Abstract
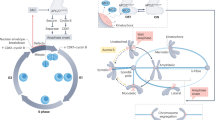
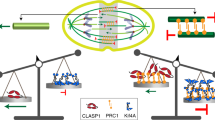
The microtubule-based spindle orchestrates chromosome segregation during cell division. Following more than a century of study, many components and pathways contributing to spindle assembly have been described, but how the spindle robustly assembles remains incompletely understood. This process involves the self-organization of a large number of molecular parts — up to hundreds of thousands in vertebrate cells — whose local interactions give rise to a cellular-scale structure with emergent architecture, mechanics and function. In this Review, we discuss key concepts in our understanding of spindle assembly, focusing on recent advances and the new approaches that enabled them. We describe the pathways that generate the microtubule framework of the spindle by driving microtubule nucleation in a spatially controlled fashion and present recent insights regarding the organization of individual microtubules into structural modules. Finally, we discuss the emergent properties of the spindle that enable robust chromosome segregation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Flemming, W. Zellsubstanz, kern und zelltheilung (Vogel, 1882).
Santaguida, S. & Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485 (2015).
Potapova, T. & Gorbsky, G. J. The consequences of chromosome segregation errors in mitosis and meiosis. Biology https://doi.org/10.3390/biology6010012 (2017).
Thawani, A. & Petry, S. Molecular insight into how gamma-TuRC makes microtubules. J. Cell Sci. https://doi.org/10.1242/jcs.245464 (2021).
Voter, W. A. & Erickson, H. P. The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. J. Biol. Chem. 259, 10430–10438 (1984).
Zupa, E., Liu, P., Wurtz, M., Schiebel, E. & Pfeffer, S. The structure of the gamma-TuRC: a 25-years-old molecular puzzle. Curr. Opin. Struct. Biol. 66, 15–21 (2021).
Liu, P. et al. Insights into the assembly and activation of the microtubule nucleator gamma-TuRC. Nature 578, 467–471 (2020).
Wieczorek, M. et al. Asymmetric molecular architecture of the human gamma-tubulin ring complex. Cell 180, 165–175.e16 (2020).
Consolati, T. et al. Microtubule nucleation properties of single human gammaTuRCs explained by their Cryo-EM structure. Dev. Cell 53, 603–617.e8 (2020).
Thawani, A. et al. The transition state and regulation of gamma-TuRC-mediated microtubule nucleation revealed by single molecule microscopy. eLife https://doi.org/10.7554/eLife.54253 (2020).
Thawani, A., Kadzik, R. S. & Petry, S. XMAP215 is a microtubule nucleation factor that functions synergistically with the gamma-tubulin ring complex. Nat. Cell Biol. 20, 575–585 (2018).
Flor-Parra, I., Iglesias-Romero, A. B. & Chang, F. The XMAP215 ortholog Alp14 promotes microtubule nucleation in fission yeast. Curr. Biol. 28, 1681–1691.e4 (2018).
King, B. R. et al. XMAP215 and gamma-tubulin additively promote microtubule nucleation in purified solutions. Mol. Biol. Cell 31, 2187–2194 (2020).
Gunzelmann, J. et al. The microtubule polymerase Stu2 promotes oligomerization of the gamma-TuSC for cytoplasmic microtubule nucleation. eLife https://doi.org/10.7554/eLife.39932 (2018).
Tsuchiya, K. et al. Ran-GTP is non-essential to activate NuMA for mitotic spindle-pole focusing but dynamically polarizes HURP near chromosomes. Curr. Biol. 31, 115–127.e3 (2021).
Sampaio, P., Rebollo, E., Varmark, H., Sunkel, C. E. & Gonzalez, C. Organized microtubule arrays in gamma-tubulin-depleted Drosophila spermatocytes. Curr. Biol. 11, 1788–1793 (2001).
Brunet, S. et al. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol. Biol. Cell 15, 5318–5328 (2004).
Imasaki, T. et al. CAMSAP2 organizes a gamma-tubulin-independent microtubule nucleation centre through phase separation. eLife https://doi.org/10.7554/eLife.77365 (2022).
McKinley, K. L. & Cheeseman, I. M. Large-scale analysis of CRISPR/Cas9 cell-cycle knockouts reveals the diversity of p53-dependent responses to cell-cycle defects. Dev. Cell 40, 405–420.e2 (2017).
Gudimchuk, N. B. & McIntosh, J. R. Regulation of microtubule dynamics, mechanics and function through the growing tip. Nat. Rev. Mol. Cell Biol. 22, 777–795 (2021).
Choi, Y. K., Liu, P., Sze, S. K., Dai, C. & Qi, R. Z. CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J. Cell Biol. 191, 1089–1095 (2010).
Liu, P., Choi, Y. K. & Qi, R. Z. NME7 is a functional component of the gamma-tubulin ring complex. Mol. Biol. Cell 25, 2017–2025 (2014).
Cota, R. R. et al. MZT1 regulates microtubule nucleation by linking gammaTuRC assembly to adapter-mediated targeting and activation. J. Cell Sci. 130, 406–419 (2017).
Kollman, J. M. et al. Ring closure activates yeast gammaTuRC for species-specific microtubule nucleation. Nat. Struct. Mol. Biol. 22, 132–137 (2015).
Woodruff, J. B. et al. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077.e10 (2017).
Baumgart, J. et al. Soluble tubulin is significantly enriched at mitotic centrosomes. J. Cell Biol. 218, 3977–3985 (2019).
King, M. R. & Petry, S. Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat. Commun. 11, 270 (2020).
Haren, L., Stearns, T. & Luders, J. Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE 4, e5976 (2009).
Lee, K. & Rhee, K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J. Cell Biol. 195, 1093–1101 (2011).
Haren, L. et al. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505–515 (2006).
Luders, J., Patel, U. K. & Stearns, T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137–147 (2006).
