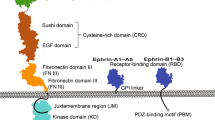
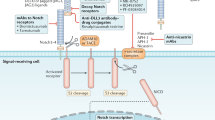
Abstract
Evidence implicating Eph receptor tyrosine kinases and their ephrin ligands (that together make up the ‘Eph system’) in cancer development and progression has been accumulating since the discovery of the first Eph receptor approximately 35 years ago. Advances in the past decade and a half have considerably increased the understanding of Eph receptor–ephrin signalling mechanisms in cancer and have uncovered intriguing new roles in cancer progression and drug resistance. This Review focuses mainly on these more recent developments. I provide an update on the different mechanisms of Eph receptor–ephrin-mediated cell–cell communication and cell autonomous signalling, as well as on the interplay of the Eph system with other signalling systems. I further discuss recent advances in elucidating how the Eph system controls tumour expansion, invasiveness and metastasis, supports cancer stem cells, and drives therapy resistance. In addition to functioning within cancer cells, the Eph system also mediates the reciprocal communication between cancer cells and cells of the tumour microenvironment. The involvement of the Eph system in tumour angiogenesis is well established, but recent findings also demonstrate roles in immune cells, cancer-associated fibroblasts and the extracellular matrix. Lastly, I discuss strategies under evaluation for therapeutic targeting of Eph receptors–ephrins in cancer and conclude with an outlook on promising future research directions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the graphs shown in Fig. 2 are available in cBioPortal.
References
Pasquale, E. B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 6, 462–475 (2005).
Pasquale, E. B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10, 165–180 (2010).
Boyd, A. W., Bartlett, P. F. & Lackmann, M. Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug Discov. 13, 39–62 (2014).
Wimmer-Kleikamp, S. H., Janes, P. W., Squire, A., Bastiaens, P. I. & Lackmann, M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 164, 661–666 (2004).
Seiradake, E., Harlos, K., Sutton, G., Aricescu, A. R. & Jones, E. Y. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat. Struct. Mol. Biol. 17, 398–402 (2010).
Seiradake, E. et al. Structurally encoded intraclass differences in EphA clusters drive distinct cell responses. Nat. Struct. Mol. Biol. 20, 958–964 (2013).
Himanen, J. P. et al. Architecture of Eph receptor clusters. Proc. Natl Acad. Sci. USA 107, 10860–10865 (2010).
Liang, L. Y., Patel, O., Janes, P. W., Murphy, J. M. & Lucet, I. S. Eph receptor signalling: from catalytic to non-catalytic functions. Oncogene 38, 6567–6584 (2019).
Hattori, M., Osterfield, M. & Flanagan, J. G. Regulated cleavage of a contact-mediated axon repellent. Science 289, 1360–1365 (2000).
Janes, P. W. et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123, 291–304 (2005).
Janes, P. W. et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol. 7, e1000215 (2009).
Solanas, G., Cortina, C., Sevillano, M. & Batlle, E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat. Cell Biol. 13, 1100–1107 (2011).
Batlle, E. & Wilkinson, D. G. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 4, a008227 (2012).
Janes, P. W., Nievergall, E. & Lackmann, M. Concepts and consequences of Eph receptor clustering. Semin. Cell Dev. Biol. 23, 43–50 (2012).
Cai, C. et al. ADAM10-cleaved ephrin-A5 contributes to prostate cancer metastasis. Cell Death Dis. 13, 453 (2022).
Schaupp, A. et al. The composition of EphB2 clusters determines the strength in the cellular repulsion response. J. Cell Biol. 204, 409–422 (2014).
Chen, Z., Oh, D., Biswas, K. H., Zaidel-Bar, R. & Groves, J. T. Probing the effect of clustering on EphA2 receptor signaling efficiency by subcellular control of ligand-receptor mobility. eLife 10, e67379 (2021).
Zapata-Mercado, E. et al. The efficacy of receptor tyrosine kinase EphA2 autophosphorylation increases with EphA2 oligomer size. J. Biol. Chem. 298, 102370 (2022).
