Abstract
OTULIN (OTU deubiquitinase with linear linkage specificity) removes linear polyubiquitin from proteins that have been modified by LUBAC (linear ubiquitin chain assembly complex) and is critical for preventing auto-inflammatory disease1,2 and embryonic lethality during mouse development3. Here we show that OTULIN promotes rather than counteracts LUBAC activity by preventing its auto-ubiquitination with linear polyubiquitin. Thus, knock-in mice that express catalytically inactive OTULIN, either constitutively or selectively in endothelial cells, resembled LUBAC-deficient mice4 and died midgestation as a result of cell death mediated by TNFR1 (tumour necrosis factor receptor 1) and the kinase activity of RIPK1 (receptor-interacting protein kinase 1). Inactivation of OTULIN in adult mice also caused pro-inflammatory cell death. Accordingly, embryonic lethality and adult auto-inflammation were prevented by the combined loss of cell death mediators: caspase 8 for apoptosis and RIPK3 for necroptosis. Unexpectedly, OTULIN mutant mice that lacked caspase 8 and RIPK3 died in the perinatal period, exhibiting enhanced production of type I interferon that was dependent on RIPK1. Collectively, our results indicate that OTULIN and LUBAC function in a linear pathway, and highlight a previously unrecognized interaction between linear ubiquitination, regulators of cell death, and induction of type I interferon.
Similar content being viewed by others
Main
Mouse embryos carrying the OTULIN(W96R) or OTULIN(D336E) mutations have vascularization defects that have been attributed to impaired Wnt signalling3, whereas OTULIN loss in mouse haematopoietic cells or homozygous hypomorphic OTULIN mutations in humans cause severe inflammation that has been attributed to enhanced NF-κB-dependent gene transcription1,2. It has been proposed that OTULIN counteracts LUBAC activity within TNFR1, NOD2 (nucleotide binding oligomerization domain containing 2), and Wnt signalling complexes3,5,6,7,8; however, even though LUBAC and OTULIN form a stable complex3,5,7,9, only LUBAC is recruited to TNFR1 and NOD2 signalling complexes10,11. Therefore, the key substrates of OTULIN and where OTULIN acts to limit inflammation remain unclear.
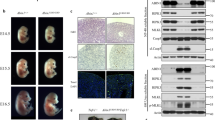
To determine the physiological function of OTULIN enzymatic activity, we analysed OtulinC129A/C129A knock-in mice expressing the active site cysteine mutant OTULIN(C129A) (Extended Data Fig. 1a). While OtulinC129A/+ mice were viable (Extended Data Fig. 1b), OtulinC129A/C129A embryos were resorbed after exhibiting abnormal yolk sac vasculature at embryonic day 10.5 (E10.5) (Fig. 1a, Extended Data Fig. 1c–e). Staining for cleaved caspase 3, a marker of apoptosis, or RIPK3 autophosphorylated on Thr231 and Ser232, a marker of necroptosis, revealed extensive cell death in both the yolk sac and placenta, with some dying cells having endothelial morphology (Fig. 1b, Extended Data Fig. 1f–i). Linear polyubiquitin was also increased in E10.5 OtulinC129A/C129A embryos (Extended Data Fig. 1j), consistent with OTULIN being the main deubiquitinase for linear polyubiquitin3,5.
a, E10.5 embryos (n = 15 Otulin+/+, n = 23 OtulinC129A/C129A). b, d, E10.5 yolk sacs stained for PECAM-1 (red) and cleaved caspase 3 (green) (n = 5 Otulin+/+, n = 9 OtulinC129A/C129A in b; n = 3 Cdh5-Cre+ OtuliniC129A/+, n = 4 Cdh5-Cre+ OtuliniC129A/iC129A in d). Scale bar, 50 μm. c, E11.5 embryos (n = 4 Cdh5-Cre+ OtuliniC129A/+, n = 3 Cdh5-Cre+ OtuliniC129A/iC129A). e, Body weight of tamoxifen-treated mice plotted as a percentage of initial body weight. Lines represent the mean. P values, repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. f, Livers from day 5 in e stained for cleaved caspase 3 (brown) (n = 5 per genotype). Scale bar, 100 μm.
Restricting expression of OTULIN(C129A) to endothelial cells and some haematopoietic cells using an inducible OtuliniC129A allele (Extended Data Fig. 1k) and a Cdh5-Cre transgene12 also caused embryonic lethality (Extended Data Fig. 1l). Disruption of the yolk sac vasculature at E11.5 (Fig. 1c, Extended Data Fig. 1m) was preceded by extensive apoptosis at E10.5 (Fig. 1d, Extended Data Fig. 1n), consistent with OTULIN activity promoting endothelial cell survival during embryogenesis.
Inactivation of OTULIN in adult mice using a tamoxifen-inducible Rosa26 (R26)-CreERT2 allele13 caused rapid weight loss (Fig. 1e). Neutrophils and pro-inflammatory cytokines and chemokines were elevated in the blood, and there was increased cell death and tissue degeneration in the bone marrow, thymus, liver, small intestine, and heart (Fig. 1f, Extended Data Fig. 2). Radiation-induced bone marrow chimaeras with tamoxifen-induced OTULIN(C129A) expression restricted to the haematopoietic compartment also exhibited weight loss, elevated pro-inflammatory cytokines and chemokines, and immune cell deregulation, but they lacked extensive cell death in the liver and small intestine (Extended Data Fig. 3). Therefore, OTULIN activity is important in non-haematopoietic as well as haematopoietic cells. Indeed, when R26-CreERT2 OtuliniC129A/iC129A mice reconstituted with wild-type bone marrow were treated with tamoxifen, they showed mild weight loss, mildly elevated cytokines, and degeneration in the liver and small intestine (Extended Data Fig. 4).
Similar to OtulinC129A/C129A embryos (Extended Data Fig. 1j), primary mouse embryo fibroblasts (MEFs) and immortalized haemato-poietic progenitor cells expressing OTULIN(C129A) contained abundant linear polyubiquitin (Extended Data Fig. 5a–c). This polyubiquitin was protein-conjugated because it was not cleaved by the deubiquitinase IsoT (Extended Data Fig. 5d). Mass spectrometry of the proteins modified with linear polyubiquitin in OtulinC129A/C129A cells identified the core LUBAC components HOIP, HOIL-1 and SHARPIN (Extended Data Fig. 5e), which is consistent with regulation of LUBAC auto-ubiquitination by OTULIN6,10.
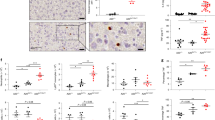
Enhanced linear ubiquitination of LUBAC in OtulinC129A/C129A MEFs coincided with a reduction in unmodified HOIP, HOIL-1 and SHARPIN (Fig. 2a). A similar reduction in HOIP and SHARPIN has been reported for OTULIN-deficient lymphocytes1. LUBAC disappearance required its E3 ligase activity because SHARPIN, HOIL-1 and HOIP were maintained in fibroblasts expressing catalytically inactive HOIP(C879S) and OTULIN(C129A) (Extended Data Fig. 5f, g). Note that OtulinC129A/C129A lysates treated with recombinant OTULIN or the promiscuous deubiquitinase USP2 still lacked LUBAC, despite successful elimination of the linear polyubiquitin signal (Extended Data Fig. 5h). Therefore, the drop in unmodified LUBAC did not simply reflect the retarded migration of increasingly ubiquitinated LUBAC. Both transcriptional and post-translational mechanisms may contribute to the decrease in LUBAC because Hoip (also known as Rnf31), Hoil-1 (also known as Rbck1) and Sharpin mRNAs were reduced slightly in OtulinC129A/C129A cells (Extended Data Fig. 5i–k), and proteasome inhibition caused a subtle increase in LUBAC components (Extended Data Fig. 5l). Similar results have been reported for OTULIN-deficient lymphocytes1.
a, b, d, e, Western blots of MEFs or their immunoprecipitates (IP). Treatments were for 5 min (d) or 4 h (e). Results are representative of two (a, e) or three (b, d) independent experiments. A FLAG-tagged TUBE (tandem ubiquitin binding entity) reagent was used to pull-down linear polyubiquitin. c, Graph indicates the percentage of MEFs that were viable and not stained by propidium iodide (PI) after overnight treatment. Bars represent the mean. Circles represent MEFs from individual mice. P values, one-way ANOVA followed by Tukey’s multiple comparison test.
