Abstract
The human cerebral cortex is distinguished by its large size and abundant gyrification, or folding. However, the evolutionary mechanisms that drive cortical size and structure are unknown. Although genes that are essential for cortical developmental expansion have been identified from the genetics of human primary microcephaly (a disorder associated with reduced brain size and intellectual disability)1, studies of these genes in mice, which have a smooth cortex that is one thousand times smaller than the cortex of humans, have provided limited insight. Mutations in abnormal spindle-like microcephaly-associated (ASPM), the most common recessive microcephaly gene, reduce cortical volume by at least 50% in humans2,3,4, but have little effect on the brains of mice5,6,7,8,9; this probably reflects evolutionarily divergent functions of ASPM10,11. Here we used genome editing to create a germline knockout of Aspm in the ferret (Mustela putorius furo), a species with a larger, gyrified cortex and greater neural progenitor cell diversity12,13,14 than mice, and closer protein sequence homology to the human ASPM protein. Aspm knockout ferrets exhibit severe microcephaly (25–40% decreases in brain weight), reflecting reduced cortical surface area without significant change in cortical thickness, as has been found in human patients3,4, suggesting that loss of ‘cortical units’ has occurred. The cortex of fetal Aspm knockout ferrets displays a very large premature displacement of ventricular radial glial cells to the outer subventricular zone, where many resemble outer radial glia, a subtype of neural progenitor cells that are essentially absent in mice and have been implicated in cerebral cortical expansion in primates12,13,14,15,16. These data suggest an evolutionary mechanism by which ASPM regulates cortical expansion by controlling the affinity of ventricular radial glial cells for the ventricular surface, thus modulating the ratio of ventricular radial glial cells, the most undifferentiated cell type, to outer radial glia, a more differentiated progenitor.
Similar content being viewed by others
Main
We injected 148 ferret zygotes with genome editing constructs that targeted Aspm exon 15, mutations in which cause severe microcephaly in humans17, and recovered 11 kits born at full term, all carrying insertions or deletions in the targeted exon (Fig. 1a–d). We established three stable Aspm germline knockout ferret lines, which showed comparable phenotypes. Loss of ASPM protein was confirmed in embryonic fibroblasts (Fig. 1e).
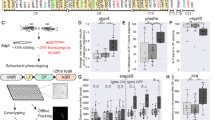
a, NPC diversity in humans, ferrets and mice. b, c, ASPM protein is highly similar between humans and ferrets, including the number of calmodulin-binding (IQ) domains (c, in parentheses). d, Ferret Aspm gene showing the targeted sequences (blue highlights) and founder frameshift deletions. e, Loss of Aspm in knockout ferret embryonic fibroblasts. f, Brains of Aspm+/− and Aspm−/− ferret littermates. g–k, MRI segmentations of grey and white matter (g), gyri grouped into four regions (h), horizontal and coronal sections (i) and quantification of volume (j) and cortical surface area (k). *P < 0.05; n = 3 ferrets per genotype. l–p, Aspm−/− ferrets show reduced brain weight (n, **P < 0.005; *P < 0.01; n = 3–17 ferrets per genotype per age group), but cytoarchitecture (l), laminar organization (m), cortical thickness (o, n = 6 ferrets per genotype) and body weight (p, n = 3 ferrets per genotype) are preserved. q, Loss of Aspm decreases outer cortical surface area in ferrets, but not in mice (n = 3 ferrets and 3 mice per genotype). *P = 0.0217. Data are mean ± s.e.m. (j, k, o–q); box plots show maximum, third quartile, median, first quartile and minimum (n). See Methods, Extended Data Table 1 and Source Data for statistics and reproducibility. Scale bars, 10 μm (e), 100 μm (m), 1 mm (l) and 5 mm (f–i). AEG, anterior ectosylvian gyrus; ASG, anterior sigmoid gyrus; cb, cerebellum; cc, corpus callosum; cd, caudate; CG, cingulate gyrus; CH, calponin homology; CNG, coronal gyrus; CP, cortical plate; GR, gyrus rectus; GM, grey matter; hpc, hippocampus; hy, hypothalamus; IZ, intermediate zone; IP, intermediate progenitor; LG, lateral gyrus; OBG, orbital gyrus; PEG, posterior ectosylvian gyrus; PL, piriform lobe; PSG, posterior sigmoid gyrus; SSG, suprasylvian gyrus; th, thalamus; VZ, ventricular zone; WM, white matter.
Aspm knockout ferrets displayed robust microcephaly (Fig. 1f–i), with up to 40% reduced brain weight (Fig. 1n), but no change in body weight (Fig. 1p), closely modelling the effects of human mutations2,3,4,17. Magnetic resonance imaging18 (MRI) showed that, similar to humans4, loss of cortical volume and surface area followed an anterior-to-posterior gradient, and the frontal cortex was affected the most (Fig. 1f–k and Extended Data Table 1). By contrast, the thickness of the cortex in knockout ferrets was preserved, similar to the cortex of human patients with mutations in ASPM2,3,4, and the cytoarchitecture and lamination of neurons was also preserved (Fig. 1l, m, o and Extended Data Fig. 1). This phenotype is distinct from Aspm knockout mice, which show approximately 10% reduced brain weight, variable body weight reduction, variable cortical thinning, and no discernible change in cortical surface area5,6,7,8,9 (Fig. 1q). Therefore, the Aspm loss-of-function phenotype is more similar in ferrets than in mice to the phenotype of human patients with mutations in ASPM.
To elucidate the developmental mechanism by which microcephaly occurs, we analysed Aspm knockout ferrets during cortical neurogenesis (Fig. 2a–o and Extended Data Figs. 2, 3), which begins around embryonic day 24 (E24) and continues for two weeks after birth, at E41. In the embryonic cortex of wild-type ferrets, undifferentiated ventricular radial glial cells (VRGs) divide symmetrically to expand the pool of VRGs or divide asymmetrically to produce two distinct, more differentiated progenitor subtypes, intermediate progenitors and outer radial glia (ORGs; Fig. 1a). ORGs are multipotent, proliferative, unipolar progenitors, which are abundant in the outer subventricular zone (OSVZ), that express molecular markers, which are also expressed by VRGs, including SOX2, PAX6 and vimentin (VIM); whereas intermediate progenitors are neuronally fated, multipolar transit amplifying cells that predominate in the inner subventricular zone (ISVZ) and express TBR2 (which is encoded by Eomes)12,13,14,16. All three neural progenitor cell (NPC) populations express the mitotic marker Ki-67 and produce neurons that migrate radially into the cortical plate12,13,14,16,19. The cortex of Aspm+/− ferrets at E35 and postnatal day 0 (P0) displayed a ventricular zone that was densely packed with PAX6+ or SOX2+ VRGs, and a less-dense zone of Ki-67+ NPCs that expressed SOX2, TBR2 or both in the SVZ (Fig. 2d–g and Extended Data Figs. 2, 3). By contrast, the cortex of Aspm knockout ferrets contained overabundant Ki-67+ NPCs in the basal SVZ and intermediate zone (Fig. 2e, f), reminiscent of the positioning of ORGs that normally populate the OSVZ12,13,14,20. Discontinuous clusters of basal NPCs were accompanied by thinning of the ventricular zone, suggesting that precocious OSVZ progenitors were derived by premature withdrawal from the ventricular zone (Fig. 2d–f and Extended Data Figs. 2, 3). Displaced OSVZ progenitors were more abundant frontally and dorsally (Fig. 2a–c), matching the topography of cortical volume reduction in the adult (Fig. 1f–k).
a–f, Nuclear staining of Aspm−/− ferret brains shows that there is a premature OSVZ-like zone (a–c, arrowheads), which contains NPCs that express PAX6, SOX2, and Ki-67 (d–f). g–k, Displaced NPCs include SOX2+pVIM+ ORG (g, arrowheads) with a basal process (g, arrows; h, i, k) and TBR2+ intermediate progenitors. The number of abventricular pVIM+ NPCs are increased threefold in Aspm−/− ferrets (j). *P = 0.006; analysed using a one-tailed t-test; data are mean ± s.e.m.; n = 3 Aspm+/− and n = 4 Aspm−/− animals. l–o, Displaced NPCs express Vim, Eomes or Ptprz1 (l, m), have ARL13B+ cilia (o) and are either SOX2+NEUROG2+HOPX+ (arrowheads) or SOX2+NEUROG2+HOPX– (arrows) (n). p, q, Aspm−/− mice lack displaced NPCs. See Methods for statistics and reproducibility. Scale bars, 10 μm (g–i, n, o), 50 μm (d–f), 100 μm (k–m, p, q) and 500 μm (a–c).
Many displaced progenitors in the OSVZ of Aspm knockout ferrets expressed VRG/ORG markers including VIM, phosphorylated vimentin (pVIM), phosphorylated histone H3 (pH3), SOX2 and PAX6; as well as the ciliary marker ARL13B and the human ORG-enriched genes Ptprz1 and Hopx21, whereas other cells expressed the intermediate progenitor marker, as shown by TBR2 protein and Eomes mRNA analysis (Fig. 2g–o and Extended Data Figs. 2, 3, 6). Some of the displaced cells had an ORG-like unipolar morphology, with basal radial fibres that were immunoreactive to VIM, pVIM or HOPX antibodies (Fig. 2g–i, k, o). Quantification of pVIM+ mitotic NPCs revealed a threefold increase in the number of ORG-like progenitors in the knockout ferrets at E35 (P = 0.006; 3 Aspm+/− and 4 Aspm−/− littermates; Fig. 2j). The intermingled presence of NEUROG2+HOPX+ ORGs, TBR2+ intermediate progenitors and DCX+ newborn neurons together indicated preserved neurogenesis within clusters of displaced NPCs (Fig. 2n and Extended Data Fig. 6). These data demonstrate that the loss of Aspm in the ferret cortex causes VRGs to prematurely detach from the ventricular zone and relocate to the OSVZ, where many dislocated cells exhibit ORG morphology, molecular profile and neurogenic potential.
These marked changes in NPC populations in the knockout ferret contrast with six previously reported Aspm knockout mouse lines5,6,7,8,9, which have consistently shown limited changes in NPC identity and organization. Aspm knockout mice show a trend towards an increased number of intermediate progenitors at the expense of VRGs9, but lack ectopic basal SOX2+ or PAX6+ NPCs (Fig. 2p, q and Extended Data Fig. 4). Aspm knockout ferrets also showed increased cell apoptosis in telencephalic germinal zones that was not seen in Aspm knockout mice5,6,7,9 (Extended Data Fig. 5), further highlighting that loss of Aspm elicits divergent brain phenotypes in ferrets and mice.
Single-cell RNA-sequencing22 (scRNA-seq) of around 21,000 cells from the telencephalons of seven E35 embryos (3 Aspm+/+ or Aspm+/− and 4 Aspm−/− animals) reinforced the conclusion that NPC proportions were altered in the Aspm knockout animals, although their transcriptional programs were mostly preserved (Fig. 3, Extended Data Fig. 7 and Extended Data Table 2). We identified cell clusters corresponding to excitatory and inhibitory progenitor and neuronal subtypes, as well as non-neural cells (Fig. 3a, b) and found that the cell type composition of the E35 Aspm knockout forebrain was significantly altered (χ2 = 267.27, degrees of freedom = 12, P = 2.2 × 10−16), yet cells still clustered by cell type, not by genotype or batch (Extended Data Fig. 7). Consistent with immunohistochemical observations, scRNA-seq analysis suggested that VRGs, wild-type ORGs and prematurely displaced knockout ORGs were transcriptionally indistinguishable, and the total proportion of radial glial cells (cycling radial glial cells and interphase radial glial cells) was unchanged in Aspm knockout cells (Extended Data Table 2). A 30% increase in the proportion of intermediate progenitors in knockout ferrets (P = 0.0002, false discovery rate of < 0.01; Fig. 3c and Extended Data Table 2) was consistent with the increased number of intermediate progenitors that were detected by immunostaining (Fig. 2l, m and Extended Data Fig. 6) and was further validated by single-molecule fluorescence in situ hybridization (Fig. 3d, e). A doubling of the small proportion of cells that expressed the oligodendrocyte precursor cell markers Apod and Olig1 suggested limited but significant premature differentiation towards the glial lineage (Fig. 3c, e, f and Extended Data Table 2). These scRNA-seq data suggest that the gene expression programs of cortical neurogenesis are mostly preserved, but the proportions of developmental cell types are changed, in microcephaly associated with ASPM mutations.
a, scRNA-seq identifies major cell types at E35. For abbreviations and statistics, see Extended Data Table 2. b, Cell type markers for each cluster. c, Proportions of each cell type, with the largest changes indicated by black outlines (bootstrap false discovery rate < 0.01). d, Aspm is enriched at the apical surface of the ventricular zone, whereas the number of Eomes+ intermediate progenitors is increased in the SVZ of Aspm knockout ferrets. Asterisks indicate blood vessels. e, f, The number of Apod+ oligodendrocyte precursor cells is increased in Aspm−/− ferrets. *P = 0.012; analysed using a one-tailed t-test; n = 4 animals per genotype. See Methods for statistics and reproducibility. Data are mean ± s.e.m. Scale bars, 100 μm.