Fong, K. W., Choi, Y. K., Rattner, J. B. & Qi, R. Z. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol. Biol. Cell 19, 115–125 (2008).
Gomez-Ferreria, M. A. et al. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 17, 1960–1966 (2007).
Joukov, V., Walter, J. C. & De Nicolo, A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol. Cell 55, 578–591 (2014).
Zhang, X. et al. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome. J. Cell Sci. 122, 2240–2251 (2009).
Zimmerman, W. C., Sillibourne, J., Rosa, J. & Doxsey, S. J. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642–3657 (2004).
Rale, M. J., Romer, B., Mahon, B. P., Travis, S. M. & Petry, S. The conserved centrosomin motif, γTuNA, forms a dimer that directly activates microtubule nucleation by the γ-tubulin ring complex (γTuRC). eLife https://doi.org/10.7554/eLife.80053 (2022).
Tungadi, E. A., Ito, A., Kiyomitsu, T. & Goshima, G. Human microcephaly ASPM protein is a spindle pole-focusing factor that functions redundantly with CDK5RAP2. J. Cell Sci. 130, 3676–3684 (2017).
Heald, R. et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996).
Matthies, H. J., McDonald, H. B., Goldstein, L. S. & Theurkauf, W. E. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 134, 455–464 (1996).
Schuh, M. & Ellenberg, J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484–498 (2007).
Zhang, H. & Dawe, R. K. Mechanisms of plant spindle formation. Chromosome Res. 19, 335–344 (2011).
Khodjakov, A., Cole, R. W., Oakley, B. R. & Rieder, C. L. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 10, 59–67 (2000).
Moutinho-Pereira, S. et al. Genes involved in centrosome-independent mitotic spindle assembly in Drosophila S2 cells. Proc. Natl Acad. Sci. USA 110, 19808–19813 (2013).
Basto, R. et al. Flies without centrioles. Cell 125, 1375–1386 (2006).
Bischoff, F. R. & Ponstingl, H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 354, 80–82 (1991).
Gorlich, D., Pante, N., Kutay, U., Aebi, U. & Bischoff, F. R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 15, 5584–5594 (1996).
Cavazza, T. & Vernos, I. The RanGTP Pathway: from nucleo-cytoplasmic transport to spindle assembly and beyond. Front. Cell Dev. Biol. 3, 82 (2015).
Gruss, O. J. et al. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83–93 (2001).
Nachury, M. V. et al. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104, 95–106 (2001).
Wiese, C. et al. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 291, 653–656 (2001).
Kalab, P., Weis, K. & Heald, R. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295, 2452–2456 (2002).
Kalab, P., Pralle, A., Isacoff, E. Y., Heald, R. & Weis, K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 440, 697–701 (2006).
Goshima, G., Mayer, M., Zhang, N., Stuurman, N. & Vale, R. D. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181, 421–429 (2008).
Lawo, S. et al. HAUS, the 8-subunit human Augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 19, 816–826 (2009).
Uehara, R. et al. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc. Natl Acad. Sci. USA 106, 6998–7003 (2009).
Ho, C. M. et al. Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant. Cell 23, 2606–2618 (2011).
Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. & Vale, R. D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777 (2013).
Hayward, D., Metz, J., Pellacani, C. & Wakefield, J. G. Synergy between multiple microtubule-generating pathways confers robustness to centrosome-driven mitotic spindle formation. Dev. Cell 28, 81–93 (2014).
David, A. F. et al. Augmin accumulation on long-lived microtubules drives amplification and kinetochore-directed growth. J. Cell Biol. 218, 2150–2168 (2019).
Tariq, A., Green, L., Jeynes, J. C. G., Soeller, C. & Wakefield, J. G. In vitro reconstitution of branching microtubule nucleation. eLife https://doi.org/10.7554/eLife.49769 (2020).
Zhang, Y., Hong, X., Hua, S. & Jiang, K. Reconstitution and mechanistic dissection of the human microtubule branching machinery. J. Cell Biol. https://doi.org/10.1083/jcb.202109053 (2022).
Alfaro-Aco, R., Thawani, A. & Petry, S. Biochemical reconstitution of branching microtubule nucleation. eLife https://doi.org/10.7554/eLife.49797 (2020).
Hsia, K. C. et al. Reconstitution of the augmin complex provides insights into its architecture and function. Nat. Cell Biol. 16, 852–863 (2014).
Song, J. G. et al. Mechanism of how augmin directly targets the gamma-tubulin ring complex to microtubules. J. Cell Biol. 217, 2417–2428 (2018).
Zhu, H., Coppinger, J. A., Jang, C. Y., Yates, J. R. III & Fang, G. FAM29A promotes microtubule amplification via recruitment of the NEDD1-gamma-tubulin complex to the mitotic spindle. J. Cell Biol. 183, 835–848 (2008).
Petry, S., Pugieux, C., Nedelec, F. J. & Vale, R. D. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl Acad. Sci. USA 108, 14473–14478 (2011).
Almeida, A. C. et al. Augmin-dependent microtubule self-organization drives kinetochore fiber maturation in mammals. Cell Rep. 39, 110610 (2022).
Verma, V. & Maresca, T. J. Direct observation of branching MT nucleation in living animal cells. J. Cell Biol. 218, 2829–2840 (2019).
Thawani, A., Stone, H. A., Shaevitz, J. W. & Petry, S. Spatiotemporal organization of branched microtubule networks. eLife https://doi.org/10.7554/eLife.43890 (2019).
Setru, S. U. et al. A hydrodynamic instability drives protein droplet formation on microtubules to nucleate branches. Nat. Phys. 17, 493–498 (2021).
Roostalu, J., Cade, N. I. & Surrey, T. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422–1434 (2015).
Reid, T. A. et al. Suppression of microtubule assembly kinetics by the mitotic protein TPX2. J. Cell Sci. 129, 1319–1328 (2016).
Luo, J. et al. The microtubule-associated protein EML3 regulates mitotic spindle assembly by recruiting the Augmin complex to spindle microtubules. J. Biol. Chem. 294, 5643–5656 (2019).
Sanchez-Huertas, C. & Luders, J. The augmin connection in the geometry of microtubule networks. Curr. Biol. 25, R294–R299 (2015).
Khodjakov, A., Copenagle, L., Gordon, M. B., Compton, D. A. & Kapoor, T. M. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J. Cell Biol. 160, 671–683 (2003).
Maiato, H., Rieder, C. L. & Khodjakov, A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J. Cell Biol. 167, 831–840 (2004).
Sikirzhytski, V. et al. Microtubules assemble near most kinetochores during early prometaphase in human cells. J. Cell Biol. 217, 2647–2659 (2018).
Renda, F. et al. Non-centrosomal microtubules at kinetochores promote rapid chromosome biorientation during mitosis in human cells. Curr. Biol. 32, 1049–1063.e4 (2022).
Orjalo, A. V. et al. The Nup107-160 nucleoporin complex is required for correct bipolar spindle assembly. Mol. Biol. Cell 17, 3806–3818 (2006).
Mishra, R. K., Chakraborty, P., Arnaoutov, A., Fontoura, B. M. & Dasso, M. The Nup107-160 complex and γ-TuRC regulate microtubule polymerization at kinetochores. Nat. Cell Biol. 12, 164–169 (2010).
Yokoyama, H. et al. The nucleoporin MEL-28 promotes RanGTP-dependent gamma-tubulin recruitment and microtubule nucleation in mitotic spindle formation. Nat. Commun. 5, 3270 (2014).
Rasala, B. A., Orjalo, A. V., Shen, Z., Briggs, S. & Forbes, D. J. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl Acad. Sci. USA 103, 17801–17806 (2006).
Tulu, U. S., Fagerstrom, C., Ferenz, N. P. & Wadsworth, P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 16, 536–541 (2006).
Euteneuer, U., Ris, H. & Borisy, G. G. Polarity of kinetochore microtubules in Chinese hamster ovary cells after recovery from a colcemid block. J. Cell Biol. 97, 202–208 (1983).
Shrestha, R. L. & Draviam, V. M. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Curr. Biol. 23, 1514–1526 (2013).
Popova, J. V. et al. Genetic control of kinetochore-driven microtubule growth in Drosophila mitosis. Cells https://doi.org/10.3390/cells11142127 (2022).
Maresca, T. J. et al. Spindle assembly in the absence of a RanGTP gradient requires localized CPC activity. Curr. Biol. 19, 1210–1215 (2009).
Gassmann, R. et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191 (2004).
Lan, W. et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273–286 (2004).
Sampath, S. C. et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187–202 (2004).
Ohi, R., Sapra, T., Howard, J. & Mitchison, T. J. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell 15, 2895–2906 (2004).
Tan, L. & Kapoor, T. M. Examining the dynamics of chromosomal passenger complex (CPC)-dependent phosphorylation during cell division. Proc. Natl Acad. Sci. USA 108, 16675–16680 (2011).
Niethammer, P., Bastiaens, P. & Karsenti, E. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science 303, 1862–1866 (2004).
Trivedi, P. et al. The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol. 21, 1127–1137 (2019).
Musacchio, A. On the role of phase separation in the biogenesis of membraneless compartments. EMBO J. 41, e109952 (2022).
Rebollo, E., Llamazares, S., Reina, J. & Gonzalez, C. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biol. 2, E8 (2004).
So, C. et al. A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian oocytes. Science https://doi.org/10.1126/science.aat9557 (2019).
Halpin, D., Kalab, P., Wang, J., Weis, K. & Heald, R. Mitotic spindle assembly around RCC1-coated beads in Xenopus egg extracts. PLoS Biol. 9, e1001225 (2011).
Wolff, I. D., Tran, M. V., Mullen, T. J., Villeneuve, A. M. & Wignall, S. M. Assembly of Caenorhabditis elegans acentrosomal spindles occurs without evident microtubule-organizing centers and requires microtubule sorting by KLP-18/kinesin-12 and MESP-1. Mol. Biol. Cell 27, 3122–3131 (2016).
Dumont, J. et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J. Cell Biol. 176, 295–305 (2007).
Ma, N. et al. Poleward transport of TPX2 in the mammalian mitotic spindle requires dynein, Eg5, and microtubule flux. Mol. Biol. Cell 21, 979–988 (2010).
Heald, R., Tournebize, R., Habermann, A., Karsenti, E. & Hyman, A. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138, 615–628 (1997).
Lecland, N. & Luders, J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat. Cell Biol. 16, 770–778 (2014).
Prosser, S. L. & Pelletier, L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat. Rev. Mol. Cell Biol. 18, 187–201 (2017).
Gaska, I., Armstrong, M. E., Alfieri, A. & Forth, S. The mitotic crosslinking protein PRC1 acts like a mechanical dashpot to resist microtubule sliding. Dev. Cell 54, 367–378.e5 (2020).
Verde, F., Berrez, J. M., Antony, C. & Karsenti, E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: requirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 112, 1177–1187 (1991).
Gaglio, T., Saredi, A. & Compton, D. A. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 131, 693–708 (1995).
Surrey, T., Nedelec, F., Leibler, S. & Karsenti, E. Physical properties determining self-organization of motors and microtubules. Science 292, 1167–1171 (2001).
Tan, R., Foster, P. J., Needleman, D. J. & McKenney, R. J. Cooperative accumulation of dynein-dynactin at microtubule minus-ends drives microtubule network reorganization. Dev. Cell 44, 233–247.e4 (2018).