OtulinC129A/C129A MEFs contained a small amount of phosphorylated IκBα, consistent with low-level, spontaneous activation of the NF-κB pathway (Fig. 2b, Extended Data Fig. 5m). However, NF-κB activation in response to TNF or lipopolysaccharide (LPS) was slightly muted in OtulinC129A/C129A MEFs (Fig. 2b, Extended Data Fig. 5m) and there was increased cell death (Fig. 2c, Extended Data Fig. 5n) with markers of both necroptosis (phospho-RIPK3 Thr231 and Ser232 and phospho-MLKL Ser345) and apoptosis (cleaved caspase 3) (Extended Data Fig. 5o). The RIPK1 inhibitor Nec-1 and/or the pan-caspase inhibitor Z-VAD-FMK (zVAD) reduced but did not completely block TNF-induced cell death (Fig. 2c). Notably, OtulinC129A/C129A MEFs were killed in a TNFR1-dependent manner by zVAD alone, with RIPK3 autophosphorylation and phospho-MLKL Ser345 indicating necroptosis (Extended Data Fig. 5n, o). Therefore, OtulinC129A/C129A MEFs were probably killed by autocrine TNF.
Collectively, these results imply that OTULIN inactivation destabilizes the TNFR1-associated complex I required for NF-κB activation and instead promotes the formation of cytosolic cell death signalling complexes. Indeed, TNF-induced complex I in OtulinC129A/C129A MEFs contained less HOIP and SHARPIN, less linear polyubiquitin, and less ubiquitinated RIPK1 than in Otulin+/+ MEFs (Fig. 2d). LUBAC was not detected after complex I was treated with USP2, consistent with impaired recruitment of LUBAC into complex I rather than a failure to detect ubiquitinated LUBAC (Extended Data Fig. 6a). These alterations coincided with enhanced formation of a cytosolic complex comprised of FADD, caspase 8, TRADD, phospho-RIPK1 Ser166 and Thr169, and phospho-RIPK3 Thr231 and Ser232 (Fig. 2e). This cytosolic complex was also more abundant in OtulinC129A/C129A MEFs treated with zVAD or TNF and zVAD. Similar results have been reported for cells deficient in LUBAC components4,14,15, suggesting that the main role of OTULIN is to maintain LUBAC function by suppressing its auto-ubiquitination.
To determine whether aberrant cell death caused embryonic lethality in OtulinC129A/C129A mice, as in HOIP-deficient mice4, we genetically ablated RIPK1 enzymatic activity (Ripk1D138N/D138N), TNFR1 (Tnfr1−/−), or RIPK3 (Ripk3−/−) and caspase 8 (Casp8−/−). Expression of catalytically inactive RIPK1 and loss of TNFR1 delayed embryonic lethality from around E10.5 to around E14.5 and E16.5, respectively (Extended Data Fig. 6b–e). Therefore, activation of RIPK1 by TNFR1 contributes to, but is not entirely responsible for, the death of OtulinC129A/C129A embryos. Neither RIPK3 deficiency nor Casp8 heterozygosity delayed lethality to E14.5 (Extended Data Fig. 6f–i), but the combined loss of caspase 8 and RIPK3 sustained OtulinC129A/C129A embryos to the perinatal period despite them having increased linear polyubiquitin (Fig. 3a–c, Extended Data Fig. 6j–m). These data imply that aberrant cell death rather than defective Wnt signalling3 triggers lethality in OtulinC129A/C129A embryos before E18.5 (Fig. 3d). OTULIN activity was also not required for the expression of Wnt target genes in intestinal organoid cultures (Extended Data Fig. 6n).
a, E10.5 embryos (n = 4 Otulin+/+ Ripk3−/− Casp8−/−, n = 7 OtulinC129A/C129A Ripk3−/− Casp8−/−). b, E10.5 yolk sacs stained for PECAM-1 (red) and cleaved caspase 3 (green) (n = 4 per genotype). Scale bar, 50 μm. c, E18.5 embryos (n = 26 Otulin+/+ Ripk3−/− Casp8−/−, n = 25 OtulinC129A/C129A Ripk3−/− Casp8−/−). d, Schematic showing when different genotypes die. e, Serum chemokines or skin Ifnb1 mRNA expression at E18.5. f–h, k, Tamoxifen-treated mice on day 5 (f–h) or 10 (k). Graphs indicate terminal body weight as a percentage of initial body weight (f), serum cytokines and chemokines (h, k) and flow cytometric analysis of whole blood and bone marrow (k). MFI, median fluorescent intensity. Liver sections were stained for cleaved caspase 3 (brown) (g) (n = 5 per genotype). Scale bar, 100 μm. i, Western blots of bone marrow-derived macrophages (BMDMs) (n = 3 per genotype). j, IFN-β secretion by BMDMs following 24 h treatment as shown on x-axis. Circles represent individual mice. Bars and lines represent means (e, f, h, j, k). P values, unpaired, two-tailed t-test (e, j) or one-way ANOVA followed by Tukey’s multiple comparison test (f, h, k). ND, not detected.
Some OtulinC129A/C129A Ripk3−/− Casp8−/− embryos were oedematous at E18.5 (Fig. 3c), and newborn pups (P0) were smaller than their littermates and often found dead (Extended Data Fig. 6m). Perinatal lethality has also been reported for Ripk3−/− Casp8−/− mice with hypomorphic LUBAC due to SHARPIN mutation16. OtulinC129A/C129A Ripk3−/− Casp8−/− mice had no major histological lesions at E18.5 or P0 (Extended Data Fig. 7a–c), but serum from these mice at E18.5 contained elevated amounts of the interferon (IFN)-inducible chemokines CXCL10 and CXCL9 (Fig. 3e, Extended Data Fig. 7d). Ifnb1 mRNA was elevated in skin from E18.5 OtulinC129A/C129A Ripk3−/− Casp8−/− mice (Fig. 3e), so we speculated that OTULIN might also suppress pathways producing type I IFN.
Aberrant cell death and TNF signalling also drove auto-inflammation in R26-CreERT2 OtuliniC129A/iC129A mice after tamoxifen treatment. TNF blockade or TNFR1 deficiency largely suppressed the previously observed weight loss, elevated serum cytokines and chemokines, loss of haematopoietic progenitor cells, and thymic atrophy in these mice. Small intestine and liver injury was also less severe (Extended Data Fig. 7e–m). The combined loss of RIPK3 and caspase 8 was even better at ameliorating these phenotypes (Fig. 3f–h, Extended Data Fig. 8) but, notably, led to elevation of Ly6Chigh blood monocytes (Extended Data Fig. 9a). In addition, although most pro-inflammatory cytokines, including G-CSF, TNF and IL-6, reverted to baseline levels in these mice (Fig. 3h, Extended Data Fig. 8b), the chemokines CXCL10 and CXCL9 remained elevated (Fig. 3h) and IFN-β trended upwards (Extended Data Fig. 8b). Therefore, auto-inflammation triggered by OTULIN inactivation stems largely from excessive cell death, but as seen during mouse development, blocking this death unveils an unexpected role for OTULIN in suppressing the production of chemokines linked to type I IFN.