Ojosnegros, S. et al. Eph-ephrin signaling modulated by polymerization and condensation of receptors. Proc. Natl Acad. Sci. USA 114, 13188–13193 (2017).
Stein, E. et al. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 12, 667–678 (1998).
Salaita, K. et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, 1380–1385 (2010).
Conway, A. et al. Multivalent ligands control stem cell behaviour in vitro and in vivo. Nat. Nanotechnol. 8, 831–838 (2013).
Dong, M. et al. Spatiomechanical modulation of EphB4-ephrin-B2 signaling in neural stem cell differentiation. Biophys. J. 115, 865–873 (2018).
Verheyen, T. et al. Spatial organization-dependent EphA2 transcriptional responses revealed by ligand nanocalipers. Nucleic Acids Res. 48, 5777–5787 (2020).
Cortina, C. et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 39, 1376–1383 (2007).
Miura, K., Nam, J. M., Kojima, C., Mochizuki, N. & Sabe, H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol. Biol. Cell 20, 1949–1959 (2009).
Nievergall, E. et al. PTP1B regulates Eph receptor function and trafficking. J. Cell Biol. 191, 1189–1203 (2010).
Wakayama, Y., Miura, K., Sabe, H. & Mochizuki, N. EphrinA1-EphA2 signal induces compaction and polarization of Madin-Darby canine kidney cells by inactivating ezrin through negative regulation of RhoA. J. Biol. Chem. 286, 44243–44253 (2011).
Stahl, S. et al. Phosphoproteomic profiling of NSCLC cells reveals that ephrin B3 regulates pro-survival signaling through Akt1-mediated phosphorylation of the EphA2 receptor. J. Proteome Res. 10, 2566–2578 (2011).
Falivelli, G. et al. Attenuation of eph receptor kinase activation in cancer cells by coexpressed ephrin ligands. PLoS ONE 8, e81445 (2013).
Lisabeth, E. M., Falivelli, G. & Pasquale, E. B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 5, a009159 (2013).
Efazat, G. et al. Ephrin B3 interacts with multiple EphA receptors and drives migration and invasion in non-small cell lung cancer. Oncotarget 7, 60332–60347 (2016).
Xu, K. et al. Insights into Eph receptor tyrosine kinase activation from crystal structures of the EphA4 ectodomain and its complex with ephrin-A5. Proc. Natl Acad. Sci. USA 110, 14634–14639 (2013).
Xu, Y. et al. The Ephb2 receptor uses homotypic, head-to-tail interactions within its ectodomain as an autoinhibitory control mechanism. Int. J. Mol. Sci. 22, 10473 (2021).
Miao, H. et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 16, 9–20 (2009).
Zhou, Y. et al. Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2. Nat. Commun. 6, 7679 (2015). This study shows that serine–threonine kinases of the RSK family, which are activated downstream of ERK, are important regulators of EPHA2 S897 phosphorylation that leads to cancer cell invasion. Thus, oncogenic EPHA2 non-canonical signalling can be induced not only by AKT, as previously known, but also by the ERK–RSK signaling pathway activated, for example, by inflammatory cytokines.
Barquilla, A. et al. Protein kinase A can block EphA2 receptor-mediated cell repulsion by increasing EphA2 S897 phosphorylation. Mol. Biol. Cell 27, 2757–2770 (2016).
Gehring, M. P. & Pasquale, E. B. Protein kinase C phosphorylates the EphA2 receptor on serine 892 in the regulatory linker connecting the kinase and SAM domains. Cell Signal. 73, 109668 (2020).
Lechtenberg, B. C. et al. Regulation of the EphA2 receptor intracellular region by phosphomimetic negative charges in the kinase-SAM linker. Nat. Commun. 12, 7047 (2021).
Pasquale, E. B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008).
Barquilla, A. & Pasquale, E. B. Eph receptors and ephrins: therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 55, 465–487 (2015).
Daar, I. O. Non-SH2/PDZ reverse signaling by ephrins. Semin. Cell Dev. Biol. 23, 65–74 (2012).
Kania, A. & Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 17, 240–256 (2016).
