibre battery with polymer gel electrolyte. Nature 629, 86–91 (2024). https://doi.org/10.1038/s41586-024-07343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07343-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.