Bieling, P., Telley, I. A. & Surrey, T. A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell 142, 420–432 (2010).
Wijeratne, S. & Subramanian, R. Geometry of antiparallel microtubule bundles regulates relative sliding and stalling by PRC1 and Kif4A. eLife https://doi.org/10.7554/eLife.32595 (2018).
Hannabuss, J. et al. Self-organization of minimal anaphase spindle midzone bundles. Curr. Biol. 29, 2120–2130.e7 (2019).
McDonald, K. L., O’Toole, E. T., Mastronarde, D. N. & McIntosh, J. R. Kinetochore microtubules in PTK cells. J. Cell Biol. 118, 369–383 (1992).
Long, A. F., Kuhn, J. & Dumont, S. The mammalian kinetochore-microtubule interface: robust mechanics and computation with many microtubules. Curr. Opin. Cell Biol. 60, 60–67 (2019).
Booth, D. G., Hood, F. E., Prior, I. A. & Royle, S. J. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 30, 906–919 (2011).
Kiewisz, R. et al. Three-dimensional structure of kinetochore-fibers in human mitotic spindles. eLife https://doi.org/10.7554/eLife.75459 (2022).
O’Toole, E., Morphew, M. & McIntosh, J. R. Electron tomography reveals aspects of spindle structure important for mechanical stability at metaphase. Mol. Biol. Cell 31, 184–195 (2020).
Drpic, D. et al. Chromosome segregation is biased by kinetochore size. Curr. Biol. 28, 1344–1356.e5 (2018).
Stumpff, J., von Dassow, G., Wagenbach, M., Asbury, C. & Wordeman, L. The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev. Cell 14, 252–262 (2008).
Varga, V. et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat. Cell Biol. 8, 957–962 (2006).
Du, Y., English, C. A. & Ohi, R. The kinesin-8 Kif18A dampens microtubule plus-end dynamics. Curr. Biol. 20, 374–380 (2010).
Stumpff, J. et al. A tethering mechanism controls the processivity and kinetochore-microtubule plus-end enrichment of the kinesin-8 Kif18A. Mol. Cell 43, 764–775 (2011).
Dudka, D., Castrogiovanni, C., Liaudet, N., Vassal, H. & Meraldi, P. Spindle-length-dependent HURP localization allows centrosomes to control kinetochore-fiber plus-end dynamics. Curr. Biol. 29, 3563–3578.e6 (2019).
Zhai, Y., Kronebusch, P. J. & Borisy, G. G. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 131, 721–734 (1995).
Nicklas, R. B. & Koch, C. A. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 43, 40–50 (1969).
DeLuca, J. G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 (2006).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Koffa, M. D. et al. HURP is part of a Ran-dependent complex involved in spindle formation. Curr. Biol. 16, 743–754 (2006).
Sillje, H. H., Nagel, S., Korner, R. & Nigg, E. A. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr. Biol. 16, 731–742 (2006).
Begley, M. A. et al. K-fiber bundles in the mitotic spindle are mechanically reinforced by Kif15. Mol. Biol. Cell 32, br11 (2021).
Nixon, F. M. et al. The mesh is a network of microtubule connectors that stabilizes individual kinetochore fibers of the mitotic spindle. eLife https://doi.org/10.7554/eLife.07635 (2015).
Nicklas, R. B., Kubai, D. F. & Hays, T. S. Spindle microtubules and their mechanical associations after micromanipulation in anaphase. J. Cell Biol. 95, 91–104 (1982).
Kirschner, M. & Mitchison, T. Beyond self-assembly: from microtubules to morphogenesis. Cell 45, 329–342 (1986).
Magidson, V. et al. Adaptive changes in the kinetochore architecture facilitate proper spindle assembly. Nat. Cell Biol. 17, 1134–1144 (2015).
Wollman, R. et al. Efficient chromosome capture requires a bias in the ‘search-and-capture’ process during mitotic-spindle assembly. Curr. Biol. 15, 828–832 (2005).
Magidson, V. et al. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 (2011).
Wynne, D. J. & Funabiki, H. Kinetochore function is controlled by a phospho-dependent coexpansion of inner and outer components. J. Cell Biol. 210, 899–916 (2015).
Sacristan, C. et al. Dynamic kinetochore size regulation promotes microtubule capture and chromosome biorientation in mitosis. Nat. Cell Biol. 20, 800–810 (2018).
Mitchison, T., Evans, L., Schulze, E. & Kirschner, M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell 45, 515–527 (1986).
Rieder, C. L. & Alexander, S. P. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 110, 81–95 (1990).
Hueschen, C. L., Kenny, S. J., Xu, K. & Dumont, S. NuMA recruits dynein activity to microtubule minus-ends at mitosis. eLife https://doi.org/10.7554/eLife.29328 (2017).
Elting, M. W., Hueschen, C. L., Udy, D. B. & Dumont, S. Force on spindle microtubule minus ends moves chromosomes. J. Cell Biol. 206, 245–256 (2014).
Sikirzhytski, V. et al. Direct kinetochore-spindle pole connections are not required for chromosome segregation. J. Cell Biol. 206, 231–243 (2014).
Mastronarde, D. N., McDonald, K. L., Ding, R. & McIntosh, J. R. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 123, 1475–1489 (1993).
Kajtez, J. et al. Overlap microtubules link sister k-fibres and balance the forces on bi-oriented kinetochores. Nat. Commun. 7, 10298 (2016).
Polak, B., Risteski, P., Lesjak, S. & Tolic, I. M. PRC1-labeled microtubule bundles and kinetochore pairs show one-to-one association in metaphase. EMBO Rep. 18, 217–230 (2017).
Mollinari, C. et al. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157, 1175–1186 (2002).
Kapitein, L. C. et al. Microtubule-driven multimerization recruits ase1p onto overlapping microtubules. Curr. Biol. 18, 1713–1717 (2008).