Wilkinson, D. G. Interplay of Eph-Ephrin signalling and cadherin function in cell segregation and boundary formation. Front. Cell Dev. Biol. 9, 784039 (2021).
Bush, J. O. Cellular and molecular mechanisms of EPH/EPHRIN signaling in evolution and development. Curr. Top. Dev. Biol. 149, 153–201 (2022).
Nievergall, E., Lackmann, M. & Janes, P. W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell Mol. Life Sci. 69, 1813–1842 (2012).
Hanover, G. et al. Integration of cancer-related genetic landscape of Eph receptors and ephrins with proteomics identifies a crosstalk between EPHB6 and EGFR. Cell Rep. 42, 112670 (2023). This study integrates genomics, proteomics and bioinformatics to define the signalling networks of individual Eph receptors and ephrins and highlights connections with the EGFR family. A strong cancer-related crosstalk between EPHB6 and EGFR was predicted and experimentally validated.
Chen, X. et al. The role of EphA7 in different tumors. Clin. Transl. Oncol. 24, 1274–1289 (2022).
Batlle, E. et al. EphB receptor activity suppresses colorectal cancer progression. Nature 435, 1126–1130 (2005).
Hua, K. T. et al. miR-519d promotes melanoma progression by downregulating EphA4. Cancer Res. 78, 216–229 (2018).
Macrae, M. et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell 8, 111–118 (2005).
Sugiyama, N. et al. EphA2 cleavage by MT1-MMP triggers single cancer cell invasion via homotypic cell repulsion. J. Cell Biol. 201, 467–484 (2013).
Dunne, P. D. et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin. Cancer Res. 22, 230–242 (2016).
Tsouko, E., Wang, J., Frigo, D. E., Aydogdu, E. & Williams, C. miR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EPHA2 oncogene. Carcinogenesis 36, 1051–1060 (2015).
Mo, J. et al. Effect of EphA2 knockdown on melanoma metastasis depends on intrinsic ephrinA1 level. Cell Oncol. 43, 655–667 (2020).
Yang, N. Y. et al. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cell Signal. 23, 201–212 (2011).
Youngblood, V. M. et al. The Ephrin-A1/EPHA2 signaling axis regulates glutamine metabolism in HER2-positive breast cancer. Cancer Res. 76, 1825–1836 (2016). This paper reports a role for EPHA2 in reprogramming cancer cell metabolism to promote cancer cell fitness through increased glutamine metabolism and de novo lipogenesis.
Edwards, D. N. et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 10, eaan4667 (2017).
Liang, S. et al. Ligand-independent EphA2 contributes to chemoresistance in small-cell lung cancer by enhancing PRMT1-mediated SOX2 methylation. Cancer Sci. 114, 921–936 (2022).
Harly, C. et al. Human γδ T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Sci. Immunol. 6, eaba9010 (2021). This study shows that interaction with EPHA2 enhances T cell receptor signalling and cytotoxicity against cancer cells. This intercellular communication involves simultaneous EPHA2 interaction with the γδ T cell receptor and EFNA4 and suggests that EPHA2 is part of a cancer stress signature with a role in immunomodulation.
Miao, B. et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 5, 274–287 (2015).
Paraiso, K. H. et al. Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype. Cancer Discov. 5, 264–273 (2015).
Hugo, W. et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 162, 1271–1285 (2015).
Koch, H., Busto, M. E., Kramer, K., Medard, G. & Kuster, B. Chemical proteomics uncovers EPHA2 as a mechanism of acquired resistance to small molecule EGFR kinase inhibition. J. Proteome Res. 14, 2617–2625 (2015).
Amato, K. R. et al. EPHA2 blockade overcomes acquired resistance to EGFR kinase inhibitors in lung cancer. Cancer Res. 76, 305–318 (2016).
Chen, Z. et al. EPHA2 blockade reverses acquired resistance to afatinib induced by EPHA2-mediated MAPK pathway activation in gastric cancer cells and avatar mice. Int. J. Cancer 145, 2440–2449 (2019).
Cioce, M. & Fazio, V. M. EphA2 and EGFR: friends in life, partners in crime. Can EphA2 be a predictive biomarker of response to anti-EGFR agents? