Abstract
Strongyloidiasis is a neglected tropical disease caused primarily by the roundworm Strongyloides stercoralis. Strongyloidiasis is most prevalent in Southeast Asia and the Western Pacific. Although cases have been documented worldwide, global prevalence is largely unknown due to limited surveillance. Infection of the definitive human host occurs via direct skin penetration of the infective filariform larvae. Parasitic females reside in the small intestine and reproduce via parthenogenesis, where eggs hatch inside the host before rhabditiform larvae are excreted in faeces to begin the single generation free-living life cycle. Rhabditiform larvae can also develop directly into infectious filariform larvae in the gut and cause autoinfection. Although many are asymptomatic, infected individuals may report a range of non-specific gastrointestinal, respiratory or skin symptoms. Autoinfection may cause hyperinfection and disseminated strongyloidiasis in immunocompromised individuals, which is often fatal. Diagnosis requires direct examination of larvae in clinical specimens, positive serology or nucleic acid detection. However, there is a lack of standardization of techniques for all diagnostic types. Ivermectin is the treatment of choice. Control and elimination of strongyloidiasis will require a multifaceted, integrated approach, including highly sensitive and standardized diagnostics, active surveillance, health information, education and communication strategies, improved water, sanitation and hygiene, access to efficacious treatment, vaccine development and better integration and acknowledgement in current helminth control programmes.
Similar content being viewed by others
Introduction
Strongyloidiasis is an infection caused by a nematode of the genus Strongyloides and is often referred to as the most neglected among the neglected tropical diseases (NTDs), having been largely ignored in helminth control programmes due in part to complexities in diagnosis and treatment, and lack of specific funding1,2. The most common species causing strongyloidiasis is Strongyloides stercoralis, which has a worldwide distribution1,3. Other species capable of infecting humans are Strongyloides fuelleborni in Africa and Asia, and Strongyloides fuelleborni kellyi in Papua New Guinea4,5, with S. fuelleborni infection recognized as a zoonosis. Infection is most common in tropical and subtropical regions of the world; however, there have been reports of infection in more temperate regions, and there have been imported cases in non-endemic areas6,7,8,9,10. Rather than an NTD, it would be better stated that strongyloidiasis is a disease of disadvantage, marginalization and neglect11. In 2016, most countries where strongyloidiasis was present were in tropical and subtropical zones, and the areas within these countries where strongyloidiasis was endemic had lower socioeconomic status11. Even in high-income countries (HICs), such as the USA and Australia, only in areas of low socioeconomic status is strongyloidiasis endemic11, although imported cases from travellers and immigrants are also a major cohort in those countries6. In all species, infection occurs via direct penetration of the skin by infectious larvae12.
The life cycle of S. stercoralis is complex and has many different stages (Fig. 1), with human disease reflecting the complex parasite life cycle. Acute strongyloidiasis occurs early in infection, with symptoms reflecting the initial parasite migration from cutaneous entry to the respiratory and gastrointestinal tracts. Due to its ability to cause autoinfection (infection caused by an agent already inside the body), chronic strongyloidiasis may result in a range of non-specific symptoms, although many individuals are asymptomatic. Severe strongyloidiasis typically occurs in the context of immunocompromise, manifesting as hyperinfection and disseminated disease, which are often fatal. Hyperinfection is characterized by a remarkable proliferation of parasites within the host, potentially causing bacterial sepsis due to perforation of the intestinal lumen, and disseminated disease, where larvae migrate to various organs and tissues outside the usual migration route.
Filariform larvae (L3) directly penetrate the skin (1), causing a rash called larva currens. The filariform larvae migrate through venous vessels and lymphatics216 to the lungs (2), where the parasite leaves the bloodstream at the alveolar–capillary barrier to enter the air-filled bronchial system. After having ascended into the pharyngeal area, the parasite is swallowed to reach the small intestine11. Larvae can also migrate to the gut by other, random pathways46. In the small intestine (3), the larvae (L4) mature into parasitic females, which reproduce by parthenogenesis, producing eggs that, in the case of Strongyloides stercoralis, hatch into rhabditiform larvae (L1) in the gut (4), which are then excreted into the environment via the faeces. For Strongyloides fuelleborni and Strongyloides fuelleborni kellyi, eggs are released into the environment and quickly hatch into rhabditiform larvae. In the free-living life cycle, once in the environment, the rhabditiform larvae (4) go through several moults becoming free-living adults (5), which reproduce to produce a single generation of rhabditiform larvae (6), which undergo successive moults (L1, L2) into infectious filariform larvae (L3) (7), which then cause infection by direct skin penetration (1). In autoinfection, rhabditiform larvae of S. stercoralis (4) can also moult into the infectious filariform larvae (5) within the human host, which can then directly penetrate the host skin causing autoinfection, migrating through the body, and developing into parasitic females producing eggs (6) and continuing the life cycle. Due to this, infections can last decades.
The latest estimates published in 2020 suggest that as many as 600 million individuals worldwide may be infected with S. stercoralis3, with Southeast Asia and the Western Pacific having the largest number of infections: 237 million and 133 million infected people, respectively3. Previous estimates from 2006 ranged only between 30 and 100 million infections, which were probably underestimates13. Accurate estimations of global infection are affected by diagnostic challenges and, hence, the paucity of quality data. Despite the inclusion of strongyloidiasis as a soil-transmitted helminth (STH) by the World Health Organization (WHO), strongyloidiasis has remained the ‘silent’ STH, with little attention in epidemiological surveys or control programmes, largely due to difficulties and complexities in accurate diagnosis. Similarly, the most widely used treatments in mass drug administration (MDA) targeting STH infection (that is, single-dose albendazole or mebendazole) have no, or only very low, efficacy against Strongyloides spp., and thus infection has been largely unchecked14.
In this Primer, we describe the main features of Strongyloides spp. biology and epidemiology, clinical features and disease management, including co-morbidities, diagnostics, treatment and control of strongyloidiasis, while identifying key areas in which improvement is required to achieve control and elimination of this disease.
Epidemiology
The parasites
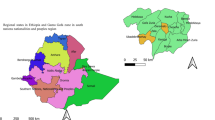
The most common species infecting humans is S. stercoralis, which is found worldwide (Fig. 2). S. fuelleborni and S. f. kellyi are endemic in Africa and Asia, and Papua New Guinea, respectively.
S. fuelleborni is a zoonotic species, found in non-human primates in both Africa and Asia. Human infections of S. fuelleborni in Asia are rare15; anthroponotic transmission (transmission from human to human; in this case meaning transmission from human faecal contamination to other humans) has not been shown to occur in Asia16,17.
It is hypothesized that S. f. kellyi evolved from a local zoonotic host, although the host has not been identified18. Infection with S. f. kellyi has been associated with ‘swollen belly syndrome’ in children in Papua New Guinea, which can be fatal5,18. Transmammary transmission has been identified in human infections of S. fuelleborni in Africa19, and presumed to occur in S. f. kellyi, although this has not been proven5. It is uncertain whether S. stercoralis infection represents a zoonosis in dogs20,21 (Box 1).
Global burden of disease
The global burden of disease is difficult to estimate due to the lack of large-scale surveys in many areas; yet, the most recent estimate from 2020 suggests 600 million infections worldwide (Fig. 2). A limitation with estimating global prevalence is bias in the number of studies available for different countries. Most publications on strongyloidiasis come from Thailand and Brazil, while other countries may have very few or no publications, which must stand as proxy for the country-wide prevalence3,22,23. Another limitation is differences in diagnostics used, and lack of standardization of diagnostics, across studies.
A meta-analysis of cross-sectional studies in Africa for the period 2008–2018 revealed a prevalence of 6.8% in rural communities, 6.4% in schools and 0.9% in health institutions24. Prevalence varied widely between studies, even within the same country, as did the diagnostics used. The most common diagnostic used was the formol–ether concentration technique (FECT) — which is not an ideal diagnosis for strongyloidiasis owing to low recovery of larvae, although it was employed in 50 of the 86 studies included in the analysis. The highest prevalence was identified in sub-Saharan countries, specifically Rwanda (17.4%), Ethiopia (10.5–20.7%), Tanzania (13.3–16.6%), Angola (12.2–21.4%), Nigeria (11.0–37.1%), Côte d’Ivoire (21.9–27.1%), Mozambique (48.5%) and Uganda (12.4%), while Egypt had the highest prevalence elsewhere in Africa (15.7%).
Estimated prevalences in different areas of Asia include 6.6% in China, 0.04% in Japan and 6.8% in South Korea3. In a meta-analysis of strongyloidiasis in Southeast Asia, the estimated prevalence was 12.7%, with the highest prevalences identified in Cambodia (24.9%) and Lao People’s Democratic Republic (PDR) (16.5%)23. Thailand had a country-wide prevalence of 11.3%, with highest burden of disease in Northeast Thailand (22.5%). In Cambodia, the prevalence was universally high across all areas (>20%) with a peak of 33.7% in the Elephant Mountains and Cardamon region. In Lao PDR, prevalence was likewise high in all areas (>14%), but highest (28%) in the Southern region23. In Timor-Leste, the Philippines and Singapore, only a single study was available to be included in the analysis; and no studies were available for Brunei23. This highlights gaps in knowledge, particularly given known high prevalence of the other STH in the Philippines and Timor-Leste25,26.
Prevalence in countries from the Pacific ranges from 0.3% in Vanuatu, to 15.9% in Fiji, and 19% in the Solomon Islands3. Prevalence in Pacific countries that participate in the Pacific Elimination of Lymphatic Filariasis (PAC-ELF) programme may be lower due to the use of ivermectin in an MDA programme. In Australia, the country-wide prevalence is very low (0.01%) but is much higher in the north of the country where the parasite is endemic: 15.5% in the Northern Territory and 10.6% in Queensland, based on seroprevalence data from pathology laboratories (2012–2016)27.
In the Americas, the prevalence is 6.9%; most infections occur in South America, although autochthonous cases also occur in the USA (0.01%)3,28. The highest prevalences in the Americas were identified in Colombia (18.4%), Costa Rica (15.7%), Panama (15.7%) and Nicaragua (15%). In Europe, the prevalence is 2.8%, with the highest prevalence in the Eastern Mediterranean region (5.8%)3, although identified cases may also be as a result of immigration or returning travellers. Autochthonous cases in the Mediterranean are rarer. Italy, Spain and Portugal have the highest number of autochthonous strongyloidiasis cases in Europe29,30,31, although this is still relatively low compared with the numbers in countries in Southeast Asia and the Pacific.
Risk factors
Strongyloidiasis is generally considered a disease of the tropics and subtropics, although autochthonous cases have occurred in more temperate regions22,27,32,33 (Fig. 2). In Australia and Africa, strongyloidiasis also occurs in dry, desert areas6,27. Infection in drier areas is assumed to occur due to domestic and farm water use, causing moist soil conditions ideal for development of Strongyloides spp. larvae. As infection is caused by direct penetration of infectious filariform larvae present in the soil, walking barefoot is a major risk factor12,22,34. In Malaysia, the risk of infection is higher in those who do not wear shoes (odds ratio (OR) 1.91) than in those who do35. In a Cambodian study, where the risk of infection in men is nearly threefold higher than in women (OR 2.79), farming was primarily in muddy rice fields; therefore, most farmers did not wear shoes while working in the fields36. Fields are more likely to be contaminated due to a lack of latrines near the fields leading to open defaecation, as wells as the use of human faeces as fertilizer37. As men are more likely to perform agricultural activities, they are at higher risk of infection than women due to higher exposure to the parasite38,39.