Subramanian, R. et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 142, 433–443 (2010).
Jagric, M., Risteski, P., Martincic, J., Milas, A. & Tolic, I. M. Optogenetic control of PRC1 reveals its role in chromosome alignment on the spindle by overlap length-dependent forces. eLife https://doi.org/10.7554/eLife.61170 (2021).
Brinkley, B. R. & Cartwright, J. Jr. Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann. N. Y. Acad. Sci. 253, 428–439 (1975).
Ohi, R., Burbank, K., Liu, Q. & Mitchison, T. J. Nonredundant functions of Kinesin-13s during meiotic spindle assembly. Curr. Biol. 17, 953–959 (2007).
Burbank, K. S., Groen, A. C., Perlman, Z. E., Fisher, D. S. & Mitchison, T. J. A new method reveals microtubule minus ends throughout the meiotic spindle. J. Cell Biol. 175, 369–375 (2006).
Yang, G. et al. Architectural dynamics of the meiotic spindle revealed by single-fluorophore imaging. Nat. Cell Biol. 9, 1233–1242 (2007).
Saxton, W. M. et al. Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 99, 2175–2186 (1984).
Wandke, C. et al. Human chromokinesins promote chromosome congression and spindle microtubule dynamics during mitosis. J. Cell Biol. 198, 847–863 (2012).
Antonio, C. et al. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102, 425–435 (2000).
Funabiki, H. & Murray, A. W. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102, 411–424 (2000).
Brouhard, G. J. & Hunt, A. J. Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc. Natl Acad. Sci. USA 102, 13903–13908 (2005).
Ke, K., Cheng, J. & Hunt, A. J. The distribution of polar ejection forces determines the amplitude of chromosome directional instability. Curr. Biol. 19, 807–815 (2009).
Rieder, C. L. & Salmon, E. D. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J. Cell Biol. 124, 223–233 (1994).
Stout, J. R. et al. Kif18B interacts with EB1 and controls astral microtubule length during mitosis. Mol. Biol. Cell 22, 3070–3080 (2011).
Tanenbaum, M. E. et al. A complex of Kif18b and MCAK promotes microtubule depolymerization and is negatively regulated by Aurora kinases. Curr. Biol. 21, 1356–1365 (2011).
Dema, A., van Haren, J. & Wittmann, T. Optogenetic EB1 inactivation shortens metaphase spindles by disrupting cortical force-producing interactions with astral microtubules. Curr. Biol. 32, 1197–1205.e4 (2022).
Barisic, M. et al. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 348, 799–803 (2015).
di Pietro, F., Echard, A. & Morin, X. Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 17, 1106–1130 (2016).
Lechler, T. & Mapelli, M. Spindle positioning and its impact on vertebrate tissue architecture and cell fate. Nat. Rev. Mol. Cell Biol. 22, 691–708 (2021).
Thery, M. et al. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953 (2005).
Fink, J. et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 13, 771–778 (2011).
Guild, J., Ginzberg, M. B., Hueschen, C. L., Mitchison, T. J. & Dumont, S. Increased lateral microtubule contact at the cell cortex is sufficient to drive mammalian spindle elongation. Mol. Biol. Cell 28, 1975–1983 (2017).
Schaefer, M., Shevchenko, A., Shevchenko, A. & Knoblich, J. A. A protein complex containing Inscuteable and the Galpha-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol. 10, 353–362 (2000).
Gotta, M., Dong, Y., Peterson, Y. K., Lanier, S. M. & Ahringer, J. Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr. Biol. 13, 1029–1037 (2003).
Du, Q. & Macara, I. G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004).
Fielmich, L. E. et al. Optogenetic dissection of mitotic spindle positioning in vivo. eLife https://doi.org/10.7554/eLife.38198 (2018).
Okumura, M., Natsume, T., Kanemaki, M. T. & Kiyomitsu, T. Dynein-dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. eLife https://doi.org/10.7554/eLife.36559 (2018).
Hornick, J. E. et al. Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr. Biol. 21, 598–605 (2011).
Sir, J. H. et al. Loss of centrioles causes chromosomal instability in vertebrate somatic cells. J. Cell Biol. 203, 747–756 (2013).
Wong, Y. L. et al. Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science 348, 1155–1160 (2015).
Chinen, T. et al. NuMA assemblies organize microtubule asters to establish spindle bipolarity in acentrosomal human cells. EMBO J. 39, e102378 (2020).
Merdes, A., Heald, R., Samejima, K., Earnshaw, W. C. & Cleveland, D. W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 149, 851–862 (2000).
Goshima, G., Nedelec, F. & Vale, R. D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 171, 229–240 (2005).
Hueschen, C. L., Galstyan, V., Amouzgar, M., Phillips, R. & Dumont, S. Microtubule end-clustering maintains a steady-state spindle shape. Curr. Biol. 29, 700–708.e5 (2019).
Echeverri, C. J., Paschal, B. M., Vaughan, K. T. & Vallee, R. B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 (1996).
Renna, C. et al. Organizational principles of the NuMA-Dynein interaction interface and implications for mitotic spindle functions. Structure 28, 820–829.e6 (2020).
Harborth, J., Wang, J., Gueth-Hallonet, C., Weber, K. & Osborn, M. Self assembly of NuMA: multiarm oligomers as structural units of a nuclear lattice. EMBO J. 18, 1689–1700 (1999).
Sun, M. et al. NuMA regulates mitotic spindle assembly, structural dynamics and function via phase separation. Nat. Commun. 12, 7157 (2021).
Ma, H. et al. NuMA forms condensates through phase separation to drive spindle pole assembly. J. Mol. Cell Biol. https://doi.org/10.1093/jmcb/mjab081 (2022).
Mountain, V. et al. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 147, 351–366 (1999).
Cai, S., Weaver, L. N., Ems-McClung, S. C. & Walczak, C. E. Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Mol. Biol. Cell 20, 1348–1359 (2009).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Sawin, K. E., LeGuellec, K., Philippe, M. & Mitchison, T. J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, 540–543 (1992).
Blangy, A. et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169 (1995).
Kashina, A. S., Scholey, J. M., Leszyk, J. D. & Saxton, W. M. An essential bipolar mitotic motor. Nature 384, 225 (1996).
Kapitein, L. C. et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435, 114–118 (2005).
Raaijmakers, J. A. et al. Nuclear envelope-associated dynein drives prophase centrosome separation and enables Eg5-independent bipolar spindle formation. EMBO J. 31, 4179–4190 (2012).
Toso, A. et al. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J. Cell Biol. 184, 365–372 (2009).
Sturgill, E. G. & Ohi, R. Kinesin-12 differentially affects spindle assembly depending on its microtubule substrate. Curr. Biol. 23, 1280–1290 (2013).
Tanenbaum, M. E. et al. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr. Biol. 19, 1703–1711 (2009).
Vanneste, D., Takagi, M., Imamoto, N. & Vernos, I. The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr. Biol. 19, 1712–1717 (2009).
Drechsler, H., McHugh, T., Singleton, M. R., Carter, N. J. & McAinsh, A. D. The Kinesin-12 Kif15 is a processive track-switching tetramer. eLife 3, e01724 (2014).
Reinemann, D. N. et al. Collective force regulation in anti-parallel microtubule gliding by dimeric Kif15 kinesin motors. Curr. Biol. 27, 2810–2820.e6 (2017).
Umbreit, N. T. et al. Mechanisms generating cancer genome complexity from a single cell division error. Science https://doi.org/10.1126/science.aba0712 (2020).
van Heesbeen, R. G., Tanenbaum, M. E. & Medema, R. H. Balanced activity of three mitotic motors is required for bipolar spindle assembly and chromosome segregation. Cell Rep. 8, 948–956 (2014).
Neahring, L., Cho, N. H. & Dumont, S. Opposing motors provide mechanical and functional robustness in the human spindle. Dev. Cell 56, 3006–3018.e5 (2021).
Sturgill, E. G., Norris, S. R., Guo, Y. & Ohi, R. Kinesin-5 inhibitor resistance is driven by kinesin-12. J. Cell Biol. 213, 213–227 (2016).
Mitchison, T. J. et al. Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol. Biol. Cell 16, 3064–3076 (2005).
Tanenbaum, M. E., Macurek, L., Galjart, N. & Medema, R. H. Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J. 27, 3235–3245 (2008).
Ferenz, N. P., Paul, R., Fagerstrom, C., Mogilner, A. & Wadsworth, P. Dynein antagonizes eg5 by crosslinking and sliding antiparallel microtubules. Curr. Biol. 19, 1833–1838 (2009).
Brugues, J., Nuzzo, V., Mazur, E. & Needleman, D. J. Nucleation and transport organize microtubules in metaphase spindles. Cell 149, 554–564 (2012).
Elting, M. W., Prakash, M., Udy, D. B. & Dumont, S. Mapping load-bearing in the mammalian spindle reveals local kinetochore fiber anchorage that provides mechanical isolation and redundancy. Curr. Biol. 27, 2112–2122.e5 (2017).
Suresh, P., Long, A. F. & Dumont, S. Microneedle manipulation of the mammalian spindle reveals specialized, short-lived reinforcement near chromosomes. eLife https://doi.org/10.7554/eLife.53807 (2020).
Franck, A. D. et al. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat. Cell Biol. 9, 832–837 (2007).
Akiyoshi, B. et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 (2010).
Skibbens, R. V. & Salmon, E. D. Micromanipulation of chromosomes in mitotic vertebrate tissue cells: tension controls the state of kinetochore movement. Exp. Cell Res. 235, 314–324 (1997).
Nicklas, R. B. & Staehly, C. A. Chromosome micromanipulation. I. The mechanics of chromosome attachment to the spindle. Chromosoma 21, 1–16 (1967).
Long, A. F., Suresh, P. & Dumont, S. Individual kinetochore-fibers locally dissipate force to maintain robust mammalian spindle structure. J. Cell Biol. 219, https://doi.org/10.1083/jcb.201911090 (2020).
Dumont, S. & Mitchison, T. J. Compression regulates mitotic spindle length by a mechanochemical switch at the poles. Curr. Biol. 19, 1086–1095 (2009).
Mitchison, T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell Biol. 109, 637–652 (1989).
Arpag, G., Lawrence, E. J., Farmer, V. J., Hall, S. L. & Zanic, M. Collective effects of XMAP215, EB1, CLASP2, and MCAK lead to robust microtubule treadmilling. Proc. Natl Acad. Sci. USA 117, 12847–12855 (2020).
Miyamoto, D. T., Perlman, Z. E., Burbank, K. S., Groen, A. C. & Mitchison, T. J. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 167, 813–818 (2004).
Brust-Mascher, I., Sommi, P., Cheerambathur, D. K. & Scholey, J. M. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol. Biol. Cell 20, 1749–1762 (2009).
Cameron, L. A. et al. Kinesin 5-independent poleward flux of kinetochore microtubules in PtK1 cells. J. Cell Biol. 173, 173–179 (2006).
Steblyanko, Y. et al. Microtubule poleward flux in human cells is driven by the coordinated action of four kinesins. EMBO J. 39, e105432 (2020).
Ganem, N. J., Upton, K. & Compton, D. A. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 15, 1827–1832 (2005).
Rogers, G. C. et al. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427, 364–370 (2004).
Matos, I. et al. Synchronizing chromosome segregation by flux-dependent force equalization at kinetochores. J. Cell Biol. 186, 11–26 (2009).
Maiato, H., Khodjakov, A. & Rieder, C. L. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat. Cell Biol. 7, 42–47 (2005).
Maffini, S. et al. Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr. Biol. 19, 1566–1572 (2009).
Girao, H. et al. CLASP2 binding to curved microtubule tips promotes flux and stabilizes kinetochore attachments. J. Cell Biol. https://doi.org/10.1083/jcb.201905080 (2020).
Maddox, P., Straight, A., Coughlin, P., Mitchison, T. J. & Salmon, E. D. Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J. Cell Biol. 162, 377–382 (2003).
Nicklas, R. B. Measurements of the force produced by the mitotic spindle in anaphase. J. Cell Biol. 97, 542–548 (1983).
Garzon-Coral, C., Fantana, H. A. & Howard, J. A force-generating machinery maintains the spindle at the cell center during mitosis. Science 352, 1124–1127 (2016).
Miller, M. P., Asbury, C. L. & Biggins, S. A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell 165, 1428–1439 (2016).
Chen, G. Y. et al. Tension promotes kinetochore-microtubule release by Aurora B kinase. J. Cell Biol. https://doi.org/10.1083/jcb.202007030 (2021).
Begg, D. A. & Ellis, G. W. Micromanipulation studies of chromosome movement. I. Chromosome-spindle attachment and the mechanical properties of chromosomal spindle fibers. J. Cell Biol. 82, 528–541 (1979).
Itabashi, T. et al. Probing the mechanical architecture of the vertebrate meiotic spindle. Nat. Methods 6, 167–172 (2009).
Shimamoto, Y., Maeda, Y. T., Ishiwata, S., Libchaber, A. J. & Kapoor, T. M. Insights into the micromechanical properties of the metaphase spindle. Cell 145, 1062–1074 (2011).
Takagi, J., Sakamoto, R., Shiratsuchi, G., Maeda, Y. T. & Shimamoto, Y. Mechanically distinct microtubule arrays determine the length and force response of the meiotic spindle. Dev. Cell 49, 267–278.e5 (2019).
Carlini, L., Brittingham, G. P., Holt, L. J. & Kapoor, T. M. Microtubules enhance mesoscale effective diffusivity in the crowded metaphase cytoplasm. Dev. Cell 54, 574–582.e4 (2020).
Biswas, A., Kim, K., Cojoc, G., Guck, J. & Reber, S. The Xenopus spindle is as dense as the surrounding cytoplasm. Dev. Cell 56, 967–975.e5 (2021).
Brugues, J. & Needleman, D. Physical basis of spindle self-organization. Proc. Natl Acad. Sci. USA 111, 18496–18500 (2014).
Oriola, D., Julicher, F. & Brugues, J. Active forces shape the metaphase spindle through a mechanical instability. Proc. Natl Acad. Sci. USA 117, 16154–16159 (2020).
Roostalu, J., Rickman, J., Thomas, C., Nedelec, F. & Surrey, T. Determinants of polar versus nematic organization in networks of dynamic microtubules and mitotic motors. Cell 175, 796–808.e14 (2018).
Novak, M. et al. The mitotic spindle is chiral due to torques within microtubule bundles. Nat. Commun. 9, 3571 (2018).
Trupinic, M. et al. The chirality of the mitotic spindle provides a mechanical response to forces and depends on microtubule motors and augmin. Curr. Biol. https://doi.org/10.1016/j.cub.2022.04.035 (2022).
Velle, K. B. et al. Naegleria’s mitotic spindles are built from unique tubulins and highlight core spindle features. Curr. Biol. 32, 1247–1261.e6 (2022).
Yajima, J., Mizutani, K. & Nishizaka, T. A torque component present in mitotic kinesin Eg5 revealed by three-dimensional tracking. Nat. Struct. Mol. Biol. 15, 1119–1121 (2008).
Maruyama, Y. et al. CYK4 relaxes the bias in the off-axis motion by MKLP1 kinesin-6. Commun. Biol. 4, 180 (2021).
Bormuth, V. et al. The highly processive kinesin-8, Kip3, switches microtubule protofilaments with a bias toward the left. Biophys. J. 103, L4–L6 (2012).
Elshenawy, M. M. et al. Cargo adaptors regulate stepping and force generation of mammalian dynein-dynactin. Nat. Chem. Biol. 15, 1093–1101 (2019).
Mitra, A. et al. Kinesin-14 motors drive a right-handed helical motion of antiparallel microtubules around each other. Nat. Commun. 11, 2565 (2020).
Nitzsche, B. et al. Working stroke of the kinesin-14, ncd, comprises two substeps of different direction. Proc. Natl Acad. Sci. USA 113, E6582–E6589 (2016).
Wuhr, M. et al. Evidence for an upper limit to mitotic spindle length. Curr. Biol. 18, 1256–1261 (2008).
Crowder, M. E. et al. A comparative analysis of spindle morphometrics across metazoans. Curr. Biol. 25, 1542–1550 (2015).
Milunovic-Jevtic, A., Jevtic, P., Levy, D. L. & Gatlin, J. C. In vivo mitotic spindle scaling can be modulated by changing the levels of a single protein: the microtubule polymerase XMAP215. Mol. Biol. Cell 29, 1311–1317 (2018).
Goehring, N. W. & Hyman, A. A. Organelle growth control through limiting pools of cytoplasmic components. Curr. Biol. 22, R330–R339 (2012).
Good, M. C., Vahey, M. D., Skandarajah, A., Fletcher, D. A. & Heald, R. Cytoplasmic volume modulates spindle size during embryogenesis. Science 342, 856–860 (2013).
Hazel, J. et al. Changes in cytoplasmic volume are sufficient to drive spindle scaling. Science 342, 853–856 (2013).
Wilbur, J. D. & Heald, R. Mitotic spindle scaling during Xenopus development by kif2a and importin alpha. eLife 2, e00290 (2013).
Rieckhoff, E. M. et al. Spindle scaling is governed by cell boundary regulation of microtubule nucleation. Curr. Biol. 30, 4973–4983.e10 (2020).
Miller, K. E., Session, A. M. & Heald, R. Kif2a scales meiotic spindle size in Hymenochirus boettgeri. Curr. Biol. 29, 3720–3727.e5 (2019).
Loughlin, R., Wilbur, J. D., McNally, F. J., Nedelec, F. J. & Heald, R. Katanin contributes to interspecies spindle length scaling in Xenopus. Cell 147, 1397–1407 (2011).
Bird, A. W. & Hyman, A. A. Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. J. Cell Biol. 182, 289–300 (2008).
Helmke, K. J. & Heald, R. TPX2 levels modulate meiotic spindle size and architecture in Xenopus egg extracts. J. Cell Biol. 206, 385–393 (2014).
Courtois, A., Schuh, M., Ellenberg, J. & Hiiragi, T. The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J. Cell Biol. 198, 357–370 (2012).
Decker, F., Oriola, D., Dalton, B. & Brugues, J. Autocatalytic microtubule nucleation determines the size and mass of Xenopus laevis egg extract spindles. eLife https://doi.org/10.7554/eLife.31149 (2018).
Reber, S. B. et al. XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat. Cell Biol. 15, 1116–1122 (2013).
Burbank, K. S., Mitchison, T. J. & Fisher, D. S. Slide-and-cluster models for spindle assembly. Curr. Biol. 17, 1373–1383 (2007).
Knouse, K. A., Lopez, K. E., Bachofner, M. & Amon, A. Chromosome segregation fidelity in epithelia requires tissue architecture. Cell 175, 200–211.e13 (2018).
Ponjavic, I., Vukusic, K. & Tolic, I. M. Expansion microscopy of the mitotic spindle. Methods Cell Biol. 161, 247–274 (2021).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Sen, O., Harrison, J. U., Burroughs, N. J. & McAinsh, A. D. Kinetochore life histories reveal an Aurora-B-dependent error correction mechanism in anaphase. Dev. Cell 56, 3405 (2021).
Pamula, M. C. et al. High-resolution imaging reveals how the spindle midzone impacts chromosome movement. J. Cell Biol. 218, 2529–2544 (2019).
Yamashita, N. et al. Three-dimensional tracking of plus-tips by lattice light-sheet microscopy permits the quantification of microtubule growth trajectories within the mitotic apparatus. J. Biomed. Opt. 20, 101206 (2015).
Author information
Authors and Affiliations
Contributions
V.A.V. and L.N. created the first draft of the text and figures. All authors contributed equally to discussion, revision and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Barrelling-type instability
-
A compression-induced deformation that is symmetric about the axis of compression such as a compression that transforms a cylinder into a barrel shape.
- Biomolecular condensates
-
Micro-scale compartments that lack surrounding membranes and are formed through weak multivalent interactions, concentrating proteins and nucleic acids.
- Biorientation
-
The state of a chromosome when both sister kinetochores are attached to microtubules linked to opposite spindle poles.
- Catastrophe
-
A parameter of microtubule dynamic instability that describes the stochastic switching of a microtubule into a rapidly shrinking, depolymerizing state.
- Chromokinesins
-
Microtubule plus-end-directed motors that bind to chromosome arms via specific DNA-binding motifs.
- CLASPs
-
TOG domain-containing proteins that recognize microtubule plus ends, where they suppress catastrophe and promote rescue.
- Elasticity
-
Ability of a material, by storing energy, to return to its original shape after being deformed. The stiffness (elastic modulus) determines the magnitude of a deformation.
- Emergent properties
-
Properties of an ensemble, produced by interactions between smaller components, that the smaller components in isolation do not exhibit.
- Metaphase plate
-
The plane formed by chromosomes after alignment at metaphase, located at the spindle equator.
- NDC80 complex
-
The core outer kinetochore protein complex that mediates direct binding to microtubules. The NDC80 complex consists of four subunits (Hec1/Ndc80, Nuf2, Spc24 and Spc25) and is present in multiple copies per kinetochore.
- Nematic
-
The liquid crystal phase in which filaments of mixed polarity are roughly aligned in parallel.
- Nucleoporin
-
(Nup). Broadly conserved family of proteins that comprise the nuclear pore complex, facilitating molecular transport between the cytoplasm and nucleus.
- Pericentriolar material
-
(PCM). The dense, structured protein matrix, composed mainly of scaffold proteins and microtubule nucleation factors, that accumulates around centrioles to create the centrosome.
- Polar ejection forces
-
Forces present during chromosome congression that push chromosome arms away from spindle poles.
- Self-organization
-
A stable order arising from local interactions between energy-consuming parts.
- Spindle assembly checkpoint
-
Signalling mechanism that monitors the attachment of kinetochores to spindle microtubules and inhibits progression of the cell cycle from metaphase to anaphase until sufficient attachments are made.
- Stathmin
-
A protein that regulates microtubule dynamics by sequestering tubulin dimers, which destabilizes microtubules and inhibits their growth.
- Rescue
-
A parameter of microtubule dynamic instability that describes the stochastic switching of a microtubule into a growing, polymerizing state.
- Rheology
-
The study of the deformation and flow of materials with both solid and fluid characteristics.
- Viscosity
-
Internal friction resisting deformation of a material, stemming from rearrangement of its components. The viscous drag coefficient determines the timescale of a deformation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valdez, V.A., Neahring, L., Petry, S. et al. Mechanisms underlying spindle assembly and robustness. Nat Rev Mol Cell Biol 24, 523–542 (2023). https://doi.org/10.1038/s41580-023-00584-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00584-0
This article is cited by
-
Ran-GTP assembles a specialized spindle structure for accurate chromosome segregation in medaka early embryos
Nature Communications (2024)
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)