Consistent with OTULIN modulating IFN induction, OtulinC129A/C129A Ripk3−/− Casp8−/− BMDMs showed spontaneous phosphorylation and activation of IKKε and TBK-1, which are the kinases required for type I IFN production17,18 (Fig. 3i). The cells also produced more IFN-β than control BMDMs after stimulation with the RIG-I ligand 5′ triphosphate double-stranded RNA (ppp-dsRNA) (Fig. 3j). CRISPR–Cas9-mediated deletion of Hoip in Otulin+/+ Ripk3−/− Casp8−/− BMDMs elicited a similar phenotype (Extended Data Fig. 9b, c), consistent with OTULIN and LUBAC19,20 functioning in a linear pathway to suppress the induction of type I IFN when intracellular dsRNA is sensed. Accordingly, when tamoxifen-treated R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/− and control mice were examined on day 10 rather than day 5, IFN-β, CXCL10, CXCL9, and Ly6Chigh monocytes remained elevated (Extended Data Fig. 9d, e). Moreover, bone marrow LSK (Lin−Sca-1+c-Kit+) cells were increased and expressed more Sca-1, which are known effects of type I IFNs21,22 (Extended Data Fig. 9f). Indeed, an IFNAR1 (IFN-α/β receptor 1)-blocking antibody reduced Ly6Chigh monocytes, bone marrow LSK cells, and chemokine levels (Fig. 3k, Extended Data Fig. 9g). Five weeks after tamoxifen treatment, R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/− mice had myeloid-driven splenomegaly, liver and gut inflammation, and more IFN-induced chemokines in their blood than R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/− mice (Extended Data Fig. 10a–c). Collectively, our results indicate that OTULIN enzymatic activity is critical for suppressing both cell death and type I IFN production.
RIPK1 has been implicated in the type I IFN response to intracellular dsRNAs23,24,25, so we investigated whether IFN signalling after OTULIN inactivation required RIPK1. CRISPR–Cas9-mediated deletion of Ripk1 in OtulinC129A/C129A Ripk3−/− Casp8−/− BMDMs reduced spontaneous activation of IKKε and TBK-1, and normalized IFN production induced by ppp-dsRNA (Fig. 4a, b). RIPK1 also contributed to the perinatal lethality of OtulinC129A/C129A Ripk3−/− Casp8−/− mice because some OtulinC129A/C129A Ripk3−/− Casp8−/− Ripk1+/− mice survived for up to 3 months, even though they were smaller than control littermates (Fig. 4c, Extended Data Fig. 10d, e). Notably, OtulinC129A/C129A Ripk3−/− Casp8−/− Ripk1−/− mice were not found and their cause of death remains unknown. This result was unexpected because Ripk3−/− Casp8−/− Ripk1−/− mice are viable26,27. Nonetheless, E18.5 OtulinC129A/C129A Ripk3−/− Casp8−/− Ripk1+/− embryos contained less CXCL9 and CXCL10 in their blood than OtulinC129A/C129A Ripk3−/− Casp8−/− embryos (Fig. 4d, Extended Data Fig. 10f). These data indicate that the combined absence of targeted linear ubiquitination, caspase 8 and RIPK3 causes detrimental RIPK1-dependent type I IFN induction. Therefore, OTULIN emerges as a key regulator of pathways that are critical for anti-viral defence by modulating cell death and type I IFN production (Extended Data Fig. 10g).
a, Western blots of BMDMs. Results are representative of four independent experiments. b, IFN-β secretion by BMDMs following 24 h treatment with ppp-dsRNA. c, Littermates aged 8 weeks (n = 5 OtulinC129A/C129A Ripk3−/− Casp8−/−Ripk1+/− mice (age 6–13 weeks) and control littermates). d, Serum chemokines at E18.5. Circles represent individual mice. Bars and lines represent means (b, d). P values, one-way ANOVA followed by Tukey’s multiple comparison test (b, d).
Methods
Mice
The Genentech institutional animal care and use committee responsible for ethical compliance approved all animal protocols. Ripk3−/− (ref. 28), Casp8−/− (ref. 29), Ripk1D138N/D138N (ref. 29), Tnfr1−/− (ref. 30), Ripk1−/− (ref. 29), Cdh5-Cre (ref. 12) and R26-CreERT2 (ref. 13) mice have been described previously. Taconic generated the OtuliniC129A/+ mice from C57BL/6 NTac embryonic stem (ES) cells. In brief, a loxP-flanked cassette containing an FRT-Neo-FRT selection marker, wild-type Otulin cDNA (exons 4–7) and 3′ UTR followed by a human growth hormone polyadenylation signal and an F3-Puro-F3 selection marker was inserted into the Otulin locus 5′ to a mutated exon 4 encoding the C129A exchange (TGT to GCA). The Neo and Puro cassettes were excised by breeding to a Flp-deleter strain (Taconic).
OtulinC129A/+ and HoipiC879S/+ mice were generated at Genentech using C57BL/6N ES cells. In brief, to generate OtulinC129A/+ mice, TGT was changed to GCT in exon 4. An FRT-flanked Neo cassette placed upstream of exon 4 was excised in ES cells using Flp, leaving a single FRT 121 bp upstream of the modified exon 4. The HoipiC879S/+ allele was obtained by inserting a cassette containing a loxP, wild-type Hoip cDNA (exons 16–21), a human growth hormone 3′ UTR followed by a 4× polyadenylation signal, an FRT-Neo-FRT selection marker and a second loxP, into the Hoip locus 57 bp 5′ to a mutated exon 16 encoding the C879S (TGC to AGC) exchange. The Neo cassette was excised in ES cells using Flp. All alleles were maintained on a C57BL/6N genetic background.
OtuliniC129A genotyping primers (5′-TGG GAA GGG ACT CTG A-3′ and 5′-TTT GCT ACT GTT GTA AAC TGT A-3′) amplified 176-bp wild-type, 284-bp iC129A and 256-bp C129A DNA fragments. OtulinC129A genotyping primers (5′-CAC TGT TGC AGA TGC TGA GAT TTC-3′ and 5′-CAC TTC CTC ATA GCC CTT GG-3′) amplified 227-bp wild-type and 261-bp C129A DNA fragments. HoipiC879S genotyping primers (5′-CTG CTG AGT GGT CTG AT-3′, 5′-CAC TAT GGA CCT CCT TCT G-3′ and 5′-CGA GGC AGA ACA AGT CT-3′) amplified 360-bp wild-type, 457-bp iC879S and 394-bp C879S DNA fragments.
The R26-CreERT2 allele was kept in a heterozygous state. CreERT2 nuclear translocation in vivo was achieved by intraperitoneal (IP) injections of 3 × 40 mg/kg (Fig. 1e, f, 3f–h, Extended Data Figs. 2–4, 7e–m, 8, 9a), 3 × 80 mg/kg (Fig. 3k, Extended Data Fig. 9d–g) or 5 × 80 mg/kg (Extended Data Fig. 10a–c) tamoxifen (Sigma-Aldrich) dissolved in sunflower seed oil (Sigma-Aldrich). Dosing was once daily on consecutive days. Animals were analysed 5 days (Fig. 1e, f, 3f–h, Extended Data Figs. 2–4, 7e–m, 8, 9, 10 days (Fig. 3k, Extended Data Fig. 9d–g) or 35 days (Extended Data Fig. 10a–c) after the first tamoxifen injection.
TNF blockade was achieved by daily IP injections with either 500 μg TNFR2-Fc protein (Genentech) or 500 μg IgG2a anti-ragweed isotype control antibody (Genentech) starting one day before the first tamoxifen injection. IFNAR1 blockade was achieved by daily subcutaneous injections of either 10 mg/kg IFNAR1 blocking antibody (Leinco Technologies) or 10 mg/kg IgG1 anti-Gp120 isotype control antibody (Genentech) starting one day before the first injection of tamoxifen. Adult mice for tamoxifen treatment studies ranged in age from 6 to 18 weeks.
To generate radiation-induced bone marrow chimaeras, CD45.1 B6.SJL-Ptprca Pepcb/BoyJ (Jackson Laboratory) or CD45.2 R26-CreERT2 Otulin+/+ and R26-CreERT2 OtuliniC129A/iC129A mice were lethally irradiated and reconstituted by intravenous injection of 5 × 106 bone marrow cells from CD45.2 R26-CreERT2 Otulin+/+ and R26-CreERT2 OtuliniC129A/iC129A or CD45.1 B6.SJL-Ptprca Pepcb/BoyJ mice (Jackson Laboratory), respectively. Reconstitution was confirmed by flow cytometric analysis of peripheral blood 6–8 weeks after irradiation, and then mice were dosed with 3 × 40 mg/kg IP injections of tamoxifen.
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin were measured in a serum chemistry analyser (Beckman Coulter AU480) and haematology assays were run on a Sysmex XT-2000iV automated haematology analyser (cell numbers and proportions for neutrophils, monocytes, eosinophils and basophils were combined and shown as myeloid cells).