While examining potential molecular mechanisms for the detachment of VRGs from the ventricular surface in the knockout ferret, we identified a novel interaction between ASPM, which is localized at the centrosome, and the apical polarity complex (Fig. 4). Together with other centrosomal proteins, ASPM is essential for normal stem cell behaviour, such as centriole biogenesis and maternal centriole structure (Extended Data Fig. 8), and interactions between the mother centriole and the apical membrane have been implicated in the maintenance of NPC stem cell character9,23,24,25. In VRGs, the centrosome is localized to the ventricular endfeet, which are linked by adherens junctions to form a polarized neuroepithelium, which expresses apical polarity complex proteins at the ventricular surface26. Aspm−/− mice showed abrogated staining of the core apical complex protein aPKCζ along the disrupted ventricular surface at E14.5 (Fig. 4a). Intriguingly, we found that depletion of ASPM by RNA interference in H4 human neuroglioma cells resulted in the loss of PKCζ and another critical apical complex protein, PAR6α, from the centrosome (Fig. 4b). Furthermore, we found an interaction between ASPM and PKCζ, as indicated by mutual co-immunoprecipitation (Fig. 4c), that may mediate centrosomal recruitment of the apical polarity complex, providing a new mechanistic insight into the link between centrosomal microcephaly-related proteins and apical progenitor identity.
a, aPKCζ at the ventricular surface is decreased in Aspm−/− mice. b, Depletion of ASPM in H4 cells prevents recruitment of PKCζ and PAR6α to the centrosomes. RNAi, RNA interference. c, ASPM and PKCζ co-immunoprecipitate in extracts of HeLa cells. d, e, Loss of ASPM decreases ventricular surface staining for ninein in mice (d) and ferrets (e). f, Depletion of ASPM and PKCζ prevents recruitment of ninein to the centrosomes in H4 cells. g, Levels of aPKCζ, PAR6α, and ninein are unchanged in Aspm−/− mouse embryonic fibroblasts. See Methods for reproducibility. For gel source data, see Supplementary Data. Scale bars, 5 μm (b, d-f) and 50 μm (a).
Finally, we found sharply reduced staining for ninein, another microcephaly-associated centrosomal protein27, at both the E14.5 mouse and E35 ferret ventricular surface (Fig. 4d, e). Ninein localizes to the mother centriole and is critical for NPC maintenance25,28, and depletion of either ASPM or PKCζ in H4 cells reduced centrosomal localization of ninein (Fig. 4f). Importantly, Aspm−/− mouse embryonic fibroblasts expressed normal levels of aPKCζ, PAR6, and ninein proteins, suggesting that loss of aPKCζ and ninein from the ventricular surface of Aspm knockout mice and ferrets is primarily because of mislocalization, rather than downregulation. These data show that loss of ASPM disturbs the organization and function of the centrosome at multiple levels, and suggest disruption of the centrosome–apical polarity complex interface as a mechanism underlying the displacement of VRGs from the ventricular zone in the Aspm knockout ferret.
Collectively, our data show that ASPM regulates the affinity of VRGs for the ventricular surface. Displaced mutant progenitors show many features of ORGs, indicating that ASPM has a central role in the regulation of the normal timing of the transition from VRG to ORG, and thus the ratio of VRGs to ORGs over the course of development. Premature basal displacement deprives VRGs of proliferation-inducing factors obtained from the cerebrospinal fluid29, increases the proportions of less-proliferative ORGs and intermediate progenitors, and results in a smaller cerebral cortex. The frontal predominance that characterizes both the loss of cortical surface area and VRG displacement further indicates that the premature transformation of VRG to ORG leads directly to reduced cortical units and surface area.
Our results support the idea that expansion of cortical surface area during human evolution may have arisen in part from changes to the proliferative time window of VRGs. Changes in the amino acid sequence of ASPM and other microcephaly-associated centrosomal proteins10 may have affected the timing of the VRG proliferative window by altering interactions between maternal centriole components and the apical polarity complex. Finally, we find that for human brain disorders that are poorly recapitulated in the mouse or in cell culture, the ferret is an efficient and accurate genetic model that demonstrates robust phenotypes and can be used to investigate the mechanisms underlying disorders of the brain.
Methods
We complied with all relevant ethical regulations and the experiments that we performed were approved by the Institutional Animal Care and Use Committees (IACUCs) at the University of Iowa, Boston Children’s Hospital, Yale School of Medicine and Marshall BioResources.
ASPM protein homology and domain analysis
ASPM protein sequences of 16 mammals were extracted from NCBI GenBank. Global pairwise alignment was performed with EMBOSS Needleall (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). The percentage of homology to human ASPM was calculated for each animal based on the alignment score using the Needleman–Wunsch algorithm (gap opening penalty, 10; gap extension penalty, 0.5). The phylogenetic tree was generated using http://timetree.org. CH and IQ domains were counted using the simple modular architecture research tool (http://smart.embl.de/), for NP_060606.3 (human), NP_033921.3 (mouse) and ENSMPUT00000010205.1 (ferret).
TALEN assembly and mRNA synthesis
We assembled three pairs of transcription activator-like effector nucleases (TALENs) that target exon 15 of ferret Aspm, which encodes the second CH domain, and cloned the TALENs into a mammalian expression vector with CMV and T7 promoters through a commercial service (PNA Bio). Gene targeting efficiency of each TALEN pair was tested in HEK293T cells using a split GFP-based reporter30. The most efficient pair, targeting TGAGAGCATAAAGCTGTTGATGGAGTGGGTAAATGCTGTTTGTGCTTTCTATA (target–spacer–target) was chosen for genome editing in vivo. These plasmids are available from Addgene. For mRNA synthesis, endotoxin-free TALEN plasmids were prepared using NucleoBond Xtra Midi EF kit (Clontech), ethanol-precipitated three times, linearized using ScaI digestion (New England BioLabs) and gel-purified. mRNAs were synthesized using the mMessage mMachine T7 ULTRA kit (ThermoFisher Scientific) and cleaned up using the MEGAclear transcription clean-up kit (ThermoFisher Scientific). Of note, we performed the optional ammonium acetate precipitation to improve the quality of the mRNAs. The TALEN mRNAs were diluted in sterile EmbryoMax injection buffer (Millipore) at 50 ng μl−1, aliquoted and kept frozen at −150 °C until use.
Embryonic targeting of the ferret Aspm gene
Zygotes were collected from the mating of ferrets with a sable coat colour (Marshall BioResources) as previously described31. TALEN mRNA (50 ng μl−1) was injected into the cytoplasm of zygotes using a micromanipulator and injector (Eppendorf) and a phase-contrast microscope. Initially 79 ferret zygotes were injected and cultured in vitro for five days so that they reached the blastocyst stage. Twelve zygotes developed to blastocysts, from each of these zygotes genomic DNA was extracted and whole-genome multiple displacement amplification was performed. The targeted genomic region was amplified by PCR (primers: 5′-TTTGTGTGTGTTTCAGGTGGA-3′ and 5′-TGCATTATACAACTGGTGACAGA-3′ with a 430-bp product size), gel-purified and cloned using a TOPO-TA cloning kit (ThermoFisher Scientific). Twelve plasmid clones were sequenced from individual bacterial colonies from each blastocyst. These studies demonstrated an 87% targeting efficiency (14 insertions or deletions in 16 alleles or 8 blastocysts that we were able to analysis). Next, we injected 148 zygotes, incubated them at 39 °C for 24 h, and transferred 116 two-cell-stage embryos into the oviduct of pseudopregnant female sable ferrets as previously described31. Twenty-three ferrets were born and eleven survived. All 11 ferrets had insertions or deletions (100% efficiency). The F0 ferrets suckled and swallowed milk normally and grew without gross abnormalities.
Germline transmission
Six Aspm mutant ferrets were shipped to Marshall BioResources at three months of age and maintained according to the protocol approved by IACUC. Two compound heterozygous males, Δ23;Δ22 (c.3364_3386del;c.3363_3384del) and Δ22;Δ16 (c.3367_3388del;c.3364_3379del), and one heterozygous female, Δ22;WT (c.3363_3384del;WT), (Fig. 1d) were chosen as founders, because they had similar frameshift, early truncating mutations. They were bred to each other or wild-type ferrets. Germline transmission was confirmed by T7 endonuclease I assay (New England BioLabs) and sequencing of both alleles. Eventually animals with a specific Δ22 mutation (c.3363_3384del) were maintained for breeding. Routine genotyping was carried out with PCR (primers: 5′-ATCAATAAGAAAAAAGACAAAAGAAATAGTGG-3′ and 5′-CTTAAGTCAGTGAGCTTAAACAGAAAT-3′ with a 150-bp product size for the wild-type allele and 128-bp from the knockout allele). Aspm knockout males mated successfully, and knockout kits were born at expected Mendelian ratios.
Semen analysis
Every mating was closely monitored at Marshall BioResources. Sperm samples were collected from mated females directly after mating. The concentration, motility and morphology of the sperm were analysed by an experienced technician. Each male received a sperm check evaluation at least once a month. Samples from Aspm+/+, Aspm+/− and Aspm−/− males showed similar sperm counts.
Ferret colony management and tissue handling
The Aspm knockout ferret colony was maintained at Marshall BioResources. For embryonic ages and < P8, timed pregnant jills were shipped to Boston Children’s Hospital and euthanized before embryo extraction, at which point brains were removed from the embryos and drop-fixed in 4% paraformaldehyde (PFA) at 4 °C overnight. For ≥P8 ferrets, all animals were deeply anaesthetized and weighed before transcardial perfusion with cold PBS followed by 4% PFA, after which the brains were extracted and placed in 4% PFA at 4 °C overnight. The brains were subsequently washed and stored in PBS before processing for immunohistochemistry. All brain weight measurements were made post-fixation, before sucrose infiltration.
MRI
Ferrets
Three Aspm+/− and three Aspm−/− ferrets ( > 8 months of age) were perfused using 4% PFA in PBS. The brains were dissected and post-fixed in PFA and PBS containing 4 mM gadolinium contrast Magnevist (Bayer) at 4 °C for two weeks. The brains were scanned using a 9.4 T at Yale Magnetic Resonance Center and the Martinos Center for Biomedical Imaging, Massachusetts General Hospital. At Yale, a custom-made 1H radiofrequency coil (40-mm diameter) was used for diffusion tensor imaging (DTI). DTI acquisition was obtained with a Stejskal and Tanner spin-echo diffusion-weighted sequence with a diffusion gradient δ = 5 ms and a delay Δ = 15 ms between diffusion gradients32. Sixty-four slices of 500-μm thickness, field of view of 25.6 × 25.6 mm2 and 128 × 128 resolution were acquired with a repetition time (TR) of 4 s, echo time (TE) of 30 ms and four averages. Each of the six MR images was first corrected for B1 shading artefacts using a slice inhomogeneity correction33 and an inverse covariance mapping of grey matter density (D.C., X.P., L.H.S & F.H., unpublished observations). Next, the Ferret Atlas18 was registered to each of the MRI images using a tensor b-spline normalized mutual information nonlinear intensity-based registration algorithm34,35 with a control point spacing of 1 mm. The result of the registration was used to warp the atlas regions to each individual MRI, and from this we calculated the volume of each of the warped regions as shown in Fig. 1 and Extended Data Table 1. Anterior sigmoid gyrus, orbital gyrus and posterior sigmoid gyrus were considered to be frontal cortex; anterior ectosylvian gyrus, coronal gyrus, posterior ectosylvian gyrus, and suprasylvian gyrus were considered to be lateral cortex; cingulate gyrus, gyrus rectus and piriform lobe were considered to be medical cortex; and lateral gyrus was considered to be the parietal/occipital cortex. The name of each brain part in Fig. 1 is as previously described36. For DTI tensor measurement, a total of 15 different non-collinear diffusion weighted directions (b = 1,000 s mm−2) and 1 without diffusion weighting were obtained. The six elements of the diffusion tensor were calculated from the signal intensity of the diffusion-weighed images. Tensor eigenvalues and their corresponding eigenvectors were computed, along with fractional anisotropy, at each voxel. The images were colour-coded by the principal direction (eigenvector) of diffusion using BioImage Suite37 (http://www.bioimagesuite.org/). At Massachusetts General Hospital, we acquired anatomically accurate brain volume images with minimal distortion using FLASH (fast low angle shot) MRI sequence with TR = 100 ms, TE = 30 ms and 150-μm isotropic resolution. Cortical grey and white matter were manually segmented using FreeView (http://surfer.nmr.mgh.harvard.edu) and their volumes were measured.
Mice
Three Aspm+/+ and three Aspm−/− mouse brains were dissected from P30 animals perfused with 4% PFA and post-fixed as described above. Brains were submerged into perfluorocarbon oil (Fomblin, Fisher Scientific) at 4 °C for three days, and imaged in this oil using a Bruker BioSpec 70/30 7 T MRI scanner (a sub-millimetre MRI with a 30-cm bore and 450 mT m−1 gradient) in the Small Animal Imaging Facility at Boston Children’s Hospital. MRI scans were isotropic 63-μm voxels across the entire brain. Cortical surface area was visualized and measured using the FreeView, Osyrix and ImageJ 3D projection.
Fluorescent immunohistochemistry
Fixed ferret brains were infiltrated with a series of 10%, then 20% and finally 30% w/v sucrose solutions in PBS until sunk, then embedded in optimal cutting temperature (OCT) compound and frozen in isopentane cooled to −40 °C, after which they were stored long-term at −80 °C. Brains were sectioned at 10- to 20-μm thickness on a Leica Cryostat, mounted immediately onto warm charged SuperFrost Plus slides (Fisher Scientific) and dried at 37 °C for 10 to 30 min before storage at −80 °C. After applying a hydrophobic barrier around the tissue (ImmEdge Pen, Vector Labs), slides were washed in cold 0.1 M PBS followed by antigen retrieval in Retrievagen A pH 6.0 (BD Biosciences) at 80–90 °C in a hybridization oven for 45 min. Sections were then cooled to room temperature in Retrievagen, washed in cold 0.1 M PBS, and blocked for 1 h at room temperature (5% normal donkey serum, 1% w/v BSA, 0.2% w/v glycine and 0.2% w/v lysine, in PBS). Slides were incubated with primary antibodies for two nights on a rotary shaker at 4 °C in blocking buffer plus 0.3% Triton X-100. Sections were then washed in PBS and incubated for 2 h at room temperature in blocking buffer containing secondary antibodies at 1:500 (Jackson Immunoresearch). Finally, slides were washed in PBS, counterstained with DAPI at 1 μg ml−1 in PBS for 15 min, washed again and coverslipped with Fluoromount-G (Southern Biotech). Images were obtained with a Zeiss LSM700 confocal microscope and Leica MZ16 F fluorescence stereomicroscope. The following antibodies were used at 1:200–1:2,000: PAX6 (Abcam ab5790), FoxP2 (Abcam ab16046), CTIP2 (Abcam ab18465), SATB2 (Bethyl A301-864A), SATB2 (Abcam ab51502), SOX2 (SCBT sc-17320), TBR2 (Millipore AB15894), Ki-67 (BD 550609), pVIM (MBL D076-3), VIM (Abcam ab8978), pH3 (Millipore 07-145), HOPX (SCBT sc-30216), ARL13B (Abcam ab136648), ARL13B (ProteinTech 17711-1-AP), NEUROG2 (R&D MAB3314) and DCX (SCBT sc-8066).
Apoptosis assay
We examined apoptosis on cryosections using the ApopTag Red In situ Apoptosis Detection Kit (Millipore) according to the manufacturer’s protocol.
scRNA-seq
Cell capture and sequencing
Cell capture and sequencing were performed using the Drop-seq method22 (http://mccarrolllab.com/dropseq/). Forebrain tissue was isolated from 2 Aspm+/+, 1 Aspm+/− and 4 Aspm−/− E35 ferret embryos and cryopreserved38, then shipped to Cornell and processed there for single-cell capture, library preparation and sequencing.
Read alignment and digital gene expression matrix generation
Ferret reference gene annotations were expanded using bulk RNA-seq data from the cortex of two P2 ferrets. Bulk data were first mapped to the Ensembl ferret reference genome and transcriptome using TopHat2, and a transcriptome was assembled with cufflinks; this assembled transcriptome and the Ensembl reference transcriptome version 1.0.85 were merged using cuffmerge39. The Drop-Seq Core Computational Protocol version 1.0.1 was followed22. Fastq reads were converted to BAM using the ‘FastqToSam’ command in Picard (http://broadinstitute.github.io/picard/). Read pairs for which more than one base in the barcode had a quality below 10 were discarded. Adaptor sequences were trimmed from the 5′ end of the read, along with polyA tails. Star-2.5.2a40 was used to map reads to the custom transcriptome reference. The digital gene expression matrix was extracted using the ‘DigitalExpression’ program of the Drop-seq protocol22, keeping only cells with at least 200 reads per cell for clustering analysis.
Single cell clustering
Seurat software was used for dimensionality reduction, clustering and obtaining cluster markers41. Cells from Aspm+/+ or Aspm+/− and Aspm−/− ferrets were merged in a single matrix. An initial run showed that one of the Aspm+/− samples contained low Unique Molecular Identifier (UMI) and gene counts compared to the other samples, and clustered differently, so this sample was removed from downstream analyses (Extended Data Fig. 7). Genes were included if they were expressed in ≥3 cells and cells were included if they expressed > 200 genes and < 2,000 genes. This resulted in 22,211 cells and 21,962 genes from 8,037 Aspm+/+ or Aspm+/− cells and 14,174 Aspm−/− cells. The data were log-normalized per cell, scaling each cell to 10,000 molecules as described previously22. The ‘MeanVarPlot’ Seurat function was used to identify the most variable genes, obtaining 3,555 variable genes. Negative binomial regression was performed on the variable genes, using the number of UMIs per cell as a confounder variable before clustering. The ‘PCAFast’ function in Seurat was used to implement principal component analysis using the IRLBA package. Twenty-five principal components were selected for clustering and as input for t-distributed stochastic neighbour embedding (t-SNE) in Seurat. These were selected by plotting the standard deviation of the principal components and setting a cutoff at the ‘elbow’ of the graph using the ‘PCElbowPlot’ function in Seurat. Clustering was performed using the Seurat function ‘FindClusters’, which implements a shared nearest neighbour modularity optimization based algorithm using k.param = 30 for defining the k of the k-nearest neighbour algorithm and a resolution of 0.5 as previously described41,42. The Barnes–Hut implementation of t-SNE was used for visualizing the clusters using the ‘RunTSNE’ and ‘TSNEPlot’ Seurat functions. We observed a co-localization in the t-SNE plot of cells clustered together by the graph-based clustering algorithm (Fig. 3a). Cluster markers were obtained with the ‘FindAllMarkers’ Seurat function using a likelihood-ratio test, using the parameter min.pct = 0.25 to test only genes expressed in at least 25% of cells in either all cells or the cells in a specific cluster, and testing only genes with at least 0.25-fold difference on a log-scale between cells in a cluster and all cells using the parameter thresh.use = 0.25. P values were adjusted for multiple-comparison testing using the p.adjust function in R for the Benjamini–Hochberg false discovery rate (FDR), selecting an FDR threshold of 0.01. Known markers were used to determine the corresponding cell type of each cluster. The heat map in Fig. 3b shows expression data for the top ten cluster markers for each cluster, in a random sample of 10% of the cells of each cluster. Plotting cells onto the t-SNE plot based on their batch (three batches with Aspm+/+ or Aspm+/− as well as Aspm−/− animals each) suggested that batches did not strongly influence clustering (Extended Data Fig. 7). Plotting cells from each sample onto the t-SNE plot suggested that two non-neuronal clusters of blood and choroid plexus epithelial cells were primarily from a single sample (a likely dissection artefact) and these two clusters were removed from further analysis (grey clusters in Fig. 3a and Extended Data Fig. 7c, d). Plotting the number of genes and UMIs in each cluster revealed that one of the excitatory neuronal clusters had almost three times as many genes and UMIs per cell (Extended Data Fig. 7c, d). This, along with the fact that this cluster expressed a combination of markers from the other two excitatory neuronal clusters suggested that this cluster may contain doublets or other technical or batch artefacts; therefore we also removed this cluster from further analysis.
Statistical analysis of cell type composition by genotype
A χ2 test was performed using the ‘chisq.test’ function in R to test the association of cluster composition with genotype. We also quantified the fraction of cells corresponding to each cluster with the assumption that Drop-seq captures and sequences cells in an unbiased manner, and that the frequencies of cells are representative of their frequency in the tissue22. The fraction of cells corresponding to each cluster was obtained by counting the number of cells assigned to each cluster for Aspm+/+ or Aspm+/− and Aspm−/− samples and dividing over the total number of cells that passed the filters described above and excluding the three clusters that were removed, for a total of 7,645 Aspm+/+ and Aspm+/− cells and 13,725 Aspm−/− cells. Empirical P values were obtained by permuting a genotype 10,000 times and obtaining the fraction of cells corresponding to each cluster for each permutation. These fractions were sorted and the P value was obtained by counting the number of times a fraction was more extreme or equal to the observed fraction in the non-permuted data divided by 10,000 and multiplied by 2 for a two-tailed test. P values were adjusted for multiple comparison testing using the p.adjust function in R for the Benjamini–Hochberg FDR, selecting an FDR threshold of 0.01.
Single-molecule fluorescence in situ hybridization
Using RNAscope fluorescence detection assays and probes (ACDbio), we performed single-molecule fluorescence in situ hybridization according to the manufacturer’s protocols. Cryosections on SuperFrost Plus slides (Fisher Scientific) were dried at −20 °C, rather than at room temperature or 37 °C, for 15 min after mounting, and were used within a week of sectioning. Target retrieval was performed at 80 °C in a hybridization oven for 30–40 min before proceeding with the RNAscope multiplex fluorescence detection protocol.
Cell culture and siRNA transfection
H4 and HeLa cells authenticated by short tandem-repeat profiling were obtained from ATCC, cultured in Advanced DMEM (ThermoFisher Scientific) supplemented with 3% FBS (Altantis) and Glutamax-I (ThermoFisher Scientific), and used within five passages with routine mycoplasma screening. Ferret embryonic fibroblasts (FEFs) and mouse embryonic fibroblasts (MEFs) were derived from post-fertilization day 35 and 14.5 embryos, respectively. FEFs and MEFs were cultured in AmnioMAX (ThermoFisher Scientific). H4 cells were transfected with validated siRNAs against human ASPM or PRKCZ, which encodes PKCζ (ThermoFisher Scientific), using Oligofectamine and OptiMEM (ThermoFisher Scientific) according to the manufacturer’s instructions and were analysed 48 h later.
Immunoprecipitation and immunoblotting
Immunoprecipitation experiments were performed as previously described9. In brief, HeLa cells were collected in Dulbecco’s PBS (DPBS, ThermoFisher Scientific) and lysed in lysis buffer (50 mM Tris-HCl pH 7.4, 266 mM NaCl, 2.27 mM KCl, 1.25 mM KH2PO4, 6.8 mM Na2HPO4-7H2O and 1% NP-40) supplemented with EDTA-free protease inhibitors cocktail set III (Calbiochem). For each immunoprecipitation, 1 mg of lysate was incubated with 2 μg of antibody for 2 h and then incubated with magnetic protein G–sepharose beads (GE Healthcare Life Sciences) for another 1 h at 4 °C. Complexes were washed and then boiled in 2× Laemmli reducing buffer with β-mercaptoethanol (Bio-Rad). Samples were separated on 4–15% TGX gels (Bio-Rad), transferred onto BA85-supported nitrocellulose (GE Healthcare Life Sciences) at 100 V for 30–45 min using a plate electrode Trans-Blot cell with cooling coil (Bio-Rad) and then subjected to immunoblot analysis using ECL Lightening Plus (Perkin-Elmers) or Western Pico (ThermoFisher Scientific). All immunoprecipitation and immunoblotting experiments were replicated three times.
Fluorescent immunocytochemistry
Cells were fixed in ice-cold methanol for 3 min, permeabilized in blocking buffer (2.5% BSA or FBS, 0.1% Triton X-100, 0.03% NaN3 in DPBS). Primary and secondary antibodies were diluted in blocking buffer and incubated for 2 h at room temperature. Coverslips were mounted using Gelvatol or Prolong Diamond (ThermoFisher Scientific) and imaged with an inverted confocal microscope (Zeiss LSM700). Images were processed with ImageJ/FIJI. For 3D-structured illumination microscopy (SIM) (Fig. 1e), wild-type and knockout FEFs were plated on 1.5-mm coverslips and immunostained as above. Coverslips were mounted with Vectashield (Vectorlabs). 3D-SIM imaging was performed on a Zeiss Elyra PS.1 microscope equipped with a 100×/1.40 NA oil objective. Exciting light was directed through a movable optical grating to generate a fine-striped interference pattern on the same plane. z stacks of 15 optical sections with a step size of 0.1 μm were acquired to generate images in maximum intensity projection. The epitope of the ASPM (216-1) antibody43, NDNYGLNQDLESES, is located before the TALEN target site. The following antibodies were used at 1:100–1:2,000: centrin (Millipore 20H5), PAR6α (SCBT sc-14405), PAR6α (Abcam ab180159), β-actin (Proteintech 20536-1-AP), ASPM (SCBT sc-98903), ASPM (gift from J. Bond, 216-1), ninein (Biolegend Poly6028) and aPKCζ (SCBT sc-216).
Statistics and reproducibility
All experiments in Fig. 1e, 1, m, 2a–i, l–q, 3d, 4a–g were repeated independently three times with similar results. No statistical method was used to predetermine sample size. At least three animals or samples were generally analysed per genotype or age. Two-tailed t-tests were performed for most data using Prism 7, unless otherwise stated. Ferret kits were born at a Mendelian ratio but the genotype of each individual kit was random, which inherently randomized our experiments. To perform blinded experiments, the genotype of each animal was revealed only after the analysis was completed. In ferrets, sex was undifferentiated up to P21, after which only male ferrets were analysed. In mice, sex was undifferentiated up to P0, after which only male mice were analysed. Figure 1j, k, n = 3 male ferrets per genotype of > 8 months of age. Individual P values can be found in the Source Data associated with this figure. Figure 1n, box plot elements are maximum, third quartile, median, first quartile and minimum. E35: n = 17 Aspm+/+ or Aspm+/− ferrets and n = 10 Aspm−/− ferrets from three litters (significant differences in brain weight between Aspm+/+ or Aspm+/− and Aspm−/− ferrets are indicated: P = 0.0023); P0: n = 9 Aspm+/+ or Aspm+/− ferrets and n = 6 Aspm−/− ferrets from one litter (P = 0.0003); P21/22 (3 weeks): n = 8 Aspm+/+ or Aspm+/− ferrets and n = 3 Aspm−/− ferrets from two litters (P = 0.0010); P41 and older animals ( > 6 weeks): n = 7 Aspm+/+ or Aspm+/− ferrets and n = 7 Aspm−/− ferrets (P = 0.0094). Because brain weight was not found to be significantly different after P41, both adult and P41 animals were combined into a ‘ > 6 weeks’ group. Ferrets display considerable variability in body weight and brain weight at birth, related to variance in the exact time of birth post-conception and to litter size, which can vary from 3 to 15 kits. Thus, one small litter of three P0 kits, including one wild-type and two Aspm+/− young, which had body weights ~2× of the other P0 litters collected, were excluded from brain weight analysis. Because the y axis is log scale, overlaying each data point as dot plots for n < 10 does not indicate the distribution of the data efficiently. Instead, brain weight of individual animals can be found in the Source Data associated with this figure. Figure 1o, using the whole-brain images of coronal sections stained with Nissl or DAPI from n = 6 animals per genotype, we manually measured mean cortical thickness of the posterior sigmoid gyrus. No significant difference was found (P = 0.0843). Figure 1p, the same animals used for MRI (3 Aspm+/− and 3 Aspm−/− ferrets as described above) were used for body weight analysis. No significant difference was found (P = 0.4481). Figure 2j, immunofluorescence images were coded and counted blind to genotype by four individuals, and the four independent counts were then averaged for each brain section. The inter-individual correlation was r ≥ 0.89. Four to six brain sections were imaged and counted per animal, and n = 3 Aspm+/+ or Aspm+/− and n = 4 Aspm−/− littermate E35 animals were analysed. Figure 3f, Apod+ cells from single-molecule fluorescence in situ hybridization were segmented and counted using ImageJ, in an area of dorsal cortex 400 × 400 μm2 centred on the intermediate zone, in multiple sections per animal, with n = 4 Aspm+/+ or Aspm+/− ferrets and n = 4 Aspm−/− ferrets at E35. Per brain average counts were then compared using a one-tailed t-test.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Code availability
The code used in this study is available from the corresponding author upon reasonable request.
Data availability
scRNA-seq data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE110010. All other data are included in the paper (Source Data for Figs. 1–3) and in the Supplementary Information.
References
Faheem, M. et al. Molecular genetics of human primary microcephaly: an overview. BMC Med. Genomics 8, S4 (2015).
Bond, J. et al. ASPM is a major determinant of cerebral cortical size. Nat. Genet. 32, 316–320 (2002).
Passemard, S. et al. Abnormal spindle-like microcephaly-associated (ASPM) mutations strongly disrupt neocortical structure but spare the hippocampus and long-term memory. Cortex 74, 158–176 (2016).
Desir, J., Cassart, M., David, P., Van Bogaert, P. & Abramowicz, M. Primary microcephaly with ASPM mutation shows simplified cortical gyration with antero-posterior gradient pre- and post-natally. Am. J. Med. Genet. A 146A, 1439–1443 (2008).
Pulvers, J. N. et al. Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc. Natl Acad. Sci. USA 107, 16595–16600 (2010).
Fujimori, A. et al. Disruption of Aspm causes microcephaly with abnormal neuronal differentiation. Brain Dev. 36, 661–669 (2014).
Capecchi, M. R. & Pozner, A. ASPM regulates symmetric stem cell division by tuning cyclin E ubiquitination. Nat. Commun. 6, 8763 (2015).
Williams, S. E. et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development 142, 3921–3932 (2015).
Jayaraman, D. et al. Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron 92, 813–828 (2016).
Montgomery, S. H. & Mundy, N. I. Microcephaly genes evolved adaptively throughout the evolution of eutherian mammals. BMC Evol. Biol. 14, 120 (2014).
Bae, B. I., Jayaraman, D. & Walsh, C. A. Genetic changes shaping the human brain. Dev. Cell 32, 423–434 (2015).
Fietz, S. A. et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699 (2010).
Reillo, I., de Juan Romero, C., García-Cabezas, M. A. & Borrell, V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694 (2011).
Johnson, M. B. et al. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat. Neurosci. 18, 637–646 (2015).
Smart, I. H., Dehay, C., Giroud, P., Berland, M. & Kennedy, H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53 (2002).
Hansen, D. V., Lui, J. H., Parker, P. R. & Kriegstein, A. R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010).
Bond, J. et al. Protein-truncating mutations in ASPM cause variable reduction in brain size. Am. J. Hum. Genet. 73, 1170–1177 (2003).
Hutchinson, E. B. et al. Population based MRI and DTI templates of the adult ferret brain and tools for voxelwise analysis. Neuroimage 152, 575–589 (2017).
Martínez-Cerdeño, V. et al. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS ONE 7, e30178 (2012).
Martínez-Martínez, M. A. et al. A restricted period for formation of outer subventricular zone defined by Cdh1 and Trnp1 levels. Nat. Commun. 7, 11812 (2016).
Pollen, A. A. et al. Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67 (2015).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Kodani, A. et al. Centriolar satellites assemble centrosomal microcephaly proteins to recruit CDK2 and promote centriole duplication. eLife 4, e07519 (2015).
Paridaen, J. T., Wilsch-Bräuninger, M. & Huttner, W. B. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 155, 333–344 (2013).
Wang, X. et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955 (2009).
Singh, S. & Solecki, D. J. Polarity transitions during neurogenesis and germinal zone exit in the developing central nervous system. Front. Cell. Neurosci. 9, 62 (2015).
Dauber, A. et al. Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J. Clin. Endocrinol. Metab. 97, E2140–E2151 (2012).
Zhang, X. et al. Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell 166, 1147–1162 (2016).
Lehtinen, M. K. et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905 (2011).
Mashiko, D. et al. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3, 3355 (2013).
Li, Z., Sun, X., Chen, J., Leno, G. H. & Engelhardt, J. F. Factors affecting the efficiency of embryo transfer in the domestic ferret (Mustela putorius furo). Theriogenology 66, 183–190 (2006).
Chahboune, H. et al. Neurodevelopment of C57B/L6 mouse brain assessed by in vivo diffusion tensor imaging. NMR Biomed. 20, 375–382 (2007).
Petersen, K. F. et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl Acad. Sci. USA 104, 12587–12594 (2007).
Rueckert, D. et al. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18, 712–721 (1999).
Papademetris, X., Jackowski, A. P., Schultz, R. T., Staib, L. H. & Duncan, J. S. Integrated intensity and point-feature nonrigid registration. In International Conference on Medical Image Computing and Computer-Assisted Intervention Vol. 3216 (eds Barillot, C., et al.) 763–770 (Springer, Berlin, 2001).
Sawada, K. & Watanabe, M. Development of cerebral sulci and gyri in ferrets (Mustela putorius). Congenit. Anom. (Kyoto) 52, 168–175 (2012).
Joshi, A. et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics 9, 69–84 (2011).
Rahman, A. S., Parvinjah, S., Hanna, M. A., Helguera, P. R. & Busciglio, J. Cryopreservation of cortical tissue blocks for the generation of highly enriched neuronal cultures. J. Vis. Exp 45, e2384 (2010).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc 7, 562–578 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Villani, A. C. et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017).
Higgins, J. et al. Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol 11, 85 (2010).
Acknowledgements
We thank the late R. W. Guillery, who first introduced ferrets as a model for developmental neuroscience; J. K. Joung for advice on genome editing; J. Bond for the ASPM antibody; L. Vasung, P. Herman, J. Neil and C. D. Kroenke for advice on ferret brain MRI; A. Lee, the G. M. Church laboratory (S. Biwas), the S. McCarroll laboratory (S. Burger), the P. Kharchenko laboratory (J. Fan), and the R. Satija laboratory (A. Butler) for advice on scRNA-seq; S. Wasiuk, E. Feiner, A. S. Kamumbu and M. Lee for technical assistance; Marshall BioResources for animal husbandry; and E. Pollack and the veterinary staff at Boston Children’s Hospital and Yale School of Medicine for surgical support. Animal silhouettes in Fig. 1 were designed by Freepik from https://www.flaticon.com/. This work was supported by P30NS052519 (F.H. and Yale’s QNMR Core Center), 2R01MH067528 (F.H.), 1R24MH114805 (X.P.), R21HD083956 (K.I.), R01EB017337 (P.E.G.), R24HL123482 (J.F.E.), 5R01NS032457 (C.A.W.), 5R21NS091865 (B.-I.B.) and the Allen Discovery Center program through The Paul G. Allen Frontiers Group. C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Reviewer information
Nature thanks S. Juliano, F. Tissir and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
B.-I.B., C.A.W. and J.F.E. conceived the project. B.-I.B. generated genome-editing reagents. X.S., Z.Y. and B.L. injected ferret zygotes under J.F.E.’s supervision. M.B.J., K.M.G., P.P.W. and D.M.G. performed immunohistochemistry experiments. R.S.S., M.C., K.I., J.B.M., P.E.G., D.C., X.P., L.H.S. and F.H. performed MRI analyses. M.B.J., R.B.-M., Y.M.W. and H.K. performed scRNA-seq. M.B.J. and R.B.-M. performed single-molecule fluorescence in situ hybridization. A.K. and S.C.R. characterized molecular defects with K.P. and B.-I.B. With input from all authors, M.B.J., B.-I.B. and C.A.W interpreted the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
X.P. is a consultant for Electrical Geodesics Inc. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended Data Figures and Tables
Extended Data Fig. 1 Cytoarchitecture and neuronal subtype lamination in the cortex of mature Aspm knockout ferrets.
a, Nissl stains of coronal sections from the brains of P41 littermates, as shown in Fig. 1l, with additional Aspm+/− and Aspm−/− littermates shown. b, c, Brain sections of P41 littermates immunostained for cortical layer-specific projection neurons including SATB2 (layer II–IV), CTIP2 (layer V), and FoxP2 (layer VI). The experiments were repeated independently three times with similar results. Scale bars, 2 mm (a, top), 200 μm (a, bottom) and 100 μm (b, c).
Extended Data Fig. 2 SOX2 and Ki-67 immunostaining in additional E35 and P0 littermates dorsal cortex.
Additional results to Fig. 2e, f. Each set of panels is from the brain of a different littermate, showing the high penetrance of the neural progenitor cell basal displacement phenotype. The experiments were repeated independently three times with similar results. Scale bar, 200 μm.
Extended Data Fig. 3 Displaced progenitors in Aspm knockout ferrets have basal fibres.
Additional results for Fig. 2h, i. Immunostaining of SOX2, Ki-67 and VIM shows that displaced neural progenitors have basal radial fibres. The experiments were repeated independently three times with similar results. Scale bars, 100 μm.
Extended Data Fig. 4 Aspm knockout mice do not demonstrate displaced progenitors in the intermediate zone.
a, b, Unlike Aspm−/− ferrets, Aspm−/− mice do not have displaced NPCs in the intermediate zone. However, they show a variable increase in the number of intermediate progenitors (PAX6−Ki-67+ cells in a and TBR2+ cells in b), which is enhanced by heterozygous, compound mutation in Wdr62, a microcephaly gene causing more severe microcephaly9. The experiments were repeated independently three times with similar results. Scale bars, 100 μm.
Extended Data Fig. 5 Modest increase in apoptosis throughout the germinal zones of the Aspm knockout telencephalon.
Apoptotic cells (yellow) are indicated by enzymatic fluorescence detection of double-stranded DNA damage with DAPI nuclear counterstaining (blue). The experiments were repeated independently three times with similar results. a, Whole section. b, c, Cortical wall columns. Scale bars, 500 μm (a) and 100 μm (b, c).
Extended Data Fig. 6 Additional immunohistochemical analyses of displaced progenitors in the Aspm knockout cortex.
a, E35 knockout cortex stained for VRG and ORG markers SOX2 and HOPX reveals extensive co-labelling in both the ventricular zone (VZ) and SVZ, including in displaced OSVZ progenitors. b, In the E35 knockout OSVZ, clusters of supernumerary displaced neural progenitor cells include numerous TBR2+ intermediate progenitors and are surrounded by DCX+ newborn neurons, indicating preserved neurogenesis within the precocious OSVZ niche of the Aspm knockout cortex. The experiments were repeated independently three times with similar results. Scale bars, 50 μm.
Extended Data Fig. 7 scRNA-seq batch, sample and cluster analyses.
a, t-SNE plot from Fig. 3a with cells coloured by biological replicate (that is, animal). Most clusters include cells from all samples, except for a cluster expressing blood genes and a cluster expressing choroid plexus epithelial cells that are mostly from animal WT5E. These two cell clusters were not included in downstream analyses. HET, heterozygote; KO, knockout; WT, wild type. Numbers and letters indicate litter and animal identification number, respectively. b, t-SNE plot from Fig. 3a with cells coloured by the batch they were processed in. Clusters are composed of cells from all batches. c, Per-cell gene count and UMI count per sample. Each violin plot is one biological replicate and each dot is one cell. Sample WT5D was not included in the analysis due to the lower gene and UMI count compared to other samples as well as the inconsistent clustering compared to other wild-type samples (data not shown). d, Per-cell gene count and UMI count for identified clusters. Each violin plot is one cell cluster and each dot is one cell. The three clusters in grey (EN4, BL, CPE) were not included in downstream analyses. See Methods for details. This scRNA-seq experiment was performed once with n = 22,211 cells (8,037 cells from two Aspm+/+ and one Aspm+/− ferrets and 14,174 cells from four Aspm−/− ferrets). RG1, cycling radial glial progenitors; RG2, interphase radial glial progenitors; IP, intermediate progenitors; EN1, upper-layer excitatory neurons; EN2, deep-layer excitatory neurons; EN3, Cajal–Retzius cells; IN1, immature inhibitory neurons; IN2, SST+ inhibitory neurons; IN3, ventral/inhibitory progenitors; ENDO1, endothelial cells 1; ENDO2, endothelial cells 2; OPC, oligodendrocyte precursors; MG, microglia; EN4, mixed excitatory neuron identity; BL, blood cells; CPE, choroid plexus epithelial cells.
Extended Data Fig. 8 Loss of Aspm disrupts centriole duplication in FEFs.
Mitotic Aspm knockout FEFs, identified by staining for pH3 and co-stained for the centriolar marker centrin, display a significant loss of centrioles. The percentage of cells with an abnormal number (less than 4) of centrioles is increased eightfold in Aspm−/− FEFs compared to Aspm+/+ FEFs (n = 100 cells per genotype for three independent experiments; P = 0.003). The experiments were repeated independently three times with similar results. Statistical analysis was performed using a two-tailed t-test; data are mean ± s.e.m.
Rights and permissions
About this article
Cite this article
Johnson, M.B., Sun, X., Kodani, A. et al. Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 556, 370–375 (2018). https://doi.org/10.1038/s41586-018-0035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0035-0
This article is cited by
-
The growth factor EPIREGULIN promotes basal progenitor cell proliferation in the developing neocortex
The EMBO Journal (2024)
-
Embryonic mouse medial neocortex as a model system for studying the radial glial scaffold in fetal human neocortex
Journal of Neural Transmission (2023)
-
How mechanisms of stem cell polarity shape the human cerebral cortex
Nature Reviews Neuroscience (2022)
-
Endosomal trafficking defects alter neural progenitor proliferation and cause microcephaly
Nature Communications (2022)
-
Inhibiting microcephaly genes as alternative to microtubule targeting agents to treat brain tumors
Cell Death & Disease (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.