Abstract
Auxins are hormones that have central roles and control nearly all aspects of growth and development in plants1,2,3. The proteins in the PIN-FORMED (PIN) family (also known as the auxin efflux carrier family) are key participants in this process and control auxin export from the cytosol to the extracellular space4,5,6,7,8,9. Owing to a lack of structural and biochemical data, the molecular mechanism of PIN-mediated auxin transport is not understood. Here we present biophysical analysis together with three structures of Arabidopsis thaliana PIN8: two outward-facing conformations with and without auxin, and one inward-facing conformation bound to the herbicide naphthylphthalamic acid. The structure forms a homodimer, with each monomer divided into a transport and scaffold domain with a clearly defined auxin binding site. Next to the binding site, a proline–proline crossover is a pivot point for structural changes associated with transport, which we show to be independent of proton and ion gradients and probably driven by the negative charge of the auxin. The structures and biochemical data reveal an elevator-type transport mechanism reminiscent of bile acid/sodium symporters, bicarbonate/sodium symporters and sodium/proton antiporters. Our results provide a comprehensive molecular model for auxin recognition and transport by PINs, link and expand on a well-known conceptual framework for transport, and explain a central mechanism of polar auxin transport, a core feature of plant physiology, growth and development.
Similar content being viewed by others
Main
Auxins are a group of hormones that regulate nearly all growth and developmental processes in plants. Indole-3-acetic acid (IAA; pKa = 4.7) is the most prominent auxin, and is synonymously referred to as ‘auxin’. IAA provides a growth signal that orchestrates most complex environmental responses in plants, including phototropism and geotropism1.
Many of the effects on plant growth depend on the distribution of auxin in the plant body, which is controlled by the process of polar auxin transport2,3. This process relies on export of auxin out of cells by PIN transporters4,5,6,7,8,9. The physiological importance of PINs is underlined by often severe pin mutant phenotypes, which can be mimicked by auxin efflux inhibitors such as the commercially available herbicide naphthylphthalamic acid10 (NPA (also known as naptalam); pKa = 4.6).
The PIN protein family is exclusive to the plant kingdom and is classified as part of the large bile/arsenite/riboflavin transporter (BART) superfamily, which also includes transporters of bile acid, arsenite and riboflavin with members distributed across all kingdoms of life11,12. PIN proteins are predicted to have ten transmembrane helices comprising two five-transmembrane helix repeats separated by a cytosolic loop. Canonical PINs (PIN1–4 and PIN7 in A. thaliana) are characterized by a long (323–355 residue) loop and are mostly located in the plasma membrane, whereas non-canonical PINs (PIN5 and PIN8 and the intermediate PIN6 in A. thaliana) possess a much shorter loop and can be found in organellar membranes such as endoplasmic reticulum membranes13,14,15 (Extended Data Figs. 1 and 2a). The long loops of canonical PINs have phosphorylation sites that regulate activity; the loops have been shown to be auto-inhibitory, requiring kinase activity to initiate transport16.
Here we present structural and biophysical characterization of a PIN protein. In particular, cryo-electron microscopy (cryo-EM) has been used to solve structures in an outward-facing state with and without bound IAA as well as in an inward-facing state with bound NPA at resolutions between 2.9 and 3.4 Å. Combined with transport data from mutant protein, these structures suggest a molecular mechanism and model for auxin transport that is broadly applicable to the ubiquitous PIN family.
We chose to study PIN8 from A. thaliana after screening various PIN homologues for expression and purification. PIN8 is a non-canonical PIN of 40 kDa in size, with a short cytosolic loop of 43 residues that lacks the phosphorylation motifs seen in the long auto-inhibitory loops of canonical PINs. When expressed in oocytes, PIN8 exhibited robust IAA transport activity similar to that of kinase-activated PIN1. This activity is independent of activating kinases and sensitive to the inhibitor NPA, demonstrating that PIN8 is a constitutively active auxin transporter (Fig. 1a and Extended Data Fig. 2b).
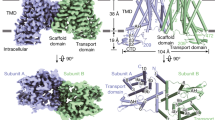
a, Relative IAA transport rates for PIN8 and PIN1 incubated with PIN-activating kinases D6PK and PID show that PIN8 is constitutively active in oocytes (internal oocyte IAA concentration = 1 µM). The centre line is the median, the box extends from the 25th to 75th percentile and whiskers extend to minimum and maximum values. Points represent biologically independent experiments (PIN8: n = 11, PIN8 D6PK: n = 5, PIN8 PID: n = 7, PIN1: n = 4, PIN1 D6PK: n = 3). For differences between PIN8 and other groups, a one-way ANOVA followed by Dunnett’s multiple comparisons test was performed. PIN8 versus PIN8 D6PK: P = 0.8508, PIN8 versus PIN8 PID: P = 0.8090, PIN8 versus PIN1: P = 0.0078, PIN8 versus PIN1 D6PK: P = 0.9968. b, Peak current response by SSM electrophysiology on PIN8 proteoliposomes. Transport is described by Michaelis–Menten kinetics (r2 = 0.98, Km = 356 ± 136 µM, maximum current (Imax) = 8.5 ± 1.9 nA; data are mean ± s.e.m.; PIN8: n = 4 different proteoliposome preparations, empty: n = 3 liposome preparations). Inset, stained SDS–PAGE analysis and size-exclusion chromatography (SEC) trace for the PIN8 purification. c, Transport current in the presence of NPA shows inhibition (Ki = 1.9 µM (95% confidence interval: 0.9–3.8 µM; n = 3 for 0 and 1 µM NPA and n = 2 for 5 and 20 µM NPA); data are mean or mean ± s.e.m. (n > 2)). d, Topology of the PIN8 monomer shows an inverted repeat of five transmembrane helices and the relation between transporter and scaffold domains. e, Cryo-EM map of the PIN8 dimer with one monomer coloured according to panel d. f, Side view of PIN8 with M1–M10 labelled. The central crossover highlighted in red in monomer B. Right, top view from the non-cytosolic side displays the dimer interface and the two domains found in each monomer: the transporter domain (green) and the scaffold domain (blue).
To characterize electrogenic transport of IAA by PIN8, we overexpressed the protein in Saccharomyces cerevisiae and, following purification, reconstituted it into proteoliposomes. We measured transport using capacitive coupling using solid supported membrane (SSM) electrophysiology, and show that PIN8 has a relatively low apparent affinity for IAA, with a Michaelis constant (Km; Methods, ‘SSM physiology assays’) of 356 ± 136 µM (n = 4) (Fig. 1b and Extended Data Fig. 2c). We measure the dissociation constant (Kd) of IAA binding to be 39.9 µM (Extended Data Fig. 2d). We observe a modest pH dependence with an optimum at 6.0–7.4 (Extended Data Fig. 2e). As in oocyte assays, transport can be inhibited by NPA, which inhibit with an inhibition constant (Ki) of 1.9 µM, suggesting an affinity one order of magnitude higher than that of IAA (Fig. 1c). We screened a number of additional PIN substrates (Extended Data Fig. 2f) and find that IAA analogues—for example, naphthaleneacetic acid (NAA) or the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D), elicit a current response in PIN8, whereas uncharged auxins as well as some endogenous auxins does not. Comparison of these substrates suggests that shape complementary has a large role in recognition: for example, the larger size of indole-3-butyric acid (IBA) and the reduced ring system of 2-phenylacetic acid (PAA) both result in reduced currents.
We solved three distinct structures of PIN8 using single-particle cryo-EM after reconstitution of the purified protein into peptidisc: an apo form at 2.9 Å resolution, PIN8 with IAA bound at 3.2 Å, and PIN8 with NPA bound at 3.4 Å resolution (Extended Data Figs. 3–5 and Extended Data Table 1). In addition, a structure of the apo form that is indistinguishable from the apo peptidisc structure was produced from a detergent-solubilized preparation at 3.3 Å (Extended Data Table 1). The highest-resolution map of the apo form was used for initial model building, but all maps display excellent density for the entire protein except for 39 residues of the disordered cytosolic loop, which were not modelled (Fig. 1d,e). We could model multiple water molecules and lipids as well as IAA and NPA in the relevant structures.
The apo form of PIN8 displays a symmetric dimer of PIN8 (Fig. 1f) characterized by a twofold rotation axis perpendicular to the membrane plane with a distinct concavity extending into the membrane along this axis from the non-cytosolic side. Within each monomer there are ten transmembrane helices (M1–M10), comprising an inverted repeat of five transmembrane helices17 (Fig. 1d). In each repeat, the fourth helix is disrupted around a conserved proline residue in the middle of the membrane plane: Pro116 in M4 and Pro325 in M9. These disrupted helices make an X-shaped crossover that marks the auxin binding pocket (Fig. 1f).
The PIN8 monomer is divided into two domains that we name the scaffold domain and the transporter domain (Fig. 1d,f and Extended Data Fig. 6a). The scaffold domain comprises helices M1, M2, M6 and M7 and creates a large interface (1,512 Å2) to the other monomer in the dimeric complex. This interface is mediated mainly by M2 and M7, and is further stabilized by a lipid in a groove between M1 and the kinked M6 (Extended Data Fig. 6b). We also observe another lipid with an aliphatic tail sticking into a pocket of the transporter domain. We tested a dependence on lipids for activity and found that PIN8 functions similarly in mixed lipid and pure phosphatidylcholine liposomes (Extended Data Fig. 6c). The transporter domain consists of helices M3–M5 and M8–M10 and harbours the central X-shaped crossover (Fig. 1f). The overall fold of the monomer is similar to that of the bile acid/sodium symporters, but the membrane topology is inverted18 (Extended Data Fig. 7). Next to the crossover, there is a well-defined water-filled binding pocket nestled between the scaffold domain and the transporter domain that is open to the non-cytosolic side of the protein via the concavity (Fig. 1f). By contrast, access to the cytosol is blocked, clearly defining the conformation of the apo-PIN8 dimer as an empty outward-open state.
The substrate-bound form of PIN8, IAA–PIN8, is almost identical to apo-PIN8 (root mean squared deviation of Cα atoms (r.m.s.d.Cα) = 0.6 Å) (Fig. 2a). There is a clear density for IAA in the binding pocket, with three surrounding water molecules (Fig. 2b,c). Thus, the IAA–PIN8 structure represents a substrate-bound outward-open state, the expected release state for auxin.
a, Cutaway view of electrostatic surface representation of IAA–PIN8 and NPA–PIN8 show the change in conformation. Whereas the concavity at the non-cytosolic side has negative potential, the binding pocket itself has a positive potential in both cases. b, View of the crossover and the position of IAA and the support site with central residues highlighted. c, Close-up view of IAA map density and the residues from the scaffold domain interacting with the indole ring. d, Peak current response evoked by 500 µM IAA determined by SSM electrophysiology for PIN8 mutants. Km values (mean ± s.d.) derived from the full measurement (Extended Data Fig. 8a) are shown above the bars; ND indicates that a Michaelis–Menten curve could not be fit. The bars show mean ± s.e.m. (n > 2); points represent biologically independent measurements (wild type (WT): n = 4 different liposome preparations; mutants: n = 6 (N117A), n = 5 (T288A), n = 4 (Q320A), n = 3 (Q145A, D75N, K79A, K79Q, Q78A, I51Y, I120Y and Y150A), n = 2 (Y150F, S146A, D75A and V328Y)).
IAA is bound with its carboxylate group oriented towards the crossover; although only two residues are within hydrogen-bonding distance (Asn117 and Gln145), IAA is stabilized by the positive dipole from M4b and M9b helices. The backbone carbonyl of Pro116 creates a polar pocket that is also lined by Tyr150 and Ser146. Here we observe three well-defined water molecules that may reflect partial disassociation of IAA from the binding pocket in the release state. Mutating either Asn117 and Gln145 to alanine abolishes transport, supporting their importance (Fig. 2d). Tyr150 mutants display mixed results: Y150F retains activity, affinity and sensitivity to NPA, whereas removal of the bulky side chain in Y150A results in very low activity and affinity (Extended Data Fig. 8a,b). By contrast, mutation of Ser146 had no effect on activity (Fig. 2d and Extended Data Fig. 8a).
In the transporter domain, the IAA carbon backbone contacts Leu119(M4b) and Ile120(M4b) towards the non-cytosolic side, whereas the indole ring contacts Val327(M9b) and Val328(M9b) towards the cytosolic side. These four hydrophobic residues are symmetrically located on the crossover immediately after the two key prolines as part of a duplicated and conserved crossover sequence motif (P(N/Q)XΦΦ; where Φ is a hydrophobic residue) (Fig. 2b–d and Extended Data Figs. 1 and 8b). The hydrophobic residues of the crossover motif provide affinity for the auxin substrate. This is supported by the bulky I120Y and V328Y mutants, which both reduce apparent affinity by interfering with substrate binding but still retain NPA sensitivity (Fig. 2d and Extended Data Fig. 8a,b). Together, the interactions between the transporter domain and IAA emphasize that PIN8 selects for IAA on the basis of shape complementarity, as also suggested by the SSM electrophysiology results. In the scaffold domain, the indole ring has additional non-specific hydrophobic interactions with Ile51 (M2), Leu54 (M2) and the pseudo-symmetrically related Leu260 (M7) and Ala263 (M7). Bulky mutations in these hydrophobic residues (such as I51Y) lead to a considerably reduced transport current (Fig. 2d and Extended Data Fig. 8a,b). All the residues defining the binding pocket show high sequence conservation across different plant species and are fully conserved in all A. thaliana PIN proteins except PIN5 (Extended Data Figs. 1 and 8c).
The NPA-bound form of PIN8 adopts an inward-open conformation (Fig. 2a). The scaffold domains and dimeric interface is unchanged relative to the outward-open conformation (r.m.s.d.Cα = 0.9 Å), but the two transporter domains are rotated by approximately 20° to expose the auxin binding site and Asn117 to the cytosolic side. This rotation results in a translation of the binding site by approximately 5 Å (Fig. 3a and Supplementary Video 1). NPA has more extensive interaction with the protein compared with IAA in the outward-open state (Fig. 3b,c and Extended Data Fig. 8c,d). Similar to IAA, the carboxylate group of NPA points towards the crossover, but has several stronger interactions that are not observed in the outward-open state. In addition to interactions seen for IAA, NPA interacts with main chain nitrogen atoms of Val327 and Val328, as well as with Gln145 and Tyr150 in a network that does not involve water. The benzene ring and naphthyl ring of NPA still interact with the two crossover motifs of the transporter domain, similar to IAA. Several new interactions are also observed with the scaffold domain, many of which are mediated by the naphthyl ring of NPA and are probably unique to the larger, more complex NPA molecule (Fig. 3b,c and Extended Data Fig. 8a–d). Inhibition by NPA can thus be explained by two components: (1) stronger binding due to engagement of additional residues from the scaffold domain, and (2) the larger size of NPA that prevents transition to the outward state.
a, Inward and outward structures superposed on the scaffold domain (blue) reveal an elevator-type movement, with the substrate binding site moving 5 Å. b, Close-up view of the NPA map density and the residues interacting with it. c, The hydrogen-bonding network linking the binding site to the support site through Gln145 in the outward state (top) and inward state (bottom).
Adjacent to the primary auxin binding site, an accessory ‘support site’ is apparent on the other side of the crossover between M3, M5 and M9. This support site is linked to the primary auxin binding site via an extensive hydrogen bond network bridged by the central Gln145 and the backbone carbonyl of Pro116. The higher-resolution apo-PIN8 map reveals two peaks in the site, which are modelled as water (Extended Data Fig. 8e). In the lower-resolution IAA–PIN8 and NPA–PIN8 map, the same site contains one single weak peak that is also modelled as water (Fig. 3c and Extended Data Fig. 8c–e). The presence of Na+ at analogous sites in bile acid/sodium symporters led us to probe ion dependence by comparing PIN8 transport in sodium- and potassium-exclusive buffers. In both cases, PIN8 retains full activity, suggesting that specific counter-transport of ions does not take place (Extended Data Fig. 9a). In all structures, the water molecules in the support site engage in a hydrogen bond network with Asp75 (M3), Gln78 (M3), Lys79 (M3), Gln320 (M9a) and Gln145 (M5) (Fig. 3c). Mutational analysis indicates that all of these residues except Gln320 are absolutely essential for activity (Fig. 2d and Extended Data Fig. 8a). Notably, Asp75 and Lys79 are fully conserved and constitute a proton donor–acceptor pair with potential for proton transport; indeed, this idea is supported by isosteric mutations that remove the charge from either residue (D75N or K79Q) and abolish transport (Fig. 2d and Extended Data Fig. 8a,c,d). The distance from Asp75 to Lys79 is below 3 Å in all structures, consistent with an unprotonated state for Asp75. However, activity of PIN8 in proteoliposomes is not sensitive to proton-motive force decouplers and has minimal pH dependence, suggesting that a proton-motive force is not obligatory for transport (Extended Data Figs. 2e and 9b). Furthermore, export rates in oocytes are also indifferent to external pH (Extended Data Fig. 9c).
Discussion
Plant growth and morphology are largely governed by polar auxin transport as mediated by canonical PINs. Comparison of all the PINs from A. thaliana with PIN8 studied here indicates that—with an exception of the unusual non-canonical PIN5—the auxin and support sites are perfectly conserved. This conservation, which also extends to other plant species, indicates that our observations can be generalized19(Extended Data Figs. 1, 2a and 8c,d). The low apparent affinity for IAA measured in proteoliposome assays is 5–500-fold lower than the physiological concentrations of auxin in plant tissues20 (0.1–10 µM). Although we cannot rule out experimental artifacts, this implies that distinct functions of A. thaliana PINs arise from differing localization, abundance and auto-inhibition properties rather than direct modulation of substrate affinity3. Some studies have suggested that ABCB transporters interact with PINs to generate selectivity in IAA transport21,22,23. Our work suggests that this interaction is not needed for activity in vitro, and is most probably not required in planta.
The PIN family is part of the BART superfamily, which includes the structurally characterized ASBT bile acid/sodium symporters from the BASS family18. Although PIN8 and ASBT adopt the same fold, ASBT assumes an inverted orientation and does not appear to dimerize (Extended Data Fig. 7). In addition, at least three other families of proteins adopt this same fold (DALI Z-score > 10), namely two Na+/H+ antiporter families (CPA1 and CPA2) and the HCO3−/Na+ symporter family24,25,26,27,28,29. Similar to the bile acid/sodium symporters, these other protein families all share negligible sequence homology with PINs. The HCO3−/Na+ symporters adopt the same membrane orientation as PIN8, whereas the Na+/H+ antiporters share the inverted orientation with the bile acid/sodium symporters, perhaps explaining why the structural link between PINs and these divergent protein families has not been noted previously (Extended Data Fig. 7).
These other protein families are all secondary active transporters that use sodium or protons to drive transport, and all are proposed to function using an elevator mechanism in which the scaffold domain is fixed and the transporter domain pivots about the conserved proline crossover motif. Notably, the site occupied by the driving sodium and protons in these families is located at the same position as the support site in PINs (Extended Data Fig. 7), and it is clear from this work that PIN8 uses the same general proline crossover-based elevator mechanism (Fig. 3a and Supplementary Video 1).
Our data show that the negative charge of the IAA is sufficient for transport (Extended Data Figs. 2e and 9). However, the basic architecture of a support site is present that would allow for ion binding, as well as a conserved and functionally essential Asp75–Lys79 pair that could mediate proton translocation30. Most mutations of the support site completely abrogate activity, underlining the essential nature of this region, but neither oocyte nor SSM electrophysiology assays suggest dependence on counter-transport of either sodium or protons to drive auxin export. Our data thus support a uniport mechanism for PINs, although we cannot definitely rule out proton antiport in vivo.
On the basis of the data available, we propose the following model for auxin transport by PINs (Fig. 4): The inward-facing conformation allows an ionized auxin molecule to enter the binding site between transport and scaffold domains. The negatively charged carboxylate group is stabilized by the positive dipole of M4b and M9b, while being held in place by Asn117 and interacting with the support site through Gln145. The carbon backbone and indole ring are recognized by the four hydrophobic residues from the two crossover motifs of the scaffold domain. During transition to the outward-facing conformation, the proline crossover rotates 20° and the auxin binding site in the scaffold domain is translated away from the cytosol by 5 Å. Release of IAA in the outward-facing state is facilitated by a pH shift that protonates and neutralizes the carboxylate. After substrate release, the protein reverts back to the inward-open state.
In the inward-open conformation (left), IAA enters the binding site with a deprotonated carboxylate. The positive dipole of the M4b and M9b helices helps diffuse the charge. During rotation of the transporter domain, the binding site moves 5 Å towards the non-cytosolic side (second from left). At the non-cytosolic side, IAA is released, probably assisted by a protonation event (second from right). The support site could also become protonated before reverting to the inward-facing conformation (right), but our data indicate that this is not obligatory for function.
It has been suggested that the oligomeric state of PINs might have a role in regulation, but the large dimer-interaction surface in PIN8 argues against a dynamic equlibrium31,32. Nevertheless, it is conceivable that the monomers operate independently and also that PINs could form hetero-oligomers33.
We have not directly addressed auto-inhibition by the cytosolic loop in canonical PINs, but the connection to other known protein families provides some hints: For HCO3−/Na+ symporters, it has been shown that a loop from a cytosolic regulatory partner locks the protein in an inward conformation by interacting with the binding site24,29. By analogy, it seems plausible that the auto-inhibitory loop in canonical PINs operates by a similar mechanism.
In conclusion, we have presented in vitro biochemical characterization of a PIN as well as structures representing two key conformational states in the presence and absence of auxin and the herbicide NPA. The structure with NPA demonstrates competitive inhibition in PIN proteins, and could provide the basis for structure-based development of novel herbicides. We describe the molecular mechanism of auxin transport by PINs that can function independently of monovalent ions or protons, thus expanding our understanding of the crossover elevator mechanism used by proteins from diverse protein superfamilies from all kingdoms of life. This work provides a comprehensive foundation for future studies aiming to elucidate PIN function in polar auxin transport, which is essential for plant growth and development.
Methods
Protein purification
A. thaliana protein sequences used in this study are publicly available at Uniprot (https://www.uniprot.org/) with the following accession codes. PIN1: Q9C6B8, PIN2: Q9LU77, PIN3: Q9S7Z8, PIN4: Q8RWZ6, PIN5: Q9FFD0, PIN6: Q9SQH6, PIN7: Q940Y5 and PIN8: Q9LFP6.
PIN genes were cloned into an S. cerevisiae overexpression plasmid based on p423_GAL1 and tested for expression and purification properties. The A. thaliana PIN8 gene (Uniprot: Q9LFP6) was selected and put in frame with a tobacco etch virus (TEV) protease cleavage site and a deca-histidine affinity tag. This construct was used as the template for site-directed mutagenesis using the Quickchange commercial protocol (Agilent) for all point mutants.
Transformed S. cerevisiae strain DSY-5 were grown in 5 l shaking flasks or culture vessels, grown to high cell density and collected after 22 h induction with galactose34. Collected cells were washed three times in water and re-suspended in buffer A (0.1 M Tris pH 7.5, 0.6 M NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 1.2 mM phenylmethylsulphonyl fluoride). Cells were lysed by bead beating and lysate was clarified by centrifugation at 5,000g for 20 min. Membrane fractions were pelleted by ultracentrifugation at 200,000g for 2 h and re-suspended in buffer B (0.05 M Tris pH 7.5, 0.5 M NaCl, 20% glycerol) before being frozen in liquid nitrogen.
For protein purification, 3–4 g of membrane was thawed and solubilized for 45 min in a total volume of 50 ml of buffer C (0.05 M Tris pH 7.5, 0.5 M NaCl, 10% glycerol) supplemented with 1% n-dodecyl-β-d-maltoside (DDM) and 0.1% cholesterol hemisuccinate (CHS). Insoluble material was discarded by centrifugation at 17,000g for 30 min following by filtration using a 1.2 µm filter. 20 mM imidazole pH 7.5 was added and the sample loaded on a 1 ml nickel-nitrilotriacetic (Ni-NTA) column. A two-step wash was performed with buffer D (buffer A with 20 mM imidazole, 0.1% DDM, 0.01% CHS) and buffer E (buffer A with 70 mM imidazole, 0.05% DDM, 0.005% CHS).
For SSM electrophysiology assays, the sample was eluted with buffer F (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.05% DDM, 0.005% CHS, 500 mM imidazole). The eluate was incubated with TEV protease and dialysed against buffer F supplemented with 0.5 mM EDTA and 0.5 mM tris(2-carboxyethyl)phosphine (TCEP) overnight. The sample was then filtered and re-run on a Ni-NTA column to adsorb the His-tagged proteins consisting of TEV protease, cleaved tag and uncleaved tagged protein. The flow-through fraction, containing tag-free PIN8, was concentrated on a 100 kDa cut-off centricon (Vivaspin) and polished by SEC on a Biorad650 or Superdex200 10/300 column pre-equilibrated with buffer G optimized by a thermostability assay35 (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.05% DDM, 0.005% CHS, 0.5 mM EDTA).
For cryo-EM, peptidisc sample preparation followed general protocols36,37. In brief, after the two-step wash, proteins were re-lipidated using buffer I (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.03% DDM, 0.003% CHS, 0.06 mg ml−1 soybean extract polar lipids (Avanti)). Prior to starting the on-bead peptidisc reconstitution, the column was washed with buffer J (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.008% DDM, 0.0008% CHS). Peptidisc reconstitution was initiated by washing the column with detergent-free buffer K (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol) containing 1 mg ml−1 peptidisc (Genscript). An additional washing step with buffer K was performed to eliminate residual free peptidisc prior to elution using buffer K supplemented with 500 mM imidazole. After this the sample was incubated with TEV protease and dialysed against buffer K supplemented with 0.5 mM EDTA and 0.5 mM TCEP.
For the cryo-EM detergent sample, immediately after the re-lipidation step with buffer I, the DDM detergent was exchanged to lauryl maltose neopentyl glycol (LMNG) using buffer L (0.05 M Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.006% LMNG, 0.0006% CHS) prior to protein elution using buffer L supplemented with 500 mM imidazole. The sample was then incubated with TEV protease and dialysed against buffer L supplemented with 0.5 mM EDTA and 0.5 mM TCEP. After dialysis, cryo-EM sample purification continued identically to the SURFE2R sample protocol described in ‘SSM electrophysiology assays’, with the exception that the SEC buffer was replaced with buffer K (peptidisc sample) or buffer L (LMNG sample) without glycerol and supplemented with 0.5 mM EDTA.
SSM electrophysiology assays
For SSM electrophysiology, a SURFE2R N1 from Nanion Technologies was used. In brief, Soy Phospholipid Mixture (38% phosphatidylcholine, 30% phosphatidyl ethanolamine, 18% phosphatidyl inositol, 7% phosphatidic acid and 7% other soy lipids) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) were purchased from Avanti. Liposomes were prepared in Ringer solution without Ca2+ (115 mM NaCl, 2.5 mM KCl, 1 mM NaHCO3, 10 mM HEPES pH 7.4, 1 mM MgCl2) and homogenized using a Lipsofast (Avestin Inc) with a 400 nM pore size. Triton X-100 was added to the liposomes to a final concentration of 1% (v/v). Protein was added to liposomes to a calculated liposome:protein ratio (LPR) of 10:1. The detergent was removed using 400 mg ml−1 Bio Beads (BioRad) overnight at 4 °C in a rotary shaker. Proteoliposomes were frozen in liquid nitrogen and kept at −80 °C until use.
Sensor coating was performed as described38. Proteoliposomes were diluted 1:5 in Ringer solution without Ca2+, sonicated five times and then applied to the sensors by centrifugation (30 min, 3,000g, 4 °C). Non-activating buffer was Ringer solution without Ca2+ as described unless specified otherwise and activating buffer contained the substrate of interest. To substitute Na+, K+-Ringer without CaCl2 (117.5 mM KCl, 10 mM HEPES pH 7.4, 1 mM MgCl2) and to substitute K+, Na+-Ringer without CaCl2 (117.5 mM NaCl, 10 mM HEPES pH 7.4, 1 mM MgCl2) were used. Uncouplers: carbonyl cyanide m-chlorophenyl hydrazone (CCCP) in ethanol was used at 5 µM and 2,4-dinitrophenol (DNP) in ethanol was used at 100 µM. All other chemicals were purchased from Roth or Sigma. Each experiment was performed on at least two individual sensors. On each sensor each measurement consists of three technical replicates where the mean is calculated.
In most instances, we used a single solution exchange experiment. In this case proteoliposomes, immobilized on the supported membrane are kept in non-activating buffer as specified. At the beginning of the experiment non-activating buffer was exchanged for fresh identical non-activating buffer and after 1 s activating buffer (same buffer containing substrate) was added. After a further 1 s, buffer was again exchanged to non-activating buffer. Current response was recorded throughout the entire 3 s. For competition or inhibition, the respective compound was present in non-activating and activating solution.
Currents in response to substrate in the activating solutions are responses to electrogenic events which occur (1) when a charged molecule is crossing the membrane; (2) when a substrate, which does not necessarily have to be charged, binds to the protein and this binding leads to a conformational change by which charges become displaced in the membrane; (3) currents are shielded or neutralized by the substrate; and (iv) any combination of these possibilities. The peak current in response to substrate application was used to describe the properties of the proteins.
To describe the current response to different substrate concentrations a Michaelis–Menten curve was fit. We use Km throughout the manuscript, but since the peak current is a mixture of binding and transport signal (that is, pre-steady state and steady state currents), this parameter can also be more appropriately described as EC50. A Km derived from a biophysical assay will be specific to that experimental setup, and comparison to other types of assay or a physiological condition should be done cautiously. In the case of competitive studies, we explicitly use Kd or Ki, since in these instances the parameters were specifically determined. GraphPad Prism V 9.3 was used for statistical analyses.
Cryo-EM sample preparation
Peak fractions of freshly purified PIN8 were concentrated to 4–10 mg ml−1. C-flat Holey Carbon grids (CF-1.2/1.3, Cu-300 mesh) were glow-discharged for 45 s at 15 mA in a GloQube Plus (Quorum). A drop of 4 µl of sample was applied to the non-carbon side of the grids, and blotted with a Vitrobot Mark IV (ThermoFisher Scientific) operating at 4 °C and 100% humidity and using blot time of 4 s, before plunge-freezing into liquid ethane. The substrate-bound states were obtained by incubating the sample with 15 mM of IAA sodium salt or 2 mM of NPA for 2 h prior to grid freezing.
Image collection and data processing
A Titan Krios G3i microscope (ThermoFisher Scientific) operating at 300 kV and equipped with a BioQuantum Imaging Filter (energy slit width of 20 eV) with a K3 detector (Gatan) was used to collect the movies. The datasets containing the peptidisc samples, were acquired using automated acquisition EPU v2.11.1.11 at nominal 130,000 magnification corresponding to a physical pixel size 0.647 Å. For all datasets, the movies were saved in super-resolution pixel size and binned 2× in EPU back to the nominal pixel size.
On-the-fly gain normalized exposures were imported into cryoSPARC (v3.2.0)39 and processed in streaming mode for patch motion correction, patch contrast transfer function (CTF) estimation, particle picking and extraction. After several rounds of particle cleaning, an initial preliminary volume map was used to create templates for template picking. From a full dataset of apo-PIN8 with 7,900 movies, template picking provided a total of 2,082,448 particles. After two rounds of 2D classification, the best representative classes were selected manually. These particles served as an input for ab initio model reconstruction. After three rounds of particle sorting by heterogenous refinement using the ab initio 3D template, the remaining 327,193 particles were used for non-uniform refinement with C2 symmetry imposed and resulted in a global 2.9 Å resolution map. In parallel a C1 symmetry refinement job was performed but showed no differences between the two monomers.
To ensure the method of membrane protein stabilization did not influence oligomeric state and overall structure we solved apo PIN8 both in peptidisc (2.9 Å) and in the detergent LMNG (3.3 Å). The respective maps reveal no variation in conformation and we focus on the peptidisc-derived map given its higher resolution. There was also no evidence of monomers or higher oligomeric states in any of the grids screened.
The processing pipeline for the ligand-bound PIN8 was identical to the one from apo-PIN8. In brief, the entire IAA–PIN8 dataset comprised of 15,771 movies and template picking yielded a total of 2,639,895 particles. After several rounds of 2D classification and heterogenous refinement to obtain a final 200,061 particles, a non-uniform refinement with C2 symmetry imposition resulted in a global 3.2 Å resolution map. A full dataset of NPA–PIN8 comprised of 14,500 movies and template picking yielded a total of 3,345,146 particles. After several rounds of 2D classification and heterogenous refinement to obtain a final 77,608 particles, a non-uniform refinement with C2 symmetry imposition resulted in a global 3.4 Å resolution map. As for the apo form, a parallel C1 refinement was performed with no differences evident between the two monomers. Local resolution estimation was performed using cryoSPARC.
Model building and refinement
A PIN8 model prediction was calculated using the RoseTTAFold server40 and docked into the PIN8 map in Chimera41. Two molecules of PIN8 could be readily fitted into the map. The flexible cytoplasmic loop of PIN8 (residues 165 to 205) is not visible in the maps and was excluded from model building in Coot42. The final models include residues 1–164 and 206–367 (of 367 residues total). The initial PIN8 dimer model was analysed by molecular dynamics-based geometry fitting to the map using MDFF43 through Namdinator v2.0 (ref. 44). Models could be further improved by iterative manual model building in Coot combined with real-space refinement using Phenix, initially with an Amber force-field molecular dynamic refinement45. The coordination of lipids and the ligand IAA was prepared using ligand builder eLBOW46. In all electron microscopy maps, although the lipid belt surrounding the PIN8 dimer is visible, the electron density only allowed for the tentative modelling of two phosphatidylcholine molecules for ligand-bound PIN8 and four molecules for apo-PIN8. Geometry was validated in MolProbity v4.2 including CaBLAM and Ramachandran-Z analysis47,48,49 (Rama-Z). Figures were prepared using PyMOL Molecular Graphics System v1.5.0.4 (Schrödinger). Conservation of residues across species was analysed using ConSurf50. Sequence alignments were constructed with PROMALS3D51. Alignments were visualized using ALINE v1.0.02552. Structural similarity to other protein families were identified using DALI53. Phylogenetic analysis was made using NGPhylogeny.fr54. In brief, MAFFT was used for multiple sequence alignment (MSA), BMGE was used for MSA pruning and FastME was used for unrooted tree generation. Bootstrap values were calculated from 500 trials.
Oocyte efflux assays
Oocyte efflux experiments were carried out as described55. In brief, oocytes were injected with 150 ng transporter cRNA without or with 75 ng kinase cRNA. 3H-IAA (25 Ci mmol−1) was purchased from ARC or RC Tritec. Oocytes were injected with IAA to reach an internal IAA concentration of 1 µM, corresponding to 100%. Residual radioactivity was determined for each individual oocyte by liquid scintillation counting after the time points indicated and are expressed relative to the initial 100%. Each time point represents the mean and s.e.m. of ten oocytes. To calculate the relative transport rate in per cent per minute, linear regression was performed. Each data point in Fig. 1a and Extended Data Fig. 9c represents the transport rate of one biological replicate using oocytes collected from different X. laevis females. GraphPad Prism V 9.3 was used for statistical analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Atomic models have been deposited in the Protein Data Bank (PDB) and cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under the following accession numbers. Apo outward state in peptidisc: PDB 7QP9 and EMDB EMD-14115, IAA-bound outward state in peptidisc: PDB 7QPA and EMDB EMD-14116, NPA-bound inward state in peptidisc: PDB 7QPC and EMDB EMD-14117, and apo outward state in detergent: EMDB EMD-14118. Source data are provided with this paper.
References
Weijers, D. & Wagner, D. Transcriptional responses to the auxin hormone. Annu. Rev. Plant Biol. 67, 539–574 (2016).
Goldsmith, M. H. M. The polar transport of auxin. Annu. Rev. Plant Physiol. 28, 439–478 (1977).
Hammes, U. Z., Murphy, A. S. & Schwechheimer, C. Auxin transporters—a biochemical view. Cold Spring Harb. Perspect. Biol. 14, a039875 (2021).
Gälweiler, L. et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230 (1998).
Chen, R. et al. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl Acad. Sci. USA 95, 15112–15117 (1998).
Luschnig, C., Gaxiola, R. A., Grisafi, P. & Fink, G. R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998).
Petrásek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006).
Raven, J. A. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 74, 163–172 (1975).
Rubery, P. H. & Sheldrake, A. R. Carrier-mediated auxin transport. Planta 118, 101–121 (1974).
Okada, K., Ueda, J., Komaki, M. K., Bell, C. J. & Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684 (1991).
Chen, J. S. et al. Phylogenetic characterization of transport protein superfamilies: superiority of SuperfamilyTree programs over those based on multiple alignments. J. Mol. Microbiol. Biotechnol. 21, 83–96 (2011).
Mansour, N. M., Sawhney, M., Tamang, D. G., Vogl, C. & Saier Jr, M. H. The bile/arsenite/riboflavin transporter (BART) superfamily. FEBS J. 274, 612–629 (2007).
Ding, Z. et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 3, 941 (2012).
Ditengou, F. A. et al. Characterization of auxin transporter PIN6 plasma membrane targeting reveals a function for PIN6 in plant bolting. New Phytol. 217, 1610–1624 (2018).
Mravec, J. et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136–1140 (2009).
Zourelidou, M. et al. Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. eLife 3, e02860 (2014).
Duran, A. M. & Meiler, J. Inverted topologies in membrane proteins: a mini-review. Comput. Struct. Biotechnol. J. 8, e201308004 (2013).
Hu, N.-J., Iwata, S., Cameron, A. D. & Drew, D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 478, 408–411 (2011).
Bennett, T. et al. Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol. Biol. Evol. 31, 2042–2060 (2014).
Petersson, S. V. et al. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21, 1659–1668 (2009).
Bandyopadhyay, A. et al. Interactions of PIN and PGP auxin transport mechanisms. Biochem. Soc. Trans. 35, 137–141 (2007).
Deslauriers, S. D. & Spalding, E. P. Electrophysiological study of Arabidopsis ABCB4 and PIN2 auxin transporters: evidence of auxin activation and interaction enhancing auxin selectivity. Plant Direct 5, e361 (2021).
Noh, B., Murphy, A. S. & Spalding, E. P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13, 2441–2454 (2001).
Fang, S. et al. Molecular mechanism underlying transport and allosteric inhibition of bicarbonate transporter SbtA. Proc. Natl Acad. Sci. USA 118, e2101632118 (2021).
Hunte, C. et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435, 1197–1202 (2005).
Lee, C. et al. A two-domain elevator mechanism for sodium/proton antiport. Nature 501, 573–577 (2013).
Paulino, C., Wöhlert, D., Kapotova, E., Yildiz, Ö. & Kühlbrandt, W. Structure and transport mechanism of the sodium/proton antiporter MjNhaP1. eLife 3, e03583 (2014).
Wöhlert, D., Kühlbrandt, W. & Yildiz, O. Structure and substrate ion binding in the sodium/proton antiporter PaNhaP. eLife 3, e03579 (2014).
Liu, X.-Y. et al. Structures of cyanobacterial bicarbonate transporter SbtA and its complex with PII-like SbtB. Cell Discov. 7, 63 (2021).
Buch-Pedersen, M. J., Pedersen, B. P., Veierskov, B., Nissen, P. & Palmgren, M. G. Protons and how they are transported by proton pumps. Pflugers Arch. 457, 573–579 (2009).
Abas, L. et al. Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc. Natl Acad. Sci. USA 118, e2020857118 (2021).
Teale, W. D. et al. Flavonol-mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO J. 40, e104416 (2021).
Pařízková, B., Pernisová, M. & Novák, O. What has been seen cannot be unseen—detecting auxin in vivo. Int. J. Mol. Sci. 18, E2736 (2017).
Lyons, J. A., Shahsavar, A., Paulsen, P. A., Pedersen, B. P. & Nissen, P. Expression strategies for structural studies of eukaryotic membrane proteins. Curr. Opin. Struct. Biol. 38, 137–144 (2016).
Tomasiak, T. M. et al. General qPCR and plate reader methods for rapid optimization of membrane protein purification and crystallization using thermostability assays. Curr. Protoc. Protein Sci. 77, 29.11.1–29.11.14 (2014).
Carlson, M. L. et al. The Peptidisc, a simple method for stabilizing membrane proteins in detergent-free solution. eLife 7, e34085 (2018).
Ung, K. L., Alsarraf, H., Kremer, L. & Blaise, M. MmpL3, the trehalose monomycolate transporter, is stable in solution in several detergents and can be reconstituted into peptidiscs. Protein Express. Purif. 191, 106014 (2022).
Bazzone, A. & Barthmes, M. Functional characterization of SLC transporters using solid supported membranes. Methods Mol. Biol. 2168, 73–103 (2020).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Trabuco, L. G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Kidmose, R. T. et al. Namdinator—automatic molecular dynamics flexible fitting of structural models into cryo-EM and crystallography experimental maps. IUCrJ 6, 526–531 (2019).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D 65, 1074–1080 (2009).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Prisant, M. G., Williams, C. J., Chen, V. B., Richardson, J. S. & Richardson, D. C. New tools in MolProbity validation: CaBLAM for CryoEM backbone, UnDowser to rethink ‘waters,’ and NGL Viewer to recapture online 3D graphics. Protein Sci. 29, 315–329 (2020).
Sobolev, O. V. et al. A global Ramachandran score identifies protein structures with unlikely stereochemistry. Structure 28, 1249–1258.e2 (2020).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Pei, J., Kim, B.-H. & Grishin, N. V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 (2008).
Bond, C. S. & Schüttelkopf, A. W. ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr. D 65, 510–512 (2009).
Holm, L. DALI and the persistence of protein shape. Protein Sci. 29, 128–140 (2020).
Lemoine, F. et al. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res. 47, W260–W265 (2019).
Fastner, A., Absmanner, B. & Hammes, U. Z. Use of Xenopus laevis oocytes to study auxin transport. Methods Mol. Biol. 1497, 259–270 (2017).
Acknowledgements
We acknowledge the EMBION Cryo-EM Facility at iNANO, Aarhus University, for time under application ID 0137, where all data was collected with the assistance of A. Bøggild, J. Lykkegaard Karlsen and T. Boesen. We also thank eBIC (proposal BI27980) for data collection on the detergent PIN8 sample. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101000936) to B.P.P. U.Z.H. is funded by the Deutsche Forschungsgemeinschaft (HA3468/6-1 and HA3468/6-3) and SFB924. D.L.S. is funded by the National Institutes of Health (R35 GM144109).
Author information
Authors and Affiliations
Contributions
Sample preparation: K.L.U. and M.W. Electron microscopy data collection and analysis: K.L.U., M.W., E.D., D.L.S. and B.P.P. Activity assays: D.P.J., L.S., M.K. and U.Z.H. Manuscript preparation: K.L.U., M.W., D.L.S., U.Z.H. and B.P.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Dolf Weijers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Multiple sequence alignment A. thaliana PINs.
Alignment between AtPIN1–8 with the following UniProt accession numbers. AtPIN1: Q9C6B8, AtPIN2: Q9LU77, AtPIN3: Q9S7Z8, AtPIN4: Q8RWZ6, AtPIN5: Q9FFD0, AtPIN6: Q9SQH6, AtPIN7: Q940Y5, AtPIN8: Q9LFP6. Conserved residues are highlighted with gray-scale, where black is perfectly conserved. Colored tubes represent α-helices found in the scaffold domain (blue) and transporter domain (green). Key residues are numbered above the α-helix markings. Residues highlighted participate in IAA carboxylate recognition (orange) or IAA hydrophobic recognition (yellow), are part of the support site (blue) or form the central prolines of the crossover motif (pink).
Extended Data Fig. 2 Functional data on PIN8.
A) Dendrogram of the relationship between Arabidopsis thaliana PIN1–8. Numbers denote bootstrap values of 500 trials. PIN8 is in a clade with the canonical PIN3, PIN4, and PIN7, unlike other non-canonical PINs (PIN5, PIN6). B) Figure time course of IAA export by PIN8 from oocytes. Relative IAA content of oocytes expressing PIN8 in the presence (■□) or absence (●) of 10 µM NPA internally determined at the time indicated after substrate injection. Initial internal IAA concentration was 1 µM. n = 10 oocytes at each time point. Data points are mean ± SE. C) Raw current traces from SSM-electrophysiology for the PIN8 WT proteoliposomes. D) Relative inhibition of the peak binding current induced by 100 µM NPA in the presence of the indicated IAA concentration in non-activating as well as activating buffer. Half-maximal inhibition 39.9 ± 14.9 µM (mean ± SE, n = 3) corresponds to apparent Kd(IAA). E) Peak currents elicited by 500 µM IAA at the pH indicated (n = 3). Bars are mean ± SE. The points represent individual measurements. F) Substrate specificity of PIN8 measured at pH 7.4. Peak currents elicited by IAA (●) or a range of putative substrates tested at 100 µM (▼). Synthetic auxins: 5-fluoro-IAA, 2,4-D, NAA, TIBA, 1-NOA, 4-CPA, CVX. Endogenous auxins: PAA, IAA-Ala, IBA, Methyl-IAA. Others: Serotonin, Zeatin (a cytokinin), BA (benzoic acid). Chemical structures at pH 7.4 are shown. Current response of substrates indicated with asterisks differed significantly from IAA, indicating that they are likely not substrates for the transporter, but we note that different chemical molecules have different electrostatic potentials and this can also have an influence on the observed current (5-Fluoro IAA p = 0.011; 2,4-D p = 0.272; NAA p = 0.999; TIBA p = 0.989; 1-NOA p = 0.539; PAA p = 0.0007, 4-CPA p < 0.0001; CVX p < 0.0001; IAA-Ala p < 0.0001; IBA p < 0.0001; Methyl-IAA p < 0.0001; Serotonin p < 0.0001; Zeatin p < 0.0001; BA p < 0.0001; IAA+NPPB p < 0.0001; IAA+NPA p < 0.0001). Substrates shown in dark grey are uncharged. Two inhibitors were tested in the presence of 100 µM IAA. Bars are mean ± SE; The data points represent individual measurements. (n = 8: IAA; n = 5: 5-Fluoro IAA, 2,4-D, NAA, TIBA, 4-CPA, IAA-Ala, IBA, Serotonin, Zeatin, BA; n = 3: 1-NOA, PAA, CVX, Methyl-IAA, IAA+NPPB, IAA+NPA)
Extended Data Fig. 3 Image processing and reconstruction for apo-PIN8.
Workflow of image processing and 3D reconstruction in cryoSPARC, including a motion corrected micrograph from Titan Krios microscope using a K3 detector, 2D classes and sharpened density map from the final non-linear refinement colored by local resolution. Corrected curve of the global Fourier shell correlation (FSC) indicates 2.89 Å based on the 0.143 gold-standard criterion. The cryo-EM experiment with this sample was repeated 7 times with data collection 1 time.
Extended Data Fig. 4 Image processing and reconstruction for IAA-PIN8.
Workflow of image processing and 3D reconstruction in cryoSPARC, including a motion corrected micrograph from Titan Krios microscope using a K3 detector, 2D classes and sharpened density map from the final non-linear refinement colored by local resolution. Corrected curve of the global FSC indicates 3.18 Å based on the 0.143 gold-standard criterion. The cryo-EM experiment with this sample was repeated 6 times with data collection 1 time.
Extended Data Fig. 5 Image processing and reconstruction for NPA-PIN8.
Workflow of image processing and 3D reconstruction in cryoSPARC, including a motion corrected micrograph from Titan Krios microscope using a K3 detector, 2D classes and sharpened density map from the final non-linear refinement colored by local resolution. Corrected curve of the global FSC indicates 3.44 Å based on the 0.143 gold-standard criterion. The cryo-EM experiment with this sample was repeated 2 times with data collection 1 time.
Extended Data Fig. 6 Domains and lipids in PIN8.
A) Overview of the transporter (green) and scaffold (blue) domain in the monomer of PIN8. B) Position of lipid modeled as phosphatidylcholine in PIN8. One lipid is located in the groove between the two monomers, the other is located at the transporter domain with one aliphatic chain sticking into a cavity of the protein next to the support site. This links the support site to the lipid environment. An unidentified density was found in maps in a cavity towards to the cytosolic side. Mutating T288A did not affect activity (Fig. 2d and Extended Data Fig. 9a). C) Peak currents elicited by the indicated IAA concentrations in liposomes consisting of soy lipid mix (●) or 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (▼). The current response did not differ between the liposomes at any concentration (two-sided unpaired t-test, 100 μM IAA p = 0.61, 500 μM IAA p = 0.07, 1000 μM p = 0.17). This supports that PIN8 is not dependent on specific lipids for activity. Bars are mean ± SE. Data points are independent experiments. (n = 6: soy lipid mix 100 μM and 500 μM; n = 3 all other conditions)
Extended Data Fig. 7 Topology of transporters with a crossover elevator mechanism.
Shown are the topologies of auxin transporters (PIN), bicarbonate/sodium symporters (SBTA), Na/H antiporters (NAPA) and bile acid/sodium symporters (ASBT). NAPA and ASBT display an inverted topology compared to PINs and SBTA. All four families have a crossover with a substrate binding site to one side and a putative support site to the other side.
Extended Data Fig. 8 Details of mutants and support site.
A) Transport current using SSM-electrophysiology on PIN8 mutants in proteoliposomes. Transport can be described by Michaelis-Menten kinetics. Data points are mean or mean ± SE (n > 2)(WT n = 4 different liposome preparations, for mutants n = 5 (T288A), n = 4 (Q320A), n = 3 (I51Y, I120Y, Y150A), n = 2 (Y150F, S146A, V328Y). B) Sensitivity of WT and selected mutants to NPA inhibition. Peak current response to 2 mM IAA or 2 mM IAA and 20 µM NPA presented in non-activating as well as activating buffer. Asterisks indicate significant differences between groups (two-sided paired t-test, WT p = 0.0131, I51Y p = 0.48, N117A p = 0.07, I120Y p = 0.03, Y150F p = 0.02, V328Y p = 0.01, Y150A p = 0.22). Data points are mean ± SE; data points are individual experiments (n = 4 (V328Y), n = 3 (all other mutants and WT)). C) View from the non-cytosolic side of the side chains interacting with IAA and forming the support site. Residues are colored by sequence conservation using ConSurf. 318 unique sequences from plants with sequence identity of 35–95% to AtPIN8 were identified, sorted by E-value and 150 selected at equal intervals for the alignment. D) View from the non-cytosolic side of the side chains interacting with NPA and forming the support site. Residues are colored by sequence conservation using ConSurf. E) Map density for the peaks found in the support site modeled as water. In the case of apo-PIN two peaks could be modeled as water with one having stronger density than the other
Extended Data Fig. 9 Activity assays support PIN8 is independent of ions, pH and lipids.
A) Peak currents elicited by 100 µM IAA in Na+-free K+ buffer or K+-free Na+ buffer. The current response was independent of the principal cation. Bars are mean ± SE; n = 3. The points represent individual measurements. Means were compared by a two-tailed unpaired t-test (p = 0.62). B) Peak currents elicited by 100 µM IAA, and with proton-motive force decouplers CCCP and DNP present. The current responses were similar in all cases. Bars are mean ± SE; n = 3. The points represent individual measurements. No difference (p = 0.97) between groups was found by one-way ANOVA multiple comparisons, followed by Tukey’s Post Hoc test (IAA vs. IAA+CCCP p > 0.99, IAA vs. IAA+DNP p = 0.98, IAA+CCCP vs. IAA+DNP p = 0.98). C) Oocyte export assay using 3H-IAA and PIN1 plus kinase PID as a control. PIN8 transport rate is unchanged at two different external pH values; n = 2. Data points are biologically independent experiments. The mean is indicated
Supplementary information
Video 1
Morph between inward-facing and outward-facing conformations of PIN8.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ung, K.L., Winkler, M., Schulz, L. et al. Structures and mechanism of the plant PIN-FORMED auxin transporter. Nature 609, 605–610 (2022). https://doi.org/10.1038/s41586-022-04883-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04883-y
This article is cited by
-
Mapping the membrane orientation of auxin homeostasis regulators PIN5 and PIN8 in Arabidopsis thaliana root cells reveals their divergent topology
Plant Methods (2024)
-
Control of leaf development in the water fern Ceratopteris richardii by the auxin efflux transporter CrPINMa in the CRISPR/Cas9 analysis
BMC Plant Biology (2024)
-
3,4-Dichlorophenylacetic acid acts as an auxin analog and induces beneficial effects in various crops
Communications Biology (2024)
-
Structures and mechanisms of the Arabidopsis cytokinin transporter AZG1
Nature Plants (2024)
-
Parallel auxin transport via PINs and plasmodesmata during the Arabidopsis leaf hyponasty response
Plant Cell Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.