Cases of contaminated fresh fruit and vegetables have also been reported40,41. Open defaecation, which causes contamination of the environment, primarily due to lack of sanitation facilities, and untreated water sources are additional risk factors35,36,42. In Cambodia, the risk of infection was lower in those who had a latrine at home (OR 0.7) while in Malaysia those who practised open defaecation were much more likely to be infected than those who did not (OR 2.81)35. Due to the autoinfective life cycle of the parasite (Fig. 1), infection can also occur person-to-person, although this is rarely reported12,43.
Mechanisms/pathophysiology
Strongyloides stercoralis life cycle
Infection occurs via direct penetration of the skin by infectious L3 filariform larvae (Fig. 1). Strongyloides spp. are attracted to their host by sensing mammal-emitted odorant substances such as urocanic acid44. Larval penetration does not require any breaks in the skin, and instead the larvae use helminth-specific astacin metalloproteinases to help break down tissue and gain entry to the body; these proteins may also be involved in penetrating the gut mucosa45.
The migrating larvae enter the circulatory system and are carried to the lungs where they penetrate the alveolar spaces, migrating through the bronchioles to the trachea where they undergo tracheal migration (Fig. 1). Tracheal migration occurs as the larvae move up the trachea, eventually being coughed up and then swallowed, thereby making their way to the small intestine. Larvae may also migrate to the small intestine by random migration46. Once in the small intestine the larvae undergo two successive moults to become parasitic females; there are no males in the pathogenic part of the life cycle12. The female worms are embedded in the submucosa of the small intestine, specifically the duodenum, and reproduce by parthenogenesis, releasing eggs which hatch in the intestine as L1 rhabditiform larvae (Figs. 1,3d,e). The L1 rhabditiform larvae may then exit the host in the faeces to begin the free-living life cycle, or may undergo two moults to become infectious L3 filariform larvae and penetrate the intestine (autoinfection) or skin around the anus and skin of the trunk (contaminative infection) (Fig. 3a), beginning the parasitic life cycle again without ever leaving the host. This ability to reinfect without leaving the body is referred to as autoinfection.
Larva currens occurring in a patient with uncomplicated strongyloidiasis (panel a), and in a patient with hyperinfection (panel b); plain radiograph (panel c) showing duodenal stricture; larvae of S. stercoralis in the gut mucosa (panels d and e); larvae from bronchoalveolar lavage (panel f), and with calcofluor staining (panel g); plain radiograph of the chest (panel h) showing diffuse ground-glass opacity and consolidation in the lung; images of the duodenum (panel i) showing superficial ulceration, oozing and erythema of the mucosa, and of the descending colon (panel j) showing erythema and scattered erosions. Panel a reprinted with permission from ref. 202, W. Page. Panel b reprinted with permission from ref. 217, OUP. Panels c–g images courtesy of H. Sheorey. Panel h reprinted from ref. 121, CC BY 4.0. Panel i reprinted from ref. 218, Springer Nature Limited. Panel j reprinted from ref. 98, Springer Nature Limited.
Immunological responses to acute and chronic infection
Acute strongyloidiasis is identified more frequently in individuals from non-endemic areas than in individuals from endemic areas (possibly owing to higher reporting and health resource access in HICs), and symptoms usually develop 1–4 weeks after infection7. There are very few studies that have investigated how the immune response against strongyloidiasis mounts after initial infection; most of the knowledge of immune response has come from animal models47,48, or from individual case studies which generally are only reported in cases of hyperinfection or dissemination when the patient is likely to be immunosuppressed and, therefore, not mounting a ‘normal’ immune response49,50,51,52 (Box 1). Helminths have immunomodulatory properties that trigger a T helper 2 (TH2) cell reaction in the host; sperm-coating protein (SCP)-like extracellular proteins (SCP/TAPS (Tpx-1/Ag5/PR-1/Sc7)), identified in a range of parasitic nematodes including hookworms and Strongyloides spp., have been identified as important for transition to the parasitic stage, host invasion and immunomodulation53. In animal models of strongyloidiasis using Strongyloides ratti, a species that infects rodents and is often used in animal strongyloidiasis models, neutrophils, eosinophils and macrophages were identified in the skin as a direct response to migrating larvae and not as a cell-mediated response47. There is rapid production of IgE, IgG1, IgG2 and IgG3 by B cells, whereas parasite-specific IgG4 is produced later in infection as a more specific immune response; its levels are increased in chronic strongyloidiasis54. IgG4 can block IgE responses, thereby modulating TH2 responses55.
Eosinophilia can also be high during the chronic phase of infection, but is less likely to be found in hyperinfection due to immune dysregulation common in individuals who develop this state47. Eosinophil larval killing is mediated by major basic protein, and in mouse models with an absence of eosinophils, neutrophils are capable of killing larvae47. As with other parasites, complement activation seems to be important in larval killing. In mouse models with S. ratti, complement component C3 promotes binding of cells to larvae, and may also cause degranulation of immune cells, promoting larval killing and clearance47,56.
Immune dysregulation and risk factors for developing severe disease
The host’s immune system plays a pivotal role in the control of strongyloidiasis during the autoinfective cycle. The balance between intraintestinal autoinfection and the host’s immune response preventing acceleration of helminth replication and migration to other organs, is severely disturbed in hosts with impaired immune systems (for example, due to advanced age, malignancy and other chronic diseases, immunosuppressive medication or organ transplantation)12,57,58. In turn, this can lead to a rapid replication of S. stercoralis larvae (hyperinfection) and disseminated disease. The immune responses in hyperinfection are complicated due to immune dysregulation and the presence of co-morbidities59.
Glucocorticoid (steroid) treatment is associated with two out of three literature-reported cases of severe strongyloidiasis60,61. While the underlying mechanisms and pathways have yet to be fully elucidated, glucocorticoids impair the clearance of Strongyloides spp. by the infected host and directly expedite the helminth’s autoinfective life cycle62 (Fig. 1). Steroid-induced adverse modifications of the host’s immune response to infection are manifold. They include inhibition of histamine release by mast cells, suppression of a TH2 response with subsequent lower production of IL-4 and IL-5, reduced mucosal immunity due to inhibition of specialized intestinal effector cells (for example, goblet cells), and an upregulation of immunosuppressive effector substances (for example, IL-10). The autoinfective cycle of Strongyloides spp. is also accelerated following administration of glucocorticoid drugs, which might be explained by the observation that the development of infectious L3 larvae can be regulated by steroid ligands that bind to the parasite’s nuclear receptor DAF12 (ref. 63).
Co-morbidities associated with development of hyperinfection and disseminated strongyloidiasis
A distinct association between clinically severe strongyloidiasis and co-infection with HTLV-1, a retrovirus that causes a lifelong infection, has been described64. S. stercoralis and HTLV-1 coexist in large parts of low-income and middle-income countries (LMICs). It has been reported that HTLV-1 can modify the host’s T cell immune response from a TH2 reaction, which is required to combat helminths65, to a TH1-like reaction. This immunological switch may explain the higher rates of hyperinfection and disseminated disease in co-infected individuals65. In a recent meta-analysis, HTLV-1 carriers were more likely to be infected with S. stercoralis than those negative for HTLV-1 (OR: 3.2)66. HTLV-1 infection is associated with an increase in pro-inflammatory cytokines, particularly IFNγ; however, there are conflicting reports of IFNγ levels in helminth co-infections67,68. In symptomatic HTLV-1 infection, specifically adult T cell leukaemia/lymphoma, there is an increase in regulatory T cells expressing FOXP3 (ref. 69), which produce inhibitory cytokines, which may be a mechanism for the development of hyperinfection through immune suppression. Decreased treatment efficacy of ivermectin against strongyloidiasis in HTLV-1-co-infected individuals has also been observed, and this may have been due to reduced IgE production66,70.
Among HIV-infected individuals, recent systematic reviews identified a global prevalence of ~5% of S. stercoralis co-infection71, with a considerably higher percentage in men than in women72. However, co-infection of HIV and strongyloidiasis is not associated with a higher frequency of severe strongyloidiasis73,74.
Treatment of COVID-19 with corticosteroids and tocilizumab in patients with S. stercoralis co-infection has been associated with worsening of pre-existing strongyloidiasis, often leading to hyperinfection75,76,77. The use of steroids is a major risk factor for the development of hyperinfection and disseminated disease; thus, screening of patients with COVID-19 at risk of infection with S. stercoralis should be considered.
Alcohol use disorder (AUD) has been shown to increase susceptibility to infection with S. stercoralis, and infected patients with AUD with a high worm burden have reduced IFNγ levels78. IFNγ is a pro-inflammatory cytokine, and its reduction leads to reduced cytokine levels and parasite killing. In studies comparing an IgE enzyme-linked immunosorbent assay (ELISA) with coprodiagnostics (analysis of faeces for parasite life cycle stages), patients with AUD were more likely to test negative by IgE ELISA than individuals without AUD, even when larvae were identified in stool samples, suggesting that IgE levels may be downregulated in patients with AUD22,79. IgE is the main immunoglobulin associated with host protection against helminths; thus, reduction in IgE levels in patients with AUD may lead to increased larval load80. Patients with AUD are also more likely to be malnourished, and have gastric inflammation, reduced intestinal motility and poor hygiene, the last of which increases the risk of becoming infected81,82.
Diagnosis, screening and prevention
Clinical presentation
Acute strongyloidiasis
Acute infection with S. stercoralis reflects initial invasion and migration of L3 filariform larval that subsequently transit the respiratory system and enter the gastrointestinal tract. Most infections are unrecognized, probably due to the non-specific nature of symptoms and the low initial parasite burden. When recognized and reported, the incubation period of natural infection can be as short as 7–14 days7,83, although human infection studies have suggested that cutaneous lesions appear almost immediately after exposure, followed by a prepatent period of 23–32 days84.
Primary cutaneous lesions may cause irritation at the site of penetration. Intradermal filariform larvae seem to migrate rapidly, resulting in a pathognomonic, serpiginous (spreading with an undulating border) urticarial rash known as larva currens (from the Latin, ‘running larva’) (Fig. 3a), growing in length by up to 10 cm per day85. In an experimental infection, 300 larvae placed onto a forearm led to an immediate pruritic cutaneous eruption that lasted 21 days86. Cough and respiratory irritation developed on day 6, lasting for 3 days, with abdominal symptoms and anorexia developing on day 17 and progressing until treatment86.
Following initial infection, transpulmonary larval migration may result in transient bronchitis, with signs and symptoms including dry cough, wheeze and shortness of breath. This syndrome of transient eosinophilic pneumonia is known as Loeffler syndrome, which may also be caused by other STHs87; however, this is mostly reported to be brief and mild in acute strongyloidiasis84. Imaging typically reveals patchy, unilateral or bilateral air space opacities, usually peripherally distributed, and typically without lymphadenopathy or pleural effusion88. Acute gastrointestinal strongyloidiasis may cause epigastric discomfort, bloating, abdominal pain, constipation and diarrhoea89. Dysentery has also been described84. Examination may reveal splenomegaly, while transaminitis has also been reported7. Systemic symptoms can include fatigue and fever7.