For timed pregnancies, embryos were designated E0.5 the morning a vaginal plug was detected. Animals that were euthanized because of fight wounds, malocclusion or dystocia were excluded from survival curves (Extended Data Fig. 10e).
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Immunohistochemistry, immunofluorescence and TUNEL staining
Formalin-fixed paraffin-embedded tissue sections were labelled with rabbit anti-cleaved caspase 3 (Asp175) (cat#9661, Cell Signaling Technologies, 0.5 µg/ml), rat anti-Gr-1 (RB6-8C5, BD Biosciences, 10 µg/ml), rat anti-F4/80 (CI:A3-1, Serotec BioRad, 10 µg/ml), or rabbit anti-phosphorylated (p)-RIPK3 Thr231, Ser232 (GEN135-35-9, Genentech, 5 µg/ml). Target antigen retrieval (Dako) and ABC Peroxidase Elite (Vector Laboratories) detection with DAB chromogen were used for Gr-1 and F4/80 immunohistochemistry (IHC). IHC for cleaved caspase 3 and p-RIPK3 was performed on the Ventana Discovery XT platform with CC1 standard antigen retrieval (Ventana). Cleaved caspase 3 was detected with the Ventana OmniMap detection system and DAB chromogen (Ventana). The p-RIPK3 signal was amplified with Ventana HQ Amplification and detected with HQ Discovery detection systems and DAB chromogen. Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) was performed with the Trevigen TACS 2 TdT Blue Label Kit (Trevigen) on a Bond autostainer (Leica Biosystems). For quantitative image analysis, immunolabelled slides were digitally scanned with a Hamamatsu slide scanner. Quantitative image analysis was performed with the MatLab software package (MathWorks).
Whole-mount yolk sac immunofluorescence was performed as described29. Paraformaldehyde-fixed tissues were labelled with rat anti-PECAM-1 antibody (CD31, MEC13.3, BD Biosciences) and rabbit anti-cleaved caspase 3 antibody (cat#9661, Cell Signaling Technology) followed by Cy3-labelled donkey anti-rabbit IgG and Cy5-labelled donkey anti-rat IgG (both Jackson ImmunoResearch). Tissues were mounted with ProLong Gold anti-fade reagent with DAPI (Life Technologies) and images were acquired on a confocal microscope (Leica SPE).
Cell culture
Primary MEFs were isolated from E14.5 embryos excluding the head, intestine and liver. Dermal fibroblasts were isolated from adult tail and ear skin. Fibroblasts were grown in high-glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutaMAX (Gibco), 1× non-essential amino acids solution (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco) on tissue culture dishes pre-coated with 0.1% gelatin in PBS. CreERT2 nuclear translocation in fibroblasts from R26-CreERT2, R26-CreERT2 OtuliniC129A/iC129A, R26-CreERT2 HoipiC879S/iC879S and R26-CreERT2 OtuliniC129A/iC129A HoipiC879S/iC879S mice was achieved by 72 h treatment with 1 μM 4-hydroxytamoxifen (4-OHT, Sigma-Aldrich).
Primary BMDMs and fetal liver-derived macrophages were differentiated from bone marrow and E14.5 fetal livers, respectively, for 6 days in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 50 μM 2-mercaptoethanol (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco) and 20% L929 supernatant containing M-CSF on non-treated plates. CreERT2 nuclear translocation in BMDMs from R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/− and R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/− mice was achieved by adding 1 μM 4-OHT during differentiation.
RIPK1- or HOIP-deficient BMDMs were generated by Cas9 ribonucleoprotein electroporation. In brief, BMDMs were differentiated for 5 days in the above-mentioned DMEM medium supplemented with 50 ng/ml recombinant M-CSF (Genentech) on non-treated plates. BMDMs were harvested and extensively washed with PBS, and then 5 × 106 BMDMs were electroporated with recombinant Cas9 protein v2 (Invitrogen) complexed with Ripk1-specific guideRNAs (seed sequence/Alt-R crRNA: 5′-TAC ACG TCC GAC TTC TCC GT-3′ and 5′-AGA AGA AGG GAA CTA TTC GC-3′ annealed to Alt-R tracrRNA, IDT) or Hoip-specific guideRNAs (seed sequence/Alt-R crRNA: 5′-CCA CCG TGC TGC GAA AGA CG-3′ and 5′-CAT ACA ACC GTA GTA CAT CC-3′ annealed to Alt-R tracrRNA) in nucleofector solution P3 (Lonza). BMDMs were maintained in DMEM supplemented with 50 ng/ml recombinant M-CSF (Genentech) and 1 μM 4-OHT for the following 7 days.
BMDMs and MEFs were stimulated with 100 ng/ml murine TNF (Genentech), 20 μM zVAD (Promega), 30 μM Nec-1 (Tocris), 100 ng/ml (BMDMs) or 1 μg/ml (MEFs) ultra-pure LPS (Invivogen), 5 μg/ml HMW poly(I:C) (Invivogen), 1 μg/ml 5′ppp-dsRNA (Invivogen) transfected with Lipofectamine 2000 CD (Invitrogen), 2 μg/ml ISD (interferon stimulatory DNA, Invivogen) transfected with Lipofectamine 2000 CD (Invitrogen), 2 μg/ml 2′3′-cGAMP (Invivogen) and 1 μg/ml FLAG–TNF (Enzo Life Sciences). MEFs were treated with 1 μM Velcade and/or 100 nM Bafilomycin A1 (both Calbiochem).
Flt3L-dependent Hoxb8-conditionally immortalized haematopoietic progenitor cells were generated as previously described31 by retroviral transduction of an oestrogen-regulated version of the HoxB8 oncogene into bone marrow cells followed by culturing cells in the presence of 70 ng/ml Flt3L (Peprotech) and 1 μM β-oestradiol (Sigma-Aldrich). For recombination, immortalized haematopoietic progenitor cells from R26-CreERT2 Otulin+/+ and R26-CreERT2 OtuliniC129A/iC129A mice were treated with 1 μM 4-OHT for 72 h.
Mouse organoids were established from isolated crypts collected from the entire length of the small intestine and maintained as previously described32. Organoids were passaged at least once and grown using IntestiCult organoid growth medium (cat#06005; StemCell Technologies). For recombination, organoids from R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/− and R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/− mice were treated with 50 nM 4-OHT for 48 h. Organoids were then recovered in IntestiCult medium and expanded for 4 days.
Flow cytometry
Single-cell suspensions were prepared from organs, nonspecific antibody binding was minimized by anti-CD16/CD32 antibody (93, eBioscience), and cells were stained with fluorophore-conjugated antibodies against: CD4 (RM4-5), CD8α (53-6.7), CD11b (M1/70), CD11c (N418), CD45.1 (A20), CD45.2 (104), Ter119 (Ter119, all eBioscience), CD150 (TC15-12F12.2), Ly6C (HK1.4), Ly6G (1A8), Sca-1 (D7), TCRβ (H57-597, all BioLegend), B220 (RA3-6B2), CD3ε (145-2C11), c-Kit (2B8), Gr-1 (RB6-8C5), Siglec-F (E50-2440, all BD Biosciences). Dead cells were excluded using 7-AAD (eBioscience). Samples were acquired on FACSCantoII or Fortessa machines (BD Biosciences) and analysed with FlowJo software. Doublets were excluded based on FSC-H/A and SSC-W/A characteristics and cell populations were gated as follows: LSK cells (lineage (CD11b, CD11c, Gr-1, B220, TCRβ, Ter119)− Sca-1+ c-Kit+), LK cells (lineage (CD11b, CD11c, Gr-1, B220, TCRβ, Ter119)− Sca-1− c-Kit+), Ly6Chigh monocytes (CD11b+ Ly6G− Ly6Chigh), neutrophils (CD11b+ Ly6G+) and as indicated in the Extended Data Figure legends. Gating strategies for flow cytometric analyses can be found in Supplementary Fig. 2.
MEF survival was analysed after overnight treatment by combining adherent and floating dead cells. Samples were stained with 2 μg/ml PI (Sigma-Aldrich), and acquired on FACSCantoII or Fortessa machines (BD Biosciences).
Western blotting, immunoprecipitation and mass spectrometry
For western blotting of whole cell lysates, cells were lysed or embryo tissues were mechanically disrupted in 50 mM Tris HCL pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% (w/v) Na-Deoxycholate, 0.1% (w/v) SDS, 1% (v/v) NP40, PhosSTOP phosphatase inhibitor (Roche), complete protease inhibitor cocktail (Roche) and 1 mM PMSF (Sigma-Aldrich). For immunoprecipitation, cells were lysed in 20 mM Tris HCl pH 7.5, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% (v/v) Triton X-100, 10% (v/v) glycerol, PhosSTOP phosphatase inhibitor, and complete protease inhibitor cocktail. 10 mM N-ethylmaleimide (NEM, Sigma-Aldrich) was added to lysis buffer if ubiquitination was analysed. Insoluble material was removed by centrifugation at 20,000g before addition of LDS sample buffer or IP.
Western blotting antibodies: RIPK3 (1G6.1.4, Genentech), phosphorylated RIPK3 Thr231, Ser232 (GEN135-35-9)29, RIPK1 (10C7, Genentech), phosphorylated RIPK1 Ser166, Thr169 (GEN175-DP-A1 raised against peptide ASFKTW(pS)KL(pT)KEKD, Genentech), MLKL (3H1, Millipore), phosphorylated MLKL Ser354 (EPR9515(2), Abcam), FLAG (M2, Sigma), actin (C4, MP Biomedicals), FADD (1.28E12, Genentech), caspase 8 (1G12, Enzo Life Sciences), OTULIN (GN13.18, Genentech), HOIP (11D6H2G5, Genentech), HOIL-1 (2E2, Millipore), SHARPIN (14626-1-AP, Proteintech), linear ubiquitin (1F11/3F5/Y102L, Genentech), K48 ubiquitin (APU2.07, Genentech), LC3 (8E10, MBL) and TRADD (GN21-3, Genentech). The following antibodies were from Cell Signaling Technologies: phosphorylated ERK (cat#9101), ERK (cat#9102), phosphorylated JNK (cat#4668), JNK (cat#9258), phosphorylated IκBα (cat#2859), IκBα (cat#9242), phosphorylated p65/RelA (cat#3033), p65/RelA (cat#8242), phosphorylated p38 (cat#9211), p38 (cat#8690), RIPK1 (cat#3493), phosphorylated IKKε (cat#8766), IKKε (cat#3416), phosphorylated TBK1 (cat#5483), TBK1 (cat# 3504), caspase 3 (cat#9662), cleaved caspase 3 (cat# 9664), and cleaved caspase 8 (cat#8592).
Anti-FADD antibody (1F7, Millipore) was used for IP. Complexes were recovered with magnetic protein A/G beads (Pierce) or magnetic anti-FLAG beads (M2, Sigma-Aldrich).
To enrich for proteins modified with linear polyubiquitin, cells were lysed in 100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% Triton-X 100 containing complete protease inhibitor cocktail, 2 mM NEM and 150 nM FLAG-anti-linear polyubiquitin TUBEs (LifeSensors). Insoluble material was removed by centrifugation at 20,000g and lysates were diluted tenfold in 100 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM EDTA containing complete protease inhibitor cocktail, 2 mM NEM and 150 nM FLAG-anti-linear polyubiquitin TUBEs. FLAG–TUBEs were recovered with magnetic anti-FLAG beads, washed with 100 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.05% NP-40 containing complete protease inhibitor cocktail and 2 mM NEM, and bound proteins were eluted for 30 min with 150 μg/ml 3 × FLAG peptide (Sigma-Aldrich) by rotation at 4 °C.
For mass spectrometry-based identification of proteins modified with linear and K48-linked polyubiquitin, modified proteins were enriched sequentially with 150 nM FLAG anti-linear polyubiquitin TUBEs followed by 150 nM FLAG anti-K48-linked polyubiquitin TUBEs (LifeSensors). Samples were eluted with 3 × FLAG peptide and separated by SDS–PAGE for ~1 cm. Coomassie (Invitrogen)-stained gel lanes were each excised as a single region and subjected to in gel digestion. Gel pieces were cut into strips, with 100 μl of 50 mM ammonium bicarbonate (AMBIC) in a 50:50 mixture of acetonitrile (ACN):water, desiccated with 50 μl of 100% ACN, and then dried for 1 h in a SpeedVac. Trypsin solution (20 ng/μl in 25 mM AMBIC; Promega) was added to dried gel pieces, incubated on ice for 1 h, then incubated for 16 h at 37 °C. Digested peptides were transferred to a new tube and gel pieces extracted with 100 μl 10% ACN 0.1% trifluoroacetic acid (TFA). The peptide containing solution was dried completely in a SpeedVac and resuspended in 0.1% TFA for mass spectrometry analysis. Samples were injected onto a QExactive-HF mass spectrometer using a NanoAcquity UPLC and analysed by data dependent shotgun sequencing. MS1 scans were collected at 60,000 resolution with an AGC target of 3E6 and a maximum ion time of 60 ms and the top 10 precursor ions subjected to MS2 analysis at 15,000 resolution with an AGC target of 1E5 and max ion time of 75 ms. Raw data files were searched with semi-tryptic specificity using Mascot against the concatenated target-decoy database from UniProt (downloaded April 2015; Mus musculus taxonomy) and common contaminants. Oxidized methionine was permitted as a variable modification. Peptide spectral matches were filtered on a per run basis to 5% false discovery rate at the peptide level using linear discriminant analysis, and then in aggregate across all runs to a 2% false discovery rate at the protein level. Total and unique peptide counts are reported.
For IsoT, OTULIN or USP2 treatment, cells were lysed in 50 mM Tris pH 8.0, 150 mM NaCl, 1% Triton, 10% glycerol supplemented with EDTA-free protease inhibitors (Roche). IPs were washed three times with 20 mM Tris HCl pH 7.5, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% (v/v) Triton X-100, 10% (v/v) glycerol, PhosSTOP phosphatase inhibitor, and complete protease inhibitor cocktail and then once with 50 mM Tris pH 8.0, 150 mM NaCl. Subsequently, IPs or diluted whole cell lysates were incubated with 1 μM IsoT, OTULIN or USP2 (all Boston Biochem) in assay buffer (50 mM Tris pH 8.0, 150 mM NaCl, 5 mM DTT) for 1 h at 32 °C. IsoT was pre-incubated in 50 mM Tris, pH 8.0, 150 mM NaCl and 10 mM DTT at 23 °C for 10 min, before the deubiquitination assay. IsoT treatment of 1 μg free linear tetra-ubiquitin chains (Boston Biochem) was performed under the same conditions. Reactions were quenched by adding LDS loading buffer (Invitrogen). Samples were subjected to SDS-PAGE analysis with subsequent staining using InstantBlue Protein Stain (Expedeon).
Cytokine and chemokine detection
Sera from adult mice or E18.5 embryos and cell supernatants were analysed using Premix Panel I 32-plex (Millipore) or IFN-β ELISA (PBL).
Quantitative reverse-transcription PCR
RNA from organoids, MEFs, macrophages or mechanically disrupted skin tissue was isolated using a RNeasy mini-kit (Qiagen). Quantitative reverse-transcription–PCR was performed using One-step Real-time RT–PCR mastermix (ABI). Taqman probes were from Life Technologies: Actb (Mm01205647_g1 or Mm02619580_g1), Lgr5 (Mm01251801_m1), Axin2 (Mm00443610_m1), Ascl2 (Mm01268891_g1), Olfm4 (Mm01320260_m1), Sharpin (Mm00550584_m1), Hoil-1 (Mm00498069_m1), Hoip (Mm01313902_m1) and Ifnb1 (Mm00439552_s1). Values were normalized to Actb transcript levels.
Statistics
Statistical analysis of the results was performed by unpaired, two-tailed t-test, by one-way ANOVA followed by Tukey’s multiple comparison test or by repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. A 95% confidence interval was used for statistics and P < 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 6.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The data sets generated in this study are available from the corresponding authors upon reasonable request. Proteomics raw data files have been deposited to the UCSD MassIVE database (ftp://massive.ucsd.edu/MSV000081959). Gel source data can be found in Supplementary Fig. 1. Source data for all graphs are provided.
References
Damgaard, R. B. et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell 166, 1215–1230.e20 (2016).
Zhou, Q. et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc. Natl Acad. Sci. USA 113, 10127–10132 (2016).
Rivkin, E. et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature 498, 318–324 (2013).
Peltzer, N. et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Reports 9, 153–165 (2014).
Keusekotten, K. et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell 153, 1312–1326 (2013).
Fiil, B. K. et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol. Cell 50, 818–830 (2013).
Schaeffer, V. et al. Binding of OTULIN to the PUB domain of HOIP controls NF-κB signaling. Mol. Cell 54, 349–361 (2014).
Hrdinka, M. et al. CYLD limits Lys63- and Met1-Linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Reports 14, 2846–2858 (2016).
Elliott, P. R. et al. Molecular basis and regulation of OTULIN–LUBAC interaction. Mol. Cell 54, 335–348 (2014).
Draber, P. et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Reports 13, 2258–2272 (2015).
Elliott, P. R. & Komander, D. Regulation of Met1-linked polyubiquitin signalling by the deubiquitinase OTULIN. FEBS J. 283, 39–53 (2016).
Alva, J. A. et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev. Dyn. 235, 759–767 (2006).
Seibler, J. et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 31, e12 (2003).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Rickard, J. A. et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife 3, e03464 (2014).
Fitzgerald, K. A. et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Inn, K.-S. et al. Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction. Mol. Cell 41, 354–365 (2011).
Belgnaoui, S. M. et al. Linear ubiquitination of NEMO negatively regulates the interferon antiviral response through disruption of the MAVS-TRAF3 complex. Cell Host Microbe 12, 211–222 (2012).
Essers, M. A. G. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Sato, T. et al. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat. Med. 15, 696–700 (2009).
Balachandran, S., Thomas, E. & Barber, G. N. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432, 401–405 (2004).
Rajput, A. et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity 34, 340–351 (2011).
Michallet, M.-C. et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28, 651–661 (2008).
Rickard, J. A. et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157, 1175–1188 (2014).
Dillon, C. P. et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157, 1189–1202 (2014).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Newton, K. et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 (2014).
Peschon, J. J. et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160, 943–952 (1998).
Redecke, V. et al. Hematopoietic progenitor cell lines with myeloid and lymphoid potential. Nat. Methods 10, 795–803 (2013).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Acknowledgements
We thank B. Hough, E. Magee, M. Garcia and K. Cherry for animal husbandry; I. Peng and the Genentech genetic analysis, histology, necropsy, immunohistochemistry, clinical pathology and FACS laboratories for technical assistance; and J. Lai and S. Stawicki for managing antibody generation.
Reviewer information
Nature thanks C. Griffin and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
K.H. designed and performed experiments with assistance from K.E.W., A.N., A.Mu., D.L.D., A.Ma., and F.d.S.M.; M.R.-G., R.J.N. and S.W. generated the OtulinC129A/+ and HoipiC879S/+ mice; Y.Z., E.V., and D.S.K. performed mass spectrometry; J.Z. and W.P.L. performed in vivo antibody blocking experiments; J.H. performed quantitative image analysis; Y.-C.H. generated the phospho-RIPK1 Ser166, Thr169 antibody; L.G.K. performed confocal microscopy; J.D.W. analysed histological data; D.V., K.N. and V.M.D. contributed to experimental design; K.H. and K.N. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
All authors were employees of Genentech.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Constitutive and conditional OTULIN inactivation using a Cdh5-Cre transgene causes embryonic lethality.
a, Organization of the constitutive OtulinC129A knock-in allele. Boxes represent exons. Untranslated regions are shaded grey. b, Kaplan–Meier plot of mouse survival. &, Animal euthanized owing to dermatitis with pruritus. c, Numbers of offspring of OtulinC129A/+ parents at clipping (P0–P10). Strains were derived from independently targeted ES cell clones. d, Numbers of offspring from intercrossing OtulinC129A/+ parents. *Embryos resorbed. e, Representative embryos from d with and without their yolk sacs. f–i, E10.5 placenta (f, i) or yolk sac (g, h) stained with haematoxylin and eosin (H&E) or labelled for phospho-RIPK3 Thr231, Ser232 or cleaved caspase 3 by immunohistochemistry (IHC) (n = 4 Otulin+/+, n = 3 OtulinC129A/C129A mice in (f, i); n = 7 Otulin+/+, n = 3 OtulinC129A/C129A mice in g, h). Scale bars, 100 μm (H&E staining and cleaved caspase 3 IHC in f), 50 μm (p-RIPK3 IHC in f, g) or 25 μm (cleaved caspase 3 IHC and p-RIPK3 IHC in h, i). Arrows indicate labelling of vessel walls (h, i). j, Western blots of E10.5 embryos (n = 3 per genotype). k, Organisation of the inducible OtuliniC129A knock-in allele. Boxes represent exons. Untranslated regions are shaded grey. Arrows indicate genotyping primer locations. l, Offspring numbers from intercrossing OtuliniC129A/iC129A with Cdh5-Cre+ OtuliniC129A/+ parents. *Embryos resorbed. m, Representative embryos from l with and without their yolk sacs. n, E10.5 yolk sacs stained for PECAM-1 (red) and cleaved caspase 3 (green) (n = 2 Cdh5-Cre+ OtuliniC129A/iC129A). 3D projection of 17 confocal z stacks. The lower image shows part of an orthogonal projection. Arrows indicate cells containing cleaved caspase 3 and PECAM-1.
Extended Data Fig. 2 Systemic OTULIN inactivation using the R26-CreERT2 transgene causes spontaneous inflammation and immune cell defects.
Additional characterization of the day 5 tamoxifen-treated mice shown in Fig. 1e. a, Serum cytokine and chemokine levels. b, The percentage of liver tissue positive for cleaved caspase 3 by IHC. c, Liver stained with haematoxylin and eosin (H&E; scale bar, 50 μm; n = 8 per genotype) or labelled with Gr-1 or F4/80 IHC (scale bar, 100 μm; n = 5 per genotype). d, Serum ALT, AST, and total bilirubin levels. e, Small intestine stained with H&E (scale bar, 50 μm; n = 8 per genotype) or labelled by phospho-RIPK3 Thr231, Ser232 IHC (scale bar, 50 μm; n = 5 per genotype) or cleaved caspase 3 IHC (scale bar, 100 μm; n = 5 per genotype). f, The percentage of crypt tissue in the small intestine positive for cleaved caspase 3 by IHC. g, Heart tissue. Scale bar, 100 μm (H&E, n = 8 per genotype; and Gr-1, F4/80 and cleaved caspase 3 IHCs, n = 5 per genotype) or 50 μm (phospho-RIPK3 Thr231, Ser232 IHC; n = 5 per genotype). h, Blood parameters. Neutrophils (CD11b+ Ly6G+) are boxed in representative FACS plots. The mean ± s.d. percentage of neutrophils is indicated (n = 5 per genotype). i, Sternum stained with H&E (scale bar, 50 μm; n = 8 per genotype). Graphs indicate bone marrow cell numbers in two femurs and two tibia, the percentage of viable bone marrow cells not stained by 7-AAD, and numbers of bone marrow LK and LSK cells. LK and LSK cells are indicated in representative FACS plots with their mean ± s.d. frequency (n = 8 per genotype). j, Thymus stained with H&E (scale bar, 100 μm; n = 8 per genotype). Graphs indicate thymus weights and total thymocyte numbers. CD4+CD8+, CD4+ and CD8+ thymocytes are indicated in representative FACS plots with their mean ± s.d. frequency (n = 5 per genotype). Circles represent individual mice. Lines represent means; P values, unpaired, two-tailed t-test (a, b, d, f, h, i, j).
Extended Data Fig. 3 OTULIN inactivation in the haematopoietic compartment causes spontaneous inflammation.
a, FACS plots indicate the efficiency with which irradiated CD45.1+ mice were reconstituted with bone marrow (BM) from CD45.2+ R26-CreERT2 Otulin+/+ or R26-CreERT2 OtuliniC129A/iC129A mice. B cells (B220+ CD3ε−), T cells (B220− CD3ε+) and neutrophils (CD11b+ Ly6G+) in whole blood were examined. Numbers represent the mean percentage ± s.d. (n = 11 R26-CreERT2 Otulin+/+, n = 12 R26-CreERT2 OtuliniC129A/iC129A). b, Body weights of tamoxifen-treated mice plotted as a percentage of initial body weight. Lines represent the mean. P values, repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. c–i, Characterization of the mice in b on day 5. c, Serum cytokines and chemokines. d, Liver (scale bar, 100 μm; n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A) and serum ALT, AST, and total bilirubin. e, Small intestine (scale bar, 50 μm; n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A). f, Heart (scale bar, 100 μm; n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A). g, Blood parameters. Numbers in representative FACS plots indicate the mean ± s.d. neutrophil frequency (n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A). h, Sternum (scale bar, 50 μm; n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A). Graphs indicate bone marrow cell numbers in two femurs and two tibia, the percentage of viable bone marrow cells not stained by 7-AAD, and numbers of bone marrow LK and LSK cells. LK and LSK cells are indicated in representative FACS plots with their mean ± s.d. frequency (n = 9 R26-CreERT2 Otulin+/+, n = 10 R26-CreERT2 OtuliniC129A/iC129A). i, Thymus (scale bar, 100 μm; n = 6 R26-CreERT2 Otulin+/+, n = 6 R26-CreERT2 OtuliniC129A/iC129A). Graphs indicate thymus weights and total thymocyte numbers. CD4+CD8+, CD4+ and CD8+ thymocytes are indicated in representative FACS plots with their mean ± s.d. frequency (n = 3 R26-CreERT2 Otulin+/+, n = 4 R26-CreERT2 OtuliniC129A/iC129A). Circles represent individual mice. Lines represent means; P values, unpaired, two-tailed t-test (c, d, g–i).
Extended Data Fig. 4 OTULIN inactivation outside the haematopoietic compartment causes mild inflammation.
a, FACS plots indicate the efficiency with which irradiated CD45.2+ R26-CreERT2 Otulin+/+ and R26-CreERT2 OtuliniC129A/iC129A mice were reconstituted with BM from CD45.1+ mice. B cells (B220+ TCRβ−), T cells (B220− TCRβ+) and neutrophils (CD11b+ Ly6G+) in whole blood were examined. Numbers represent the mean percentage ± s.d. (n = 12 per genotype). b, Body weights of tamoxifen-treated mice plotted as a percentage of initial body weight. Lines represent means; P values, repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. c–i, Characterization of the mice in b on day 5. c, Serum cytokines and chemokines. d, Liver (scale bar, 100 μm; n = 5 per genotype) and serum ALT, AST and total bilirubin. e, Small intestine (scale bar, 50 μm; n = 5 per genotype). f, Heart (scale bar, 100 μm; n = 5 per genotype). g, Blood parameters. Numbers in representative FACS plots indicate the mean neutrophil frequency ± s.d. (n = 5 per genotype). h, Sternum (scale bar, 50 μm; n = 5 per genotype). Graphs indicate bone marrow cell numbers in two femurs and two tibia, the percentage of viable bone marrow cells not stained by 7-AAD, and numbers of bone marrow LK and LSK cells. LK and LSK cells are indicated in representative FACS plots with their mean ± s.d. frequency (n = 5 per genotype). i, Thymus (scale bar, 100 μm; n = 5 per genotype). Graph indicates thymus weights. Circles represent individual mice. Lines represent means; P values, unpaired, two-tailed t-test (c, d, g–i).
Extended Data Fig. 5 OTULIN inactivation leads to LUBAC auto-ubiquitination, a reduction in LUBAC components, muted NF-κB activation and enhanced cell death upon stimulation.
a, PCR on genomic DNA isolated from primary MEFs treated or not treated with 4-OHT. b, c, g, k, m, o, p, Western blots of MEFs (b, m, o, p), immortalized haematopoietic progenitors (c), dermal fibroblasts (g) and fetal liver-derived macrophages (k). d, Western blots of MEF lysates treated or not treated with IsoT (left). Coomassie-stained gel of free linear tetra-ubiquitin chains treated or not treated with IsoT (right). e, Total (unique) peptide counts by mass spectrometry after affinity purification of linear or K48-linked ubiquitin from haematopoietic progenitors. f, Organization of the conditional HoipiC879S knock-in allele. Boxes represent exons. h, Western blots of MEF lysates with or without OTULIN or USP2 treatment and with or without incubation at 32 °C. *Nonspecific band. i, j, Graphs of relative Sharpin, Hoil-1 and Hoip mRNA expression in MEFs (i) or fetal liver-derived macrophages (j). Each circle represents cells from a different embryo. Lines represent means; P values, unpaired, two-tailed t-test (i, j). l, Western blots of MEFs treated for 4 h as indicated. n, q, Graphs indicate the percentage of MEFs that were viable and not stained by PI after overnight treatment. Each circle represents cells from a different embryo. Bars represent means; P values, one-way ANOVA followed by Tukey’s multiple comparison test (n, q). Results are representative of two independent experiments (d, h, l, m, o) or cells from three mice per genotype (a–c, g, k, p).
Extended Data Fig. 6 Cell death mediated by TNFR1, RIPK1 catalytic activity, caspase 8 and RIPK3 contributes to the embryonic lethality caused by OTULIN inactivation.
a, Western blots of MEFs and their immunoprecipitates (IP) with or without USP2 treatment. Results are representative of two independent experiments. b, Offspring numbers from intercrossing OtulinC129A/+ Ripk1D138N/D138N parents. *Embryos resorbed; #embryos grossly abnormal. c, Representative embryos from b with and without their yolk sacs. d, Offspring numbers from intercrossing OtulinC129A/+ Tnfr1−/− parents. *Embryos resorbed; #Embryos grossly abnormal. e, Representative embryos from d with and without their yolk sacs. f, E14.5 embryo numbers from intercrossing OtulinC129A/+ Casp8+/− with OtulinC129A/+ Casp8+/+ parents. *Embryos resorbed. g, Representative embryos from f with and without their yolk sacs. h, E14.5 embryo numbers from intercrossing OtulinC129A/+ Ripk3−/− parents. *Embryos resorbed. i, Representative embryos from h with and without their yolk sacs. j, Offspring numbers from intercrossing OtulinC129A/+ Ripk3−/− Casp8−/− parents. &Not found at wean. k, Representative embryos from j with and without their yolk sacs. l, Western blots of E10.5 embryos (n = 1 embryo per genotype). m, A representative P0 litter from j. Graph indicates newborn body weights. Circles represent individual pups. Lines represent means. P value, unpaired, two-tailed t-test. n, PCR on genomic DNA isolated from primary intestinal organoids treated with 4-OHT or untreated (n = 3 per genotype). Graphs indicate relative Lgr5, Ascl2, Axin2 and Olfm4 mRNA levels in intestinal organoids treated with 4-OHT. Circles represent cells from different mice. Lines represent means.
Extended Data Fig. 7 OTULIN inactivation causes perinatal lethality in the absence of RIPK3 and caspase 8, and TNF signalling-dependent inflammation in adult mice.
a, b, E18.5 and P0 skin or salivary gland (scale bar, 100 μm; E18.5, n = 8 Otulin+/+ Ripk3−/− Casp8−/−, n = 5 OtulinC129A/C129A Ripk3−/− Casp8−/−; P0, n = 3 Otulin+/+ Ripk3−/− Casp8−/−, n = 3 OtulinC129A/C129A Ripk3−/− Casp8−/−). Oedema was noted in three out of five OtulinC129A/C129A Ripk3−/− Casp8−/− mice at E18.5 and in two out of three mice at P0. c, E18.5 heart and liver stained for nicked DNA by TUNEL assay (scale bar, 200 μm; n = 3 per genotype). d, E18.5 serum cytokine and chemokine levels. Each circle represents one embryo. e, Body weights of tamoxifen-treated mice plotted as a percentage of initial body weight. Lines represent means. P values, repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. P values comparing R26-CreERT2 OtuliniC129A/iC129A isotype control and TNFR2-Fc treated animals are shown. f, Body weights of tamoxifen-treated mice plotted as a percentage of initial body weight. Lines represent the mean. P values, repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test. P values comparing R26-CreERT2 OtuliniC129A/iC129A and R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/− mice are shown. g–m, Characterization of the mice in f on day 5. g, Serum cytokine and chemokine levels. h, Liver (scale bar, 100 μm; n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−) and serum ALT, AST, and total bilirubin. i, Small intestine (scale bar, 50 μm; n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−). j, Heart (scale bar, 100 μm; n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−). k, Blood parameters. Numbers in representative FACS plots indicate the mean neutrophil frequency ± s.d. (n = 6 per genotype). l, Sternum (scale bar, 50 μm; n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−). Graphs indicate bone marrow cell numbers in two femurs and two tibia, the percentage of viable bone marrow cells not stained by 7-AAD, and numbers of bone marrow LK and LSK cells. LK and LSK cells are indicated in representative FACS plots with their mean ± s.d. frequency (n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−). m, Thymus (scale bar, 100 μm; n = 5 R26-CreERT2 Otulin+/+, n = 5 R26-CreERT2 OtuliniC129A/iC129A, n = 6 R26-CreERT2 OtuliniC129A/iC129A Tnfr1−/−). Graphs indicate thymus weights. Circles represent individual mice. Lines represent means; P values, unpaired, two-tailed t-test (d) or one-way ANOVA followed by Tukey’s multiple comparison test (g, h, k–m).
Extended Data Fig. 8 RIPK3 and caspase 8 deficiency rescues spontaneous inflammation and most immune cell defects caused by OTULIN inactivation in the adult.
Additional characterization of the day 5 tamoxifen-treated mice shown in Fig. 3f. a, Graph of the percentage of liver tissue that was positive for cleaved caspase 3 by IHC (n = 5 per genotype). b, Serum cytokines and chemokines. c, Liver (scale bar, 100 μm; n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−) and serum ALT, AST, and total bilirubin. d, Small intestine (scale bar, 100 μm; n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). Lower panels indicate labelling of cleaved caspase 3 (brown, n = 5 per genotype) and the graph shows the percentage of crypt tissue that was positive. e, Sternum (scale bar, 50 μm; n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). Graphs indicate bone marrow cell numbers in two femurs and two tibia, the percentage of viable bone marrow cells not stained by 7-AAD, and numbers of bone marrow LK and LSK cells. LK and LSK cells are indicated in representative FACS plots with their mean ± s.d. frequency (n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). f, Thymus (scale bar, 100 μm; n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). Graph indicates thymus weights. Circles represent individual mice. Lines represent means; P values, one-way ANOVA followed by Tukey’s multiple comparison test (a–f).
Extended Data Fig. 9 OTULIN inactivation in adults lacking RIPK3 and caspase 8 elicits a type I IFN signature.
Immune cell phenotypes of the day 5 tamoxifen-treated mice shown in Fig. 3f. a, Blood parameters. Numbers in representative FACS plots indicate the mean ± s.d. neutrophil frequency (n = 8 R26-CreERT2 Otulin+/+, n = 8 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A, n = 7 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/−, n = 8 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8+/−, n = 9 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). b, Western blots of BMDMs. Results are representative of cells from three mice of each genotype. c, IFN-β secretion by BMDMs following 24 h treatment as indicated. Circles represent cells from individual mice. Bars represent means. d–g, Characterization of day 10 tamoxifen-treated mice. d, Graph indicates CD11b+ Ly6G− Ly6Chigh blood cells. e, Serum cytokines and chemokines. f, Graphs indicate the percentage of LSK bone marrow cells and SCA-1 expression (MFI, median fluorescent intensity) on the indicated bone marrow subsets. g, Serum cytokines and chemokines in the mice shown in Fig. 3k. Circles represent individual mice and lines represent means (a, d–g). P values, unpaired, two-tailed t-test (d–f) or one-way ANOVA followed by Tukey’s multiple comparison test (a, g).
Extended Data Fig. 10 OTULIN inactivation in the combined absence of RIPK3 and caspase 8 causes RIPK1-dependent inflammation.
a–c, Characterization of day 35 tamoxifen-treated mice. a, Representative spleen and lymph nodes (inguinal, brachial/axillary, mandibular/cervical and mesenteric lymph; n = 4 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 5 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). Graphs indicate spleen weight, total spleen or lymph node cellularity, and numbers of different cellular subsets (CD3ε+ B220+ T cells, CD11b+ myeloid cells, CD11b+ Ly6G− SiglecF+ SSC-Ahigh eosinophils, CD11b+ Ly6G+ neutrophils and CD11b+ Ly6G− SiglecF− SSC-Alow Ly6Chigh monocytes). b, Liver (scale bar, 100 μm), small intestine (scale bar, 50 μm) and caecum (scale bar, 50 μm) (n = 4 R26-CreERT2 Otulin+/+ Ripk3−/− Casp8−/−, n = 5 R26-CreERT2 OtuliniC129A/iC129A Ripk3−/− Casp8−/−). c, Serum cytokines and chemokines. d, Offspring numbers at P4–6 from intercrossing OtulinC129A/+ Ripk3−/− Casp8−/− Ripk1+/− parents. e, Kaplan–Meier plot of mouse survival. Note that some mice were obtained independent from intercrossing OtulinC129A/+ Ripk3−/− Casp8−/− Ripk1+/− parents and are not included in d. f, E18.5 serum cytokines and chemokines. Circles represent individual mice (a, c) or individual embryos (f). Lines represent means (a, c, f). P values, unpaired, two-tailed t-test (a, c) or one-way ANOVA followed by Tukey’s multiple comparison test (f). g, Model of how OTULIN limits cell death, embryonic lethality and inflammation. OTULIN counters auto-ubiquitination of the E3 ligase LUBAC. Stimulation of TNFR1 by its cognate ligand TNF initiates assembly of complex I containing TRADD, TRAF2, cIAP1/2 and RIPK1. Ubiquitin chains generated by the E3 ligases cIAP1 and cIAP2 recruit LUBAC but not OTULIN into complex I, leading to the modification of complex I components with linear polyubiquitin and activation of NF-κB and MAPK signalling for gene induction. OTULIN inactivation results in LUBAC auto-ubiquitination, a reduction in total LUBAC, and reduced LUBAC recruitment into complex I. These changes promote formation of cytosolic cell death signalling complexes containing RIPK1, RIPK3, FADD, TRADD and caspase 8. Increased necroptotic and apoptotic cell death causes both embryonic lethality and auto-inflammation in the adult. RIPK1 mediates type I IFN induction in the combined absence of OTULIN function, caspase 8 and RIPK3.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-2. Supplementary Figure 1 contains the uncropped blots for Figures 2-4, and Extended Data Figures 1, 5, 6 and 9. Supplementary Figure 2 shows the gating strategies for flow cytometric analyses.
Supplementary Table
This table contains the total (unique) peptide spectral matches for the proteomics experiment in Extended Data Figure 5e.
Source Data
Rights and permissions
About this article
Cite this article
Heger, K., Wickliffe, K.E., Ndoja, A. et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature 559, 120–124 (2018). https://doi.org/10.1038/s41586-018-0256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0256-2
This article is cited by
-
Systematic HOIP interactome profiling reveals critical roles of linear ubiquitination in tissue homeostasis
Nature Communications (2024)
-
ZBP1 and TRIF trigger lethal necroptosis in mice lacking caspase-8 and TNFR1
Cell Death & Differentiation (2024)
-
Mechanisms underlying linear ubiquitination and implications in tumorigenesis and drug discovery
Cell Communication and Signaling (2023)
-
Roles of RIPK1 as a stress sentinel coordinating cell survival and immunogenic cell death
Nature Reviews Molecular Cell Biology (2023)
-
The mechanism of linear ubiquitination in regulating cell death and correlative diseases
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.