Chronic strongyloidiasis
The clinical symptoms of chronic infection are influenced by the infection intensity and the host’s immune response to migrating parasites during the autoinfective cycle (that is, cutaneous, respiratory and gastrointestinal systems). Approximately half of individuals with chronic strongyloidiasis are asymptomatic90,91, although intermittent mild symptoms may lead to under-reporting.
Of those who do report symptoms, gastrointestinal complaints are common, although typically non-specific and mild, and include abdominal pain, altered bowel habit and diarrhoea90,91,92. In Cambodia, the most common location of abdominal pain is the peri-umbilical and epigastric region93, the latter probably reflecting the duodenal localization of adult parasites. Duodenal obstruction94 and protein-losing enteropathy (with symptoms of fluid retention due to hypoalbuminaemia) have also been reported95,96. Recurrent gastrointestinal symptoms may also mimic inflammatory bowel disease97,98,99 (Fig. 3i,j), which is also paradoxically exacerbated by immunosuppressive therapy98,100,101,102. Chronic infection has also been associated with growth stunting103 and a poor general health status104.
Cutaneous symptoms are not uncommon92,105, and include pruritus90, recurrent urticaria106,107 and larva currens90,91. Larva currens in the context of chronic infection is most likely to be reported in the perianal region, buttock, thigh or abdomen, due to larvae emerging during autoinfection and penetrating the local skin85 (Fig. 3a). Migration of larvae to more distant cutaneous sites, such as the chest wall, has also been reported108 (Fig. 3b). Respiratory symptoms are less commonly reported by individuals with chronic strongyloidiasis90,91,92. Respiratory symptoms may include recurrent cough, dyspnoea and wheeze, which imitate the common symptoms of asthma109,110, and may be paradoxically exacerbated by corticosteroid use111,112.
Severe strongyloidiasis
Unlike chronic strongyloidiasis, in which infection may be asymptomatic or may result in typically mild, chronic symptoms, severe strongyloidiasis is associated with a remarkably acute presentation and is invariably fatal if untreated49. This is usually in the context of immunosuppression, most notably due to corticosteroid therapy and HTLV-1 co-infection49,60. Severe strongyloidiasis is categorized as hyperinfection syndrome and disseminated strongyloidiasis, although these conditions probably coexist in most patients experiencing severe disease.
The symptoms of severe strongyloidiasis, therefore, reflect the consequences of exaggerated parasite proliferation and migration. Due to the large number of filariform larvae penetrating the intestinal lumen, enteric bacterial translocation frequently occurs, resulting in secondary bacterial sepsis. Symptoms of disseminated strongyloidiasis include fever, headache and altered mental status, as enteric bacterial carriage on disseminated larvae may lead to meningitis60,113.
Gastrointestinal symptoms in severe strongyloidiasis again reflect the large burden of luminal infection, with anorexia, nausea, vomiting, abdominal pain, diarrhoea, constipation and ileus reported113,114,115. Transpulmonary migration during hyperinfection may result in a wide spectrum of cardiorespiratory presentations, such as dyspnoea, cough and haemoptysis, typically due to extensive pneumonia and pulmonary haemorrhage, which is associated with the development of acute respiratory distress syndrome116,117,118. Plain radiography of the chest may show a diffuse ground glass like opacity and consolidation (Fig. 3h). Pleural effusions may occur due to parasite migration from the lung parenchyma into the pleural space118, while migration from the pleural to the pericardial space may cause pericarditis116,119. Pulmonary abscesses have also been reported, which probably develop as a result of secondary bacterial infection116. Cutaneous signs and symptoms are less commonly reported; however, a pathognomonic rash seen in immunocompromised patients with severe strongyloidiasis is periumbilical purpura120,121. Even among those who receive treatment, case fatality rates of 43–63% have been reported49,60,119, while a mortality rate of 87% has been reported in patients with severe strongyloidiasis and translocated bacterial sepsis1,83,122,123.
Diagnosis
The diagnosis of strongyloidiasis is complicated and lacks both a ‘gold standard’ and standardization of tests, and the best diagnostic varies depending on disease status (see Supplementary Table 1). A target product profile (TPP), which outlines the desired characteristic of a diagnostic for a specific disease, has yet to be developed for strongyloidiasis. In Supplementary Table 1, we compare diagnostics used in STH control programmes and epidemiological surveys, and those specifically employed for strongyloidiasis against some key TPP criteria that have been identified in reference to other parasitic NTDs.
Microscopic/coproparasitological approaches
Stool microscopy and coproparasitological methods detect excreted larvae of S. stercoralis, and are particularly useful in revealing which larval output is high in acute strongyloidiasis and in hyperinfection. Concentration techniques, which use various methods to remove faecal debris, reduce sample volume and concentrate any parasites into a smaller volume for easier detection, such as FLOTAC and FECT, have poor sensitivity in S. stercoralis infection, with the latter potentially removing larvae through processing59,124. However, a modified FECT125 has similar sensitivity to agar plate (AP) copro-culture. The Kato–Katz technique is a mainstay in the diagnosis of STH infection but is not recommended for S. stercoralis infection diagnosis59,124.
AP and the Baermann technique have high sensitivity compared to other copro-based diagnostics, such as the Harada–Mori filter paper technique, and PCR assays, although sensitivity is low compared with immunodiagnostics12,24,126. These techniques rely on viable larvae and, therefore, the use of fresh, unfixed stool. There is poor standardization with these methods — indeed AP can refer to a whole suite of different methodologies — which can limit meaningful comparisons between published studies (see Supplementary Table 1). Hookworm and strongyloidiasis are often present in the same areas, and thus culturing methods will also result in viable hookworm larvae being present, which will then need to be distinguished (Fig. 4).
a, Rhabditiform larvae of Strongyloides show a short buccal cavity (blue arrow) and a bulb oesophagus (black arrow), and a prominent genital primordium (red arrow). b, By contrast, rhabditiform larvae of hookworm show a long buccal cavity (blue arrow). c, In filariform larvae of Strongyloides, the oesophagus–intestine junction (white arrow) is halfway down the larval body. d, By contrast, in hookworm filariform larvae, the junction (white arrow) is roughly one-third of the way down the larval body. Filariform larvae of Strongyloides also possess a notched tail while those of hookworm have a pointed tail. Images in panels a and c adapted with permission from ref. 219, CDC. Images in panels b and d adapted with permission from ref. 220, CDC.
Microscopy can also be utilized in the evaluation of sputum samples and duodenal aspirates, which have high larval content in hyperinfection127 (Fig. 3f,g). Diagnosis of S. fuelleborni and S. f. kellyi infection relies on identification of eggs in the faeces15, although eggs hatch rapidly and rhabditiform larvae may be present. S. fuelleborni eggs are passed individually, while S. f. kellyi are often passed in strings5.
Serology and immunodiagnostics
There are several serological tests, including both in-house and commercially available tests for S. stercoralis detection, utilizing crude S. stercoralis, Strongyloides venezuelensis or S. ratti extracts or more sensitive S. stercoralis recombinant antigen (NIE)128 (see Supplementary Table 1). Most immunodiagnostics are based on IgG detection, although there are also IgM based diagnostics, and a commercially available IgG–IgM combination diagnostic. Sensitivity and specificity vary depending on other reference tests utilized. Immunodiagnostics identify more positives than copro-based diagnostics; however, while high sensitivity can be exhibited by serological diagnostics, cross-reactivity can be observed with other helminths depending on the antigen used129,130. Circulating antibodies persist for some months after successful treatment and thus positive serology may not indicate active infection. Antibody titre levels do drop rapidly after successful treatment, and re-testing after 6 months should determine the success of treatment131,132,133,134. This may be more useful in non-endemic areas where the rate of re-infection is low.
Serological diagnostics lend themselves to point-of-care diagnostics, which are of particular utility in LMICs124. SsRapid, a rapid diagnostic test (RDT), utilizing NIE has been developed, and has shown high sensitivity and specificity in laboratory set-ups135, and in a field-based trial in Ecuador conducted in 2021–2022 (refs. 136,137,138,139). The RDT had a higher sensitivity in that study than copro-PCR, the Bordier and Strongy Detect ELISAs and the Baermann technique, although specificity (93.6%) was lower for the RDT than all other tests except the Strongy Detect ELISA (91.7%)136; the RDT builds on a previous lateral flow dipstick137,138. Similarly, ELISA of dried blood spots has also shown comparable sensitivity and specificity to analysis of conventional serum samples140.
Urine-based ELISAs have also been developed but have generally performed less well than serum-based ELISAs. However, more recent studies have shown results comparable to those of serum-based ELISAs141,142. Urine is a non-invasive clinical sample and may be easier to obtain from patients and therefore may have greater uptake and utility for control programmes and surveys. There are fewer antigen detection tests for strongyloidiasis: a single serum-based antigen detection ELISA (SsAg-ELISA) and a stool-based antigen detection ELISA (copro-ELISA)143,144. The copro-ELISA utilizes S. ratti excretory–secretory (E–S) antigens and shows low cross-reactivity with other helminth E–S products143. The SsAg-ELISA similarly shows no cross-reactivity with other helminths144.
Molecular-based techniques
There are a range of different laboratory developed PCR assays145,146,147 and loop-mediated isothermal amplification assays (LAMP)148. In general, PCR is considered to be sensitive and specific, and while it maintains specificity for Strongyloides detection, it is not as sensitive as other copro-based diagnostics, such as the Baermann technique, or serological tests149,150. As with other diagnostics, there is a lack of standardization of methods, including sample collection and preservation, and DNA extraction methods, and sensitivity varies depending on reference tests used to calculate sensitivity and specificity17,149,150,151,152. Few molecular assays have been validated clinically or in population-based studies for strongyloidiasis. The WHO also recommends standardization of DNA extraction methods124. Most assays are based on DNA extracted from stool samples. Urine-based PCR has also been explored; however, sensitivity is low153,154. A major limitation of DNA-based methods employed in resource limited settings is the cost, and the requirements for thermocyclers and highly trained laboratory personnel.
Limitation for current diagnostics
The lack of standardization for any diagnostic for strongyloidiasis, and the lack of validation across the many in-house molecular-based and immuno-based diagnostics, is a major limitation for current diagnostics, which makes meaningful comparison of diagnostic performance across studies virtually impossible (Box 1).
Copro-diagnostics are most useful when larval output is high, during acute strongyloidiasis and in hyperinfection, while serological diagnostics are particularly useful in chronic strongyloidiasis; however, serology can give false-negative results in acute or early infections in which an immune response has not been mounted and in hyperinfection due to reduced antibody production in immunosuppressed patients despite heavy infection131,155 (see Supplementary Table 1). According to the US Centers for Disease Control and Prevention (CDC), serial stool examination is the gold standard for S. stercoralis detection, but that it may require as many as seven stool samples to reach 100% sensitivity12,59.
In view of these shortcomings, a combination of diagnostics that include a serological test with a copro-diagnostic has been proposed to more accurately detect Strongyloides infection136,156. It is particularly pertinent for clinicians to be aware of the limitations of available diagnostics, as missed infection can have potentially severe consequences. A recent cross-sectional study in Ecuador compared five different diagnostics and assessed the sensitivity and specificity of each diagnostic separately, and as combinations of immunodiagnostics (SsRapid RDT, Bordier ELISA and Strongy Detect ELISA) with copro-diagnostics (stool PCR and the Baermann technique)136. Both sensitivity and specificity were increased when two diagnostics were employed.
Peripheral eosinophilia
Individuals with or without symptoms of chronic strongyloidiasis may be referred for investigation of unexplained eosinophilia (defined as an elevated eosinophil count in peripheral blood above 500/µl). Eosinophilia is common in helminth-infected individuals, and occurs in up to 50–75% of people infected with S. stercoralis90, although co-infections are common and may overestimate rates of eosinophilia90. In migrants and travellers returning from endemic settings with otherwise unexplained eosinophilia, diagnostic work-up for strongyloidiasis is indicated, even if other symptoms are lacking157,158. In patients with eosinophilia at the time of diagnosis, eosinophil counts usually decrease within 4–6 weeks after treatment, and may be used to monitor treatment response159. Not all Strongyloides-infected individuals present with eosinophilia, and a normal eosinophil count never excludes strongyloidiasis92,160. Eosinophilia is often absent in Strongyloides hyperinfection and disseminated disease, and might be a predictor of a worse outcome in such patients161.
Prevention
Public health measures
Strongyloidiasis is the most neglected of all the NTDs with the WHO clearly stating that “no public health strategy has been developed to control strongyloidiasis”, and “no public health strategies for controlling the disease are active at the global level”34. Despite this, S. stercoralis has been recognized as a public health problem and an evidence-based comprehensive public heath strategy is urgently required. Strongyloidiasis is generally grouped with the STHs (Ascaris lumbricoides, Trichuris trichiura and hookworm) even though it is not included in STH control programmes (primarily due to a different drug and diagnostics required for strongyloidiasis); however, the recommendations for STH control should be a starting point for a strongyloidiasis control strategy (Box 1). Ivermectin as a single oral dose can reduce infection levels and morbidity as part of a community-based MDA programme; and preventive measures such as water, sanitation and hygiene (WASH) along with health promotion can augment MDA, preventing transmission and re-infection. We posit that a multicomponent integrated approach is required for the sustainable control and potential elimination of strongyloidiasis. Such a programme would combine ivermectin MDA, WASH, community engagement, potentially animal management (if S. stercoralis infection is indeed a zoonosis), and precise surveillance (Box 1).
Ivermectin was previously expensive, which was a barrier to its use for strongyloidiasis MDA unless the medication was donated or heavily subsidized (Box 1); however, an affordable generic version of ivermectin is now available. The WHO is currently developing guidelines for the control of strongyloidiasis to provide guidance to endemic countries on methods for achieving public health control of strongyloidiasis162. The existing infrastructure for STH drug distribution already facilitates distribution of >400 million albendazole or mebendazole tablets to children each year163. Of note, ivermectin is used as preventive chemotherapy in control programmes targeting scabies, onchocerciasis and/or lymphatic filariasis164,165, and has been shown to have an effect on strongyloidiasis where it is co-endemic166. Ivermectin has been donated as part of the Pacific Programme to Eliminate Lymphatic Filariasis (PacELF), and over >1,800 million tablets for onchocerciasis control have been donated as part of the Mectizan Donation Program167,168. Similarly, preventive measures, including health education and WASH, in STH control programmes, particularly against hookworm, such as the wearing of shoes, will probably have an effect — unfortunately, no data exist on this.
Health education and community-based interventions
While health education for STH, particularly hookworm, is likely to positively affect strongyloidiasis, there is limited specific health education available; and what there is largely stems from Northern Australia. A health promotion flip chart was developed with the Miwatj Health Aboriginal Corporation for East Arnhem Land, Australia, which tells the ‘Strongyloides story ’. The flip chart was developed to help explain the transmission of strongyloidiasis, its importance to health, and risk factors for infection to community members (Strongyloides Australia). A second flip chart was designed by the Aboriginal Resource Development Services, which tells about both strongyloidiasis and scabies, which is also available in the local Yolgnu language. Health information, education and communication are important aspects of integrated control. For strongyloidiasis, this should encompass not only those who may become infected, but also health professionals for diagnosis and correct treatment strategies (Box 1).
Vaccines
There are no vaccines currently available for any human helminth species. The successful identification and development of a vaccine candidate against strongyloidiasis is hampered by the myriad immune-modulating characteristics of the parasite and by the occurrence of several stages (that is, L1, L2 and L3 larvae and adult worms) with stage-specific antigen expression in the infected host. It is also unclear whether, and how, partial protective immunity might develop after natural infection compared with a possible vaccine-induced immune response. In studies of vaccine development for other STHs, vaccine effectiveness was assessed in terms of rising cytokine and/or specific IgG4 production or a documented decrease in infection intensity169. Some experimental studies used immune-reactive antigens obtained from the glandular oesophagus of S. stercoralis or heat shock protein 60 from the closely related S. ratti as antigen vaccine candidates in mice, but the specific immunological responses remain poorly understood169. Hence, it is unlikely that a Strongyloides-specific vaccine candidate will enter into clinical trials over the next 3–5 years.
Screening
Screening is performed in HICs and rarely in LMICs, including those endemic for strongyloidiasis. In many HICs, such as Australia, Canada and the USA, screening is recommended for refugees entering the country8,9,10; Australia and the USA are also endemic and have autochthonous transmission6,27,28. In the Northern Territory of Australia, which has the highest rates of autochthonous strongyloidiasis in the country, treatment with ivermectin is recommended regardless of serology results prior to immunosuppression170,171 due to the consequences of missed diagnosis and the high pre-test probability of infection. In other non-endemic countries, screening is also recommended prior to immunosuppression due to the risk from migrants with unrecognized strongyloidiasis172.
The lack of a gold-standard diagnostic hampers screening, including issues around false-negative results, depending on the test used and the disease status of the patient, as well as lengthy testing times if using culture techniques (see Supplementary Table 1). An algorithm has been developed as a diagnostic flow chart for use in immunosuppressed patients173. The flow chart incorporates epidemiological exposure risk and current immunosuppression status to help determine if the patient is at risk of strongyloidiasis, and then suggesting diagnostics to use173. In patients at high risk waiting for immunosuppression or currently immunosuppressed then a combination of serology and copro-based diagnostics are suggested173.
The only exception to no active control programmes for strongyloidiasis is a ‘test and treat’ strategy implemented in East Arnhem Land, Australia, by the Miwatj Health Aboriginal Corporation, whereby community members are tested for strongyloidiasis as part of routine health assessments and those found positive treated with ivermectin170. Prevalence in endemic communities has been reduced utilizing this strategy. This strategy is not implemented in other communities beyond those associated with the Miwatj region.
Management
Current and future drugs
Since the 1990s, the macrocyclic lactone ivermectin has been the first-line treatment for strongyloidiasis174. Ivermectin acts on glutamate-gated chloride ion channels triggering an increase in the permeability of the cell membrane in invertebrates which results in the influx of chloride ions and hyperpolarization leading to paralysis and death of the worm174. Ivermectin has good efficacy and safety profiles and outperforms the benzimidazoles albendazole and thiabendazole that serve as alternative treatments175. In two randomized controlled trials, a single oral dose of ivermectin resulted in cure rates of 86–95%176,177,178. Multiple doses of ivermectin showed the same efficacy as single dose regimens but were associated with more adverse events176. It is considered that oral ivermectin in children weighing <15 kg is safe179. Hence, the current contraindication against treating children under 5 years old might be reconsidered considering the efforts to develop an ivermectin formulation suitable for use in children. Pharmacometric modelling has indicated that a higher dose might be required in children than in adults because of higher clearance180. To overcome the challenge of a weight-based administration in resource-constrained settings, the use of a ‘tablet pole’, which uses height rather than weight to estimate dosage has also been assessed, as it would facilitate MDA in LMICs181. The use of ivermectin is currently contraindicated in pregnant women182 and research into safety in pregnant women is urgently required. Albendazole is the only alternative medication currently available, which should be avoided in the first trimester of pregnancy182 and is inferior to ivermectin in terms of efficacy14.
Current treatment guidelines are available from organizations such as the CDC182. Targeted treatment using diagnostic testing is the ideal approach to slow the development of resistance to ivermectin; however, ivermectin MDA could also form the foundation of a community-level strongyloidiasis control programme that also incorporates preventive measures as discussed below. Presumptive treatment may be required before imminent administration of immunosuppression in individuals at risk of strongyloidiasis61.
Moxidectin, a compound from the same family as ivermectin with small structural differences, was registered in 2018 for the treatment of onchocerciasis due to Onchocerca volvulus in patients aged ≥12 years, and is now being evaluated for use in treating strongyloidiasis183. Moxidectin is considerably more lipophilic and displays a longer half-life in plasma and broader distribution compared with ivermectin174,184,185. Studies of the use of moxidectin in children are underway to extend the indication to children aged 4–11 years and to obtain additional data to inform the WHO and the ministries of health in endemic countries on moxidectin use in treatment programmes186. A dose-ranging phase IIa study evaluating moxidectin in adults infected with S. stercoralis revealed cure rates of 83–88%184. Moxidectin was well tolerated at all doses. Phase IIb studies comparing the efficacy of moxidectin and ivermectin side-by-side were completed in Cambodia and Lao PDR in 2022 (refs. 187,188). A population pharmacokinetic study demonstrated equivalent exposures after fixed-dose and weight-dependent dosing, which supports the use of a weight-independent dosing, which in turn would enable MDA campaigns189. To further increase cure rates, which is a key factor to mitigate autoinfection, repeated dosing (for example, a two-dose regimen with moxidectin 3 weeks apart) should be considered, which needs to go hand-in-hand with safety investigations185.
Unique cases of ivermectin use
In patients with hyperinfection and disseminated strongyloidiasis, parenteral ivermectin treatment has been used, using a veterinary formulation not licensed for use in humans14,190. This is usually only performed due to reduced intestinal absorption which limits uptake of orally or rectally administered ivermectin tablets. Ivermectin toxicity has been reported in a small number of patients in whom ivermectin was given subcutaneously. Neurological symptoms have been described; however, these may also be attributable to the disseminated strongyloidiasis instead14,191. Ivermectin is generally blocked from crossing the blood–brain barrier by P-glycoprotein, a protein pump14,192. Thus, mutations in this pump may lead to neurotoxicity.
Management of severe disease
In the setting of severe strongyloidiasis in immunocompromised individuals, immunosuppression should be reduced or ceased, if feasible. Intensive care may be required in patients with respiratory failure, septic shock, bacterial meningitis or other organ dysfunction. In patients with suspected bacterial infection (for example, sepsis and meningitis), antimicrobial therapy should be commenced following the collection of blood cultures, with empirical therapy broadly targeting enteric bacteria193. Anthelminthic treatment with ivermectin is administered enterally, orally or via nasogastric tube193. In a number of patients unable to tolerate or absorb enteral therapy (for example, due to small-intestinal obstruction or paralytic ileus), subcutaneous treatment using an unlicensed veterinary formulation has been used194,195,196, generally administered either daily or every alternate day, and continued until enteral therapy is feasible. Rectal instillation of ivermectin has also been reported197, although systemic absorption is poor when using a suppository preparation198. In patients with persistent severe strongyloidiasis despite ivermectin monotherapy, the addition of albendazole (given concurrently with ivermectin) has also been described199. Moxidectin has not yet been used for individual management in such patients. Ivermectin in patients who recover from severe strongyloidiasis is generally continued daily until symptoms have resolved and larvae have been absent on examination of stool/sputum for at least 2 weeks (that is, one autoinfection cycle)193.
Monitoring after treatment
In patients with larvae detectable in stool prior to treatment, guidelines recommend that repeat stool examination should be performed 2–4 weeks after therapy; if larvae remain visible, re-treatment is required, although intermittent shedding of larvae makes this an unreliable measure of treatment success130. In those who report persisting symptoms despite treatment, even if stool examination is unrevealing, a repeat course of therapy may be warranted, and additional investigations for an alternative cause may be indicated. In patients who fail treatment without an apparent cause, HTLV-1 infection should be excluded. In those with eosinophilia prior to treatment, eosinophilia may persist for months200, although eosinophilia ≥1 year after treatment, without an alternative aetiology, may also warrant re-treatment12,193. In people who are IgG seropositive at the time of diagnosis, repeated testing 6–12 months after treatment has been recommended, with seroreversion suggestive of treatment success133,134. In patients who were initially diagnosed using PCR, the role of monitoring stool PCR remains unclear159,201.
Quality of life
There are currently no global burden of disease estimates for strongyloidiasis. The latest global estimate of disease burden report put the disability-adjusted life-years (DALYs) due to STH (excluding strongyloidiasis, which is not mentioned) at 25.5 DALYs per 100,000 in 2019.
As a chronic infection strongyloidiasis can be referred to as asymptomatic; however, that may not be true. Instead symptoms are often intermittent, wide ranging and general in nature, and thus may not be recognized202. Abdominal pain, diarrhoea and urticaria are the most commonly observed symptoms in chronic infections, although these are not reported in all patients106. While there are limited data available on the morbidity and quality of life in patients with chronic strongyloidiasis, long-term diarrhoea can be associated with dehydration and nutritional deficiencies, as well as irritation and inflammation of the colon and rectum.
Growth stunting in children was found in a study in Cambodia, with odds ratios of 2.5 in those with a heavy infection (one or more larvae per gram of faeces) and 1.35 in those with a light infection (less than one larva per gram of faeces)103. While only physical growth was examined, stunting can also be associated with diminished mental ability and learning capacity, a pernicious syndrome that has knock-on effects leading to poor school performance and reduced earnings in adulthood, as well as a stronger likelihood of developing chronic diseases later in life203,204. Strongyloidiasis is a disease of poverty11, and growth and mental stunting in children caused by the parasite perpetuates the cycle of poverty.
Outlook
The WHO roadmap for NTDs 2021–2030 set several specific targets for STH, including controlling morbidity due to strongyloidiasis205,206. Emphasis was put on the development of guidelines for preventive chemotherapy, prioritization of control efforts, advocacy and funding, capacity strengthening, and awareness building and diagnosis. To add to the WHO list of areas that need work, we would also add the need for specific guidelines and standardization of diagnostics, and guidelines for disease management, which currently vary from one country to another.
Strongyloidiasis has been referred to as the most neglected of the NTDs1. This observation is borne out by the general lack of accurate diagnostic tools and control programmes specifically targeting strongyloidiasis. While included in the STH group of helminths when national surveys are performed, strongyloidiasis is not identified, and in MDA campaigns, ivermectin, the drug of choice against Strongyloides is not used207. Insensitive diagnostics, such as the Kato–Katz thick smear and FECT techniques, are performed, and drugs such as albendazole and mebendazole, which have poor efficacy against strongyloidiasis, are used208. Thus, the main problem facing strongyloidiasis is a lack of recognition. Without inclusion in national surveys, the true burden of disease will not be known, and without correct treatment the disease goes unnoticed and uncontrolled.
Diagnostics
There is an urgent need for new diagnostics that are inexpensive, rapid and field-friendly (Box 1). Any new diagnostic test should be capable of detecting strongyloidiasis at all stages of the disease; however, it may be that a two-step diagnostic process will be required, with an initial population screening test that has high sensitivity, followed by a more specific test for confirmation.
In addition, there is a need for specific strongyloidiasis guidelines that include validation and standardization of available diagnostics. The WHO is developing new guidelines around strongyloidiasis, which will hopefully lead to standardization and greater uptake in national control programmes162.
New drugs and treatment
In settings with other STHs, co-administration with albendazole is recommended, which would be highly beneficial against T. trichiura infections209. However, in areas where Loa loa is co-endemic, ivermectin is contraindicated as sudden death of the worms can result in severe adverse events210. The development of drug resistance due to widespread MDA is a threat, with resistance against ivermectin already noted in helminths of veterinary importance, such as Parascaris equorum, Haemonchus contortus and Cyathostomum spp.211,212. Thus, the development of new drugs that are safe and highly efficacious against strongyloidiasis is important. An ideal drug, or drug combination, would also have activity against other STH infections.
A future drug that could be of interest for the treatment of strongyloidiasis is the cyclo-octadepsipeptide emodepside, which targets latrophilin-like receptors and calcium-dependent voltage-gated potassium slowpoke (SLO-1) channels in the parasite213. At present, emodepside is being developed against onchocerciasis and STH infection. In view of high cure rates against T. trichiura and hookworm infections at a single dose214, a phase IIa dose-ranging study with emodepside has been launched in patients with strongyloidiasis in Lao PDR214,215.
Control and prevention
Strongyloidiasis and other STHs, particularly hookworm, share common risk factors. Many prevention strategies implemented to reduce the burden of STH infection, including health information, education, communication, promoting shoe wearing, and providing WASH infrastructure, will also affect the incidence of strongyloidiasis. Vaccine development should be a major focus to advance the prevention of strongyloidiasis, and the vaccination of immunosuppressed individuals in endemic areas may be useful to prevent severe disease, although vaccine efficacy in this group will remain a challenge. Thus, new approaches should be explored to achieve protection in those most at risk of poor outcomes (Box 1).
There is a pressing need to enhance global surveillance and develop a control strategy for strongyloidiasis. Integrating Strongyloides spp. into existing STH and intestinal schistosomiasis control programmes could be a way forward given similarities in transmission (that is, hookworm) and prevention strategies (for example, provision of sanitation and hygiene education). Research is needed to develop and validate novel diagnostics, treatments and prophylactics for strongyloidiasis. Integrated, cross-sectoral control approaches are warranted to have a lasting impact on strongyloidiasis on the road towards elimination on this important, yet severely neglected NTD.
References
Olsen, A. et al. Strongyloidiasis – the most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 103, 967–972 (2009).
Schär, F. et al. Strongyloides stercoralis genotypes in humans in Cambodia. Parasitol. Int. 63, 533–536 (2014).
Buonfrate, D. et al. The global prevalence of Strongyloides stercoralis infection. Pathogens 9, 468 (2020).
Thanchomnang, T. et al. First molecular identification of Strongyloides fuelleborni in long-tailed macaques in Thailand and Lao People’s Democratic Republic reveals considerable genetic diversity. J. Helminthol. 93, 608–615 (2019).
Ashford, R. W., Barnish, G. & Viney, M. E. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol. Today 8, 314–318 (1992).
Gordon, C. A. et al. HTLV-I and Strongyloides in Australia: the worm lurking beneath. J. Adv. parasitol. 111, 119–201 (2021).
Angheben, A. et al. Acute strongyloidiasis in Italian tourists returning from Southeast Asia. J. Travel. Med. 18, 138–140 (2011).
Australian Refugee Health Practice Guide. Refugee health assessment. Refugee Health Guide refugeehealthguide.org.au/refugee-health-assessment/#Investigations (2023).
Thompson, C. & Boggild, A. K. Strongyloidiasis in immigrants and refugees in Canada. CMAJ 187, 1389 (2015).
Maskery, B. et al. Economic analysis of the impact of overseas and domestic treatment and screening options for intestinal helminth infection among US-bound refugees from Asia. PLoS Negl. Trop. Dis. 10, e0004910 (2016).
Beknazarova, M., Whiley, H. & Ross, K. Strongyloidiasis: a disease of socioeconomic disadvantage. Int. J. Env. Res. Public. Health 13, 517 (2016).
Centers for Disease Control and Prevention. Parasites – Strongyloides. CDC www.cdc.gov/parasites/strongyloides/health_professionals/index.html#:~:Text=The%20gold%20standard%20for%20the,reach%20a%20sensitivity%20of%20100%25 (2023).
Bethony, J. et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532 (2006).
Buonfrate, D. et al. Current pharmacotherapeutic strategies for strongyloidiasis and the complications in its treatment. Expert. Opin. Pharmacother. 23, 1617–1628 (2022).
Potters, I., Micalessi, I., Van Esbroeck, M., Gils, S. & Theunissen, C. A rare case of imported Strongyloides fuelleborni infection in a Belgian student. Clin. Infec Prac. 7–8, 100031 (2020).
Thanchomnang, T. et al. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol. Res. 116, 1917–1923 (2017).
Richins, T. et al. Genetic characterization of Strongyloides fuelleborni infecting free-roaming African vervets (Chlorocebus aethiops sabaeus) on the Caribbean island of St. Kitts. Int. J. Parasitol. Parasites Wildl. 20, 153–161 (2023).
King, S. E. & Mascie-Taylor, C. G. Strongyloides fuelleborni kellyi and other intestinal helminths in children from Papua New Guinea: associations with nutritional status and socioeconomic factors. P N. G. Med. J. 47, 181–191 (2004).
Brown, R. C. & Girardeau, H. F. Transmammary passage of Strongyloides sp. larvae in the human host. Am. J. Trop. Med. Hyg. 26, 215–219 (1977).
Jaleta, T. G. et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans – dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 11, e0005752 (2017).
Buonfrate, D., Bradbury, R. S., Watts, M. R. & Bisoffi, Z. Human strongyloidiasis: complexities and pathways forward. Clin. Microbiol. Rev. 36, e0003323 (2023).
Schär, F. et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl. Trop. Dis. 7, e2288 (2013).
Chan, A. H. E. et al. Prevalence of Strongyloides in Southeast Asia: a systematic review and meta-analysis with implications for public health and sustainable control strategies. Infect. Dis. Poverty 12, 83 (2023).
Hailu, T., Nibret, E., Amor, A. & Munshea, A. Strongyloidiasis in Africa: systematic review and meta-analysis on prevalence, diagnostic methods, and study settings. Biomed. Res. Int. 2020, 2868564 (2020).
Gordon, C. A. et al. Multiplex real-time PCR monitoring of intestinal helminths in humans reveals widespread polyparasitism in Northern Samar, the Philippines. Int. J. Parasitol. 45, 477–483 (2015).
Campbell, S. J. et al. Investigations into the association between soil-transmitted helminth infections, haemoglobin and child development indices in Manufahi District, Timor-Leste. Parasit. Vectors 10, 192 (2017).
Shield, J. et al. Seropositivity and geographical distribution of Strongyloides stercoralis in Australia: a study of pathology laboratory data from 2012-2016. PLoS Negl. Trop. Dis. 15, e0009160 (2021).
Singer, R. & Sarkar, S. Modeling strongyloidiasis risk in the United States. Int. J. Infect. Dis. 100, 366–372 (2020).
Martinez-Perez, A. & Lopez-Velez, R. Is Strongyloidiasis endemic in Spain? PLoS Negl. Trop. Dis. 9, e0003482 (2015).
Buonfrate, D. et al. Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014. Eur. Surveill. 21, 30310 (2016).
Ottino, L. et al. Autochthonous human and canine Strongyloides stercoralis infection in Europe: report of a human case in an Italian teen and systematic review of the literature. Pathogens 9, 439 (2020).
Bronstein, A. M., Lukashev, A. N., Maximova, M. S. & Sacharova, T. V. The autochthonous cases of acute strongyloidiasis in the Moscow region. Germs 11, 116–119 (2021).
Fraser, J. A case report suggestive of strongyloidiasis infection occurring in temperate Australia. RRH 19, 4787 (2019).
World Health Organization. Control of neglected tropical diseases: strongyloidiasis. WHO www.who.int/teams/control-of-neglected-tropical-diseases/soil-transmitted-helminthiases/strongyloidiasis (2023).
Al-Mekhlafi, H. M. et al. Prevalence and risk factors of Strongyloides stercoralis infection among Orang Asli schoolchildren: new insights into the epidemiology, transmission and diagnosis of strongyloidiasis in Malaysia. Parasitology 146, 1602–1614 (2019).
Khieu, V. et al. Prevalence and risk factors of Strongyloides stercoralis in Takeo Province, Cambodia. Parasit. Vectors 7, 221 (2014).
White, M. A. F., Whiley, H. & Ross, K. E. A review of Strongyloides spp. environmental sources worldwide. Pathogens 8, 91 (2019).
Jongwutiwes, U., Waywa, D., Silpasakorn, S., Wanachiwanawin, D. & Suputtamongkol, Y. Prevalence and risk factors of acquiring Strongyloides stercoralis infection among patients attending a tertiary hospital in Thailand. Pathog. Glob. Health 108, 137–140 (2014).
Laoraksawong, P. et al. Current high prevalences of Strongyloides stercoralis and Opisthorchis viverrini infections in rural communities in northeast Thailand and associated risk factors. BMC Public. Health 18, 940 (2018).
Punsawad, C., Phasuk, N., Thongtup, K., Nagavirochana, S. & Viriyavejakul, P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, southern Thailand. BMC Public. Health 19, 34 (2019).
Kudah, C., Sovoe, S. & Baiden, F. Parasitic contamination of commonly consumed vegetables in two markets in Ghana. Ghana. Med. J. 52, 88–93 (2018).
Durrheim, D. N. Simply wearing footwear could interrupt transmission of Strongyloides stercoralis. BMJ 347, f5219 (2013).
Czachor, J. S. & Jonas, A. P. Transmission of Strongyloides steracolis person to person. J. Travel. Med. 7, 211–212 (2000).
Mendez, P., Walsh, B. & Hallem, E. A. Using newly optimized genetic tools to probe Strongyloides sensory behaviors. Mol. Biochem. Parasitol. 250, 111491 (2022).
Gomez Gallego, S. et al. Identification of an astacin-like metallo-proteinase transcript from the infective larvae of Strongyloides stercoralis. Parasitol. Int. 54, 123–133 (2005).
Schad, G. A., Aikens, L. M. & Smith, G. Strongyloides stercoralis: is there a canonical migratory route through the host? J. Parasitol. 75, 740–749 (1989).
Bonne-Année, S., Hess, J. A. & Abraham, D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol. Res. 51, 205–214 (2011).
Nutman, T. B. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144, 263–273 (2017).
Geri, G. et al. Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection 43, 691–698 (2015).
McDonald, H. H. & Moore, M. Strongyloides stercoralis hyperinfection. N. Engl. J. Med. 376, 2376 (2017).
Pedersen, A. A., Hartmeyer, G. N., Stensvold, C. R. & Martin-Iguacel, R. Strongyloides stercoralis hyperinfection syndrome with cerebral involvement. BMJ Case Rep. 15, e247032 (2022).
Weatherhead, J. E. & Mejia, R. Immune response to infection with Strongyloides stercoralis in patients with infection and hyperinfection. Curr. Tropical Med. Rep. 1, 229–233 (2014).
Cantacessi, C. & Gasser, R. B. SCP/TAPS proteins in helminths – where to from now? Mol. Cell. Probes 26, 54–59 (2012).
Rodrigues, R. M. et al. IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen. Parasitol. Res. 101, 1209–1214 (2007).
James, L. K. & Till, S. J. Potential mechanisms for IgG4 inhibition of immediate hypersensitivity reactions. Curr. Allergy Asthma Rep. 16, 23 (2016).
Kerepesi, L. A., Hess, J. A., Nolan, T. J., Schad, G. A. & Abraham, D. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 176, 4315–4322 (2006).
Keiser, P. B. & Nutman, T. B. Strongyloides stercoralis in the immunocompromised population. Clin. Microbiol. Rev. 17, 208–217 (2004).
Vadlamudi, R. S., Chi, D. S. & Krishnaswamy, G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin. Mol. Allergy 4, 8 (2006).
Utzinger, J. et al. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 142, w13727 (2012).
Buonfrate, D. et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect. Dis. 13, 78 (2013).
World Health Organization. A parasitic infection that can turn fatal with administration of corticosteroids. WHO www.who.int/news/item/17-12-2020-a-parasitic-infection-that-can-turn-fatal-with-administration-of-corticosteroids (2020).
Herbert, D. R., Stoltzfus, J. D. C., Rossi, H. L. & Abraham, D. Is Strongyloides stercoralis hyperinfection induced by glucocorticoids a result of both suppressed host immunity and altered parasite genetics? Mol. Biochem. Parasitol. 251, 111511 (2022).
Lok, J. B., Kliewer, S. A. & Mangelsdorf, D. J. The ‘nuclear option’ revisited: confirmation of Ss-daf-12 function and therapeutic potential in Strongyloides stercoralis and other parasitic nematode infections. Mol. Biochem. Parasitol. 250, 111490 (2022).
Schierhout, G. et al. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect. Dis. 20, 133–143 (2020).
Eschbach, M. L. et al. Strongyloides ratti infection induces transient nematode-specific Th2 response and reciprocal suppression of IFN-γ production in mice. Parasite Immunol. 32, 370–383 (2010).
Ye, L., Taylor, G. P. & Rosadas, C. Human T-cell lymphotropic virus type 1 and Strongyloides stercoralis co-infection: a systematic review and meta-analysis. Front. Med. 9, 832430 (2022).
Porto, M. A., Alcântara, L. M., Leal, M., Castro, N. & Carvalho, E. M. Atypical clinical presentation of strongyloidiasis in a patient co-infected with human T cell lymphotrophic virus type I. Am. J. Trop. Med. Hyg. 72, 124–125 (2005).
Salles, F. et al. Treatment of strongyloidiasis in HTLV-1 and Strongyloides stercoralis coinfected patients is associated with increased TNFα and decreased soluble IL2 receptor levels. Trans. R. Soc. Trop. Med. Hyg. 107, 526–529 (2013).
Montes, M. et al. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl. Trop. Dis. 3, e456 (2009).
Satoh, M. et al. Reduced efficacy of treatment of strongyloidiasis in HTLV-I carriers related to enhanced expression of IFN-γ and TGF-β1. Clin. Exp. Immunol. 127, 354–359 (2002).
Akanksha, K. et al. Prevalence of soil-transmitted helminth infections in HIV patients: a systematic review and meta-analysis. Sci. Rep. 13, 11055 (2023).
Ahmadpour, E. et al. Strongyloides stercoralis infection in human immunodeficiency virus-infected patients and related risk factors: a systematic review and meta-analysis. Transbound. Emerg. Dis. 66, 2233–2243 (2019).
Viney, M. E. et al. Why does HIV infection not lead to disseminated strongyloidiasis? J. Infect. Dis. 190, 2175–2180 (2004).
Siegel, M. O. & Simon, G. L. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl. Trop. Dis. 6, e1581 (2012).
Seeger, D., Cornejo Cisneros, E., Lucar, J. & Denyer, R. Strongyloides and COVID-19: challenges and opportunities for future research. Trop. Med. Infect. Dis. 8, 127 (2023).
Lier, A. J. et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am. J. Trop. Med. Hyg. 103, 1590–1592 (2020).
Kim, J. M. & Sivasubramanian, G. Strongyloides hyperinfection syndrome among COVID-19 patients treated with corticosteroids. Emerg. Infect. Dis. 28, 1531–1533 (2022).
Souza, A. et al. Modulation of circulating cytokine production in alcoholic patients infected with Strongyloides stercoralis. Parasite Immunol. 45, e12977 (2023).
de Souza, J. N. et al. Strongyloides stercoralis in alcoholic patients: implications of alcohol intake in the frequency of infection and parasite load. Pathogens 9, 422 (2020).
Fitzsimmons, C. M., Falcone, F. H. & Dunne, D. W. Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Front. Immunol. 5, 61 (2014).
Barve, S., Chen, S. Y., Kirpich, I., Watson, W. H. & McClain, C. Development, prevention, and treatment of alcohol-induced organ injury: the role of nutrition. Alcohol. Res. 38, 289–302 (2017).
Bujanda, L. The effects of alcohol consumption upon the gastrointestinal tract. Am. J. Gastroenterol. 95, 3374–3382 (2000).
Caumes, E. & Keystone, J. S. Acute strongyloidiasis: a rarity. Chronic strongyloidiasis: a time bomb! J. Travel. Med. 18, 71–72 (2011).
Freedman, D. O. Experimental infection of human subject with Strongyloides species. Rev. Infect. Dis. 13, 1221–1226 (1991).
Arthur, R. P. & Shelley, W. B. Larva currens; a distinctive variant of cutaneous larva migrans due to Strongyloides stercoralis. AMA Arch. Derm. 78, 186–190 (1958).
Tanaka, H. Experimental and epidemiological studies on strongyloidiasis of Amami Oshima island. Jpn. J. Exp. Med. 28, 159–182 (1958).
Kunst, H. et al. Parasitic infections of the lung: a guide for the respiratory physician. Thorax 66, 528–536 (2011).
Bernheim, A. & McLoud, T. A review of clinical and imaging findings in eosinophilic lung diseases. AJR Am. J. Roentgenol. 208, 1002–1010 (2017).
Yeung, S., Bharwada, Y., Bhasker, S. & Boggild, A. Strongyloidiasis: what every gastroenterologist needs to know. Ther. Adv. Chronic Dis. 13, 20406223221137499 (2022).
Buonfrate, D., Fittipaldo, A., Vlieghe, E. & Bottieau, E. Clinical and laboratory features of Strongyloides stercoralis infection at diagnosis and after treatment: a systematic review and meta-analysis. Clin. Microbiol. Infect. 27, 1621–1628 (2021).
Ming, D. K. et al. Clinical and diagnostic features of 413 patients treated for imported strongyloidiasis at the Hospital for Tropical Diseases, London. Am. J. Trop. Med. Hyg. 101, 428–431 (2019).
Salvador, F. et al. Imported strongyloidiasis: data from 1245 cases registered in the +REDIVI Spanish collaborative network (2009-2017). PLoS Negl. Trop. Dis. 13, e0007399 (2019).
Khieu, V. et al. Strongyloides stercoralis is a cause of abdominal pain, diarrhea and urticaria in rural Cambodia. BMC Res. Notes 6, 200 (2013).
Cruz, R. J. Jr, Vincenzi, R. & Ketzer, B. M. Duodenal obstruction – an unusual presentation of Strongyloides stercoralis enteritis: a case report. World J. Emerg. Surg. 5, 23 (2010).
El Hajj, W., Nakad, G. & Abou Rached, A. Protein loosing enteropathy secondary to strongyloidiasis: case report and review of the literature. Case Rep. Gastrointest. Med. 2016, 6831854 (2016).
Junare, P. R. & Udgirkar, S. S. An uncommon presentation of strongyloidiasis. Indian. J. Med. Res. 152, S12–S13 (2020).
Gutierrez, Y. et al. Strongyloides stercoralis eosinophilic granulomatous enterocolitis. Am. J. Surg. Pathol. 20, 603–612 (1996).
Saqib, S. U., Sood, S., Wong, L. & Patel, A. Strongyloides colitis, a rare but important mimic of Crohn’s disease, resulting in coma and multi-organ failure: a case report. Surg. Case Rep. 8, 211 (2022).
Gomez-Hinojosa, P., García-Encinas, C., Carlin-Ronquillo, A., Chancafe-Morgan, R. P. & Espinoza-Ríos, J. Strongyloides infection mimicking inflammatory bowel disease. Rev. Gastroenterol. Mex. 85, 366–368 (2020).
Qu, Z., Kundu, U. R., Abadeer, R. A. & Wanger, A. Strongyloides colitis is a lethal mimic of ulcerative colitis: the key morphologic differential diagnosis. Hum. Pathol. 40, 572–577 (2009).
Boscá Watts, M. M. et al. IBD or strongyloidiasis? Rev. Esp. Enferm. Dig. 108, 516–520 (2016).
Poveda, J., El-Sharkawy, F., Arosemena, L. R., Garcia-Buitrago, M. T. & Rojas, C. P. Strongyloides colitis as a harmful mimicker of inflammatory bowel disease. Case Rep. Pathol. 2017, 2560719 (2017).
Forrer, A. et al. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Negl. Trop. Dis. 11, e0005685 (2017).
Becker, S. L. et al. Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl. Trop. Dis. 5, e1292 (2011).
Martinez-Pérez, A. et al. Clinical features associated with strongyloidiasis in migrants and the potential impact of immunosuppression: a case control study. Pathogens 9, 507 (2020).
Tamarozzi, F. et al. Morbidity associated with chronic Strongyloides stercoralis infection: a systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 100, 1305–1311 (2019).
Zubrinich, C. M., Puy, R. M., O’Hehir, R. E. & Hew, M. Strongyloides infection as a reversible cause of chronic urticaria. J. Asthma Allergy 12, 67–69 (2019).
Showler, A. & Boggild, A. K. Strongyloidiasis presenting as larva currens 38 years after presumed exposure. J. Cutan. Med. Surg. 16, 433–435 (2012).
Mokhlesi, B., Shulzhenko, O., Garimella, P. S., Kuma, L. & Monti, C. Pulmonary strongyloidiasis: the varied clinical presentations. Clin. Pulm. Med. 11, 6–13 (2004).
Nwokolo, C. & Imohiosen, E. A. Strongyloidiasis of respiratory tract presenting as “asthma”. BMJ 2, 153–154 (1973).
Sen, P., Gil, C., Estrellas, B. & Middleton, J. R. Corticosteroid-induced asthma: a manifestation of limited hyperinfection syndrome due to Strongyloides stercoralis. South. Med. J. 88, 923–927 (1995).
Salam, R., Sharaan, A., Jackson, S. M., Solis, R. A. & Zuberi, J. Strongyloides hyperinfection syndrome: a curious case of asthma worsened by systemic corticosteroids. Am. J. Case Rep. 21, e925221 (2020).
Salluh, J. I. et al. Cutaneous periumbilical purpura in disseminated strongyloidiasis in cancer patients: a pathognomonic feature of potentially lethal disease? Braz. J. Infect. Dis. 9, 419–424 (2005).
Mukaigawara, M. et al. Clinical characteristics of disseminated strongyloidiasis, Japan, 1975-2017. Emerg. Infect. Dis. 26, 401–408 (2020).
Asdamongkol, N., Pornsuriyasak, P. & Sungkanuparph, S. Risk factors for strongyloidiasis hyperinfection and clinical outcomes. Southeast. Asian J. Trop. Med. Public. Health 37, 875–884 (2006).
Tam, J. et al. Case report: central nervous system strongyloidiasis: two cases diagnosed antemortem. Am. J. Trop. Med. Hyg. 100, 130–134 (2019).
Abanyie, F. A. et al. Donor-derived Strongyloides stercoralis infection in solid organ transplant recipients in the United States, 2009-2013. Am. J. Transpl. 15, 1369–1375 (2015).
Woodring, J. H., Halfhill, H. II & Reed, J. C. Pulmonary strongyloidiasis: clinical and imaging features. AJR Am. J. Roentgenol. 162, 537–542 (1994).
Chen, Y. A. et al. Epidemiology, clinical features, and outcomes of strongyloidiasis in Taiwan from 1988 to 2020: a case series and literature review. J. Microbiol. Immunol. Infect. 56, 172–181 (2023).
Barkati, S., Greenaway, C. & Libman, M. Strongyloidiasis-related lung involvement: too much of a bad thing. Curr. Opin. Infect. Dis. 36, 203–208 (2023).
Nabeya, D. et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect. Dis. 17, 320 (2017).
Siddiqui, A. A. & Berk, S. L. Diagnosis of Strongyloides stercoralis infection. Clin. Infect. Dis. 33, 1040–1047 (2001).
Link, K. & Orenstein, R. Bacterial complications of strongyloidiasis: Streptococcus bovis meningitis. South. Med. J. 92, 728–731 (1999).
World Health Organization. Diagnostic methods for the control of strongyloidiasis, virtual meeting, 29 September 2020. WHO www.who.int/publications/i/item/9789240016538 (2021).
Anamnart, W., Intapan, P. M. & Maleewong, W. Modified formalin-ether concentration technique for diagnosis of human strongyloidiasis. Korean J. Parasitol. 51, 743–745 (2013).
Knopp, S. et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am. J. Trop. Med. Hyg. 90, 535–545 (2014).
Potter, A., Stephens, D. & De Keulenaer, B. Strongyloides hyper-infection: a case for awareness. Ann. Trop. Med. Parasitol. 97, 855–860 (2003).
Bisoffi, Z. et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 8, e2640 (2014).
Norsyahida, A. et al. Laboratory detection of strongyloidiasis: IgG-, IgG4 - and IgE-ELISAs and cross-reactivity with lymphatic filariasis. Parasite Immunol. 35, 174–179 (2013).
Requena-Mendez, A. et al. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl. Trop. Dis. 7, e2002 (2013).
Page, W. A., Dempsey, K. & McCarthy, J. S. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans. R. Soc. Trop. Med. Hyg. 100, 1056–1062 (2006).
Boscolo, M. et al. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin. Vaccin. Immunol. 14, 129–133 (2007).
Biggs, B. A. et al. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am. J. Trop. Med. Hyg. 80, 788–791 (2009).
Salvador, F. et al. Usefulness of Strongyloides stercoralis serology in the management of patients with eosinophilia. Am. J. Trop. Med. Hyg. 90, 830–834 (2014).
Noordin, R. et al. A point-of-care cassette test for detection of Strongyloides stercoralis. Acta Trop. 226, 106251 (2022).
Tamarozzi, F. et al. Accuracy, acceptability, and feasibility of diagnostic tests for the screening of Strongyloides stercoralis in the field (ESTRELLA): a cross-sectional study in Ecuador. Lancet Glob. Health 11, e740–e748 (2023).
Yunus, M. H., Arifin, N., Balachandra, D., Anuar, N. S. & Noordin, R. Lateral flow dipstick test for serodiagnosis of strongyloidiasis. Am. J. Trop. Med. Hyg. 101, 432–435 (2019).
Noordin, R. et al. Evaluation of a rapid IgG4 lateral flow dipstick test to detect Strongyloides stercoralis infection in Northeast Thailand. Am. J. Trop. Med. Hyg. 105, 688–691 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04999774?locStr=Ecuador&country=Ecuador&distance=50&cond=Strongyloidiasis&rank=1 (2022).
Mounsey, K. et al. Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE. Acta Trop. 138, 78–82 (2014).
Eamudomkarn, C. et al. Epidemiology of strongyloidiasis determined by parasite-specific IgG detections by enzyme-linked immunosorbent assay on urine samples using Strongyloides stercoralis, S. ratti and recombinant protein (NIE) as antigens in Northeast Thailand. PLoS ONE 18, e0284305 (2023).
Wongphutorn, P. et al. Diagnostic performance of Strongyloides-specific IgG4 detection in urine for diagnosis of human strongyloidiasis. Parasit. Vectors 16, 298 (2023).
Sykes, A. M. & McCarthy, J. S. A coproantigen diagnostic test for Strongyloides infection. PLoS Negl. Trop. Dis. 5, e955 (2011).
Balachandra, D. et al. A new antigen detection ELISA for the diagnosis of Strongyloides infection. Acta Trop. 221, 105986 (2021).
Verweij, J. J. et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans. R. Soc. Trop. Med. Hyg. 103, 342–346 (2009).
Pilotte, N. et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl. Trop. Dis. 10, e0004578 (2016).
Iamrod, K. et al. Development and efficacy of droplet digital PCR for detection of Strongyloides stercoralis in stool. Am. J. Trop. Med. Hyg. 106, 312–319 (2021).
Watts, M. R. et al. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am. J. Trop. Med. Hyg. 90, 306–311 (2014).
Buonfrate, D. et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection – a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 12, e0006229 (2018).
Buonfrate, D., Perandin, F., Formenti, F. & Bisoffi, Z. A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 144, 812–816 (2017).
Barda, B. et al. Evaluation of two DNA extraction methods for detection of Strongyloides stercoralis infection. J. Clin. Microbiol. 56, e01941-17 (2018).
Repetto, S. A. et al. An improved DNA isolation technique for PCR detection of Strongyloides stercoralis in stool samples. Acta Trop. 126, 110–114 (2013).
Formenti, F. et al. A diagnostic study comparing conventional and real-time PCR for Strongyloides stercoralis on urine and on faecal samples. Acta Trop. 190, 284–287 (2019).
Lodh, N. et al. Diagnosis of Strongyloides stercoralis: detection of parasite-derived DNA in urine. Acta Trop. 163, 9–13 (2016).
Osiro, S., Hamula, C., Glaser, A., Rana, M. & Dunn, D. A case of Strongyloides hyperinfection syndrome in the setting of persistent eosinophilia but negative serology. Diagn. Microbiol. Infect. Dis. 88, 168–170 (2017).
Toledo, B. et al. Screening of Strongyloides infection using an ELISA test in transplant candidates. Clinics 74, e698 (2019).
Barrett, J. et al. The changing aetiology of eosinophilia in migrants and returning travellers in the Hospital for Tropical Diseases, London 2002-2015: an observational study. J. Infect. 75, 301–308 (2017).
Cañas García-Otero, E. et al. Clinical approach to imported eosinophilia [Spanish]. Enferm. Infecc. Microbiol. Clin. 34, 661–684 (2016).
Repetto, S. A. et al. Strongyloidiasis outside endemic areas: long-term parasitological and clinical follow-up after ivermectin treatment. Clin. Infect. Dis. 66, 1558–1565 (2018).
Castillo-Fernández, N. et al. Misleading eosinophil counts in migration-associated malaria: do not miss hidden helminthic co-infections. Travel. Med. Infect. Dis. 49, 102415 (2022).
Rojas, O. C., Montoya, A. M., Villanueva-Lozano, H. & Carrion-Alvarez, D. Severe strongyloidiasis: a systematic review and meta-analysis of 339 cases. Trans. R. Soc. Trop. Med. Hyg. 66, 682–696 (2023).
Buonfrate et al. Progress towards the implementation of control programmes for strongyloidiasis in endemic areas: estimation of number of adults in need of ivermectin for strongyloidiasis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 379, 20220433 (2024).
World Health Organization. Donating high‐quality medicines and diagnostics for the control of STH in children. WHO www.who.int/activities/donating-high-quality-medicines-and-diagnostics-for-the-control-of-sth-in-children (2020).
Crump, A. & Ōmura, S. Ivermectin, ‘wonder drug’ from Japan: the human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 87, 13–28 (2011).
Weil, G. J. et al. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: a multicenter, open-label, cluster-randomized study. PLoS Med. 16, e1002839 (2019).
Barda, B. et al. Side benefits of mass drug administration for lymphatic filariasis on Strongyloides stercoralis prevalence on Pemba Island, Tanzania. Am. J. Trop. Med. Hyg. 97, 681–683 (2017).
Ichimori, K. & Crump, A. Pacific collaboration to eliminate lymphatic filariasis. Trends Parasitol. 21, 441–444 (2005).
Colatrella, B. The mectizan donation program: 20 years of successful collaboration – a retrospective. Ann. Trop. Med. Parasitol. 102, 7–11 (2008).
Wong, M. T. J., Anuar, N. S., Noordin, R. & Tye, G. J. Soil-transmitted helminthic vaccines: where are we now? Acta Trop. 239, 106796 (2023).
Page, W. A., Judd, J. A., MacLaren, D. J. & Buettner, P. Integrating testing for chronic strongyloidiasis within the Indigenous adult preventive health assessment system in endemic communities in the Northern Territory, Australia: an intervention study. PLoS Negl. Trop. Dis. 14, e0008232 (2020).
Davis, J. S. et al. Prevention of opportunistic infections in immunosuppressed patients in the tropical top end of the Northern Territory. Commun. Dis. Intell. Q. Rep. 27, 526–532 (2003).
Requena-Méndez, A. et al. Evidence-based guidelines for screening and management of strongyloidiasis in non-endemic countries. Am. J. Trop. Med. Hyg. 97, 645–652 (2017).
Carnino, L. et al. A practical approach to screening for Strongyloides stercoralis. Trop. Med. Infect. Dis. 6, 203 (2021).
Hürlimann, E., Hofmann, D. & Keiser, J. Ivermectin and moxidectin against soil-transmitted helminth infections. Trends Parasitol. 39, 272–284 (2023).
Henriquez-Camacho, C. et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst. Rev. 2016, Cd007745 (2016).
Buonfrate, D. et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 19, 1181–1190 (2019).
Barda, B. et al. Efficacy of moxidectin versus ivermectin against Strongyloides stercoralis infections: a randomized, controlled noninferiority trial. Clin. Infect. Dis. 65, 276–281 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT01570504?distance=50&cond=Strongyloidiasis&rank=1 (2018).
Jittamala, P. et al. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 15, e0009144 (2021).
Brussee, J. M., Schulz, J. D., Coulibaly, J. T., Keiser, J. & Pfister, M. Ivermectin dosing strategy to achieve equivalent exposure coverage in children and adults. Clin. Pharmacol. Ther. 106, 661–667 (2019).
Buonfrate, D. Alternative treatment strategies for trichuriasis. Lancet Infect. Dis. 23, 266–267 (2023).
Centers for Disease Control and Prevention. Resources for health professionals. CDC www.cdc.gov/parasites/strongyloides/health_professionals/index.html (2023).
Milton, P., Hamley, J. I. D., Walker, M. & Basáñez, M. G. Moxidectin: an oral treatment for human onchocerciasis. Expert. Rev. Anti Infect. Ther. 18, 1067–1081 (2020).
Hofmann, D. et al. Efficacy and safety of ascending doses of moxidectin against Strongyloides stercoralis infections in adults: a randomised, parallel-group, single-blinded, placebo-controlled, dose-ranging, phase 2a trial. Lancet Infect. Dis. 21, 1151–1160 (2021).
Hofmann, D., Smit, C., Sayasone, S., Pfister, M. & Keiser, J. Optimizing moxidectin dosing for Strongyloides stercoralis infections: insights from pharmacometric modeling. Clin. Transl. Sci. 15, 700–708 (2022).
Pfarr, K. M. et al. The pipeline for drugs for control and elimination of neglected tropical diseases: 1. Anti-infective drugs for regulatory registration. Parasit. Vectors 16, 82 (2023).
Sprecher, V. P. et al. Efficacy and safety of moxidectin compared with ivermectin against Strongyloides stercoralis infection in adults in Laos and Cambodia: a randomised, double-blind, non-inferiority, phase 2b/3 trial. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(23)00507-8 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04848688?distance=50&cond=Strongyloides&locStr=Cambodia&country=Cambodia&rank=1 (2022).
Smit, C., Hofmann, D., Sayasone, S., Keiser, J. & Pfister, M. Characterization of the population pharmacokinetics of moxidectin in adults infected with Strongyloides stercoralis: support for a fixed-dose treatment regimen. Clin. Pharmacokinet. 61, 123–132 (2022).
Konecny, P. et al. Case report: subcutaneous ivermectin pharmacokinetics in disseminated strongyloides infection: plasma and postmortem analysis. Am. J. Trop. Med. Hyg. 99, 1580–1582 (2018).
Rose, C. E., Paciullo, C. A., Kelly, D. R., Dougherty, M. J. & Fleckenstein, L. L. Fatal outcome of disseminated strongyloidiasis despite detectable plasma and cerebrospinal levels of orally administered ivermectin. J. Parasitol. Res. 2009, 818296 (2009).
Edwards, G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J. 2, S8 (2003).
Leder, K. & Weller, P. F. Strongyloidiasis. UpToDate www.uptodate.com/contents/strongyloidiasis (2023).
Zeitler, K. et al. Successful use of subcutaneous ivermectin for the treatment of Strongyloides stercoralis hyperinfection in the setting of small bowel obstruction and paralytic ileus in the immunocompromised population. BMJ Case Rep. 2018, bcr2017223138 (2018).
Barrett, J., Broderick, C., Soulsby, H., Wade, P. & Newsholme, W. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: two case reports and a discussion of the literature. J. Antimicrob. Chemother. 71, 220–225 (2016).
Marty, F. M. et al. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clin. Infect. Dis. 41, e5–e8 (2005).
Tarr, P. E. et al. Case report: rectal adminstration of ivermectin to a patient with Strongyloides hyperinfection syndrome. Am. J. Trop. Med. Hyg. 68, 453–455 (2003).
Bogoch, I. I. et al. Failure of ivermectin per rectum to achieve clinically meaningful serum levels in two cases of Strongyloides hyperinfection. Am. J. Trop. Med. Hyg. 93, 94–96 (2015).
Pornsuriyasak, P., Niticharoenpong, K. & Sakapibunnan, A. Disseminated strongyloidiasis successfully treated with extended duration ivermectin combined with albendazole: a case report of intractable strongyloidiasis. Southeast. Asian J. Trop. Med. Public. Health 35, 531–534 (2004).
Nuesch, R., Zimmerli, L., Stockli, R., Gyr, N. & Christoph Hatz, F. R. Imported strongyloidosis: a longitudinal analysis of 31 cases. J. Travel. Med. 12, 80–84 (2005).
Buonfrate, D. & Bisoffi, Z. Is ivermectin ineffective for strongyloidiasis? Clin. Infect. Dis. 67, 810–811 (2018).
Page, W. & Speare, R. Chronic strongyloidiasis – don’t look and you won’t find. Aust. Fam. Physician 45, 40–44 (2016).
UNICEF. Stop stunting. UNICEF www.unicef.org/india/what-we-do/stop-stunting (2018).
World Health Organization. Stunting in a nutshell. WHO www.who.int/news/item/19-11-2015-stunting-in-a-nutshell#:~:Text=Stunting%20is%20the%20impaired%20growth,WHO%20Child%20Growth%20Standards%20median (2015).
World Health Organization. 2030 targets for soil-transmitted helminthiases control programmes. WHO https://www.who.int/publications/i/item/9789240000315 (2020).
World Health Organization. Ending the neglect to attain the sustainable development goals – a road map for neglected tropical diseases 2021–2030 (WHO, 2020).
Mascarini-Serra, L. Prevention of soil-transmitted helminth infection. J. Glob. Infect. Dis. 3, 175–182 (2011).
World Health Organization. Soil-transmitted helminth infections. WHO www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (2023).
Hürlimann, E. et al. Efficacy and safety of co-administered ivermectin and albendazole in school-aged children and adults infected with Trichuris trichiura in Côte d’Ivoire, Laos, and Pemba Island, Tanzania: a double-blind, parallel-group, phase 3, randomised controlled trial. Lancet Infect. Dis. 22, 123–135 (2022).
World Health Organization. Onchocerciasis. WHO www.who.int/news-room/fact-sheets/detail/onchocerciasis (2022).
Tuersong, W. et al. Comparative analysis on transcriptomics of ivermectin resistant and susceptible strains of Haemonchus contortus. Parasit. Vectors 15, 159 (2022).
Prichard, R. K. Ivermectin resistance and overview of the consortium for anthelmintic resistance SNPs. Expert. Opin. Drug. Discov. 2, S41–S52 (2007).
Rubin, E. J. Making the worm turn. N. Engl. J. Med. 388, 1908–1910 (2023).
Mrimi, E. C., Welsche, S., Ali, S. M., Hattendorf, J. & Keiser, J. Emodepside for Trichuris trichiura and hookworm infection. N. Engl. J. Med. 388, 1863–1875 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05017194 (2022).
Kilarski, W. W. et al. Inherent biomechanical traits enable infective filariae to disseminate through collecting lymphatic vessels. Nat. Commun. 10, 2895 (2019).
De Kyvon, M.-A. et al. Linear cutaneous erythema in a patient with amyotrophic lateral sclerosis. Clin. Infect. Dis. 66, 1637–1638 (2018).
Ofosu, A., Higgins, J., Frye, J. S., Kumari, R. & Barakat, M. T. Strongyloides superinfection after liver transplantion. Digestive Dis. Sci. 66, 2178–2182 (2021).
DPDx. Strongyloidiasis. CDC https://www.cdc.gov/dpdx/strongyloidiasis/index.html (2019).
DPDx. Hookworm (intestinal). CDC https://www.cdc.gov/dpdx/hookworm/index.html (2019).
Author information
Authors and Affiliations
Contributions
Introduction (C.A.G. and J.U.); Epidemiology (C.A.G. and J.U.); Mechanisms/pathophysiology (S.M. and S.L.B.); Diagnosis, screening, and prevention (C.A.G., S.M. and S.L.B.); Management (V.K., D.J.G., J.K. and S.L.B.); Quality of life (J.U. and D.J.G.); Outlook (C.A.G., J.U., V.K., D.J.G., S.M., J.K. and S.L.B.); Overview of the Primer (C.A.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks S. Babu, R. Gryschek, H. Maruyama and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gordon, C.A., Utzinger, J., Muhi, S. et al. Strongyloidiasis. Nat Rev Dis Primers 10, 6 (2024). https://doi.org/10.1038/s41572-023-00490-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-023-00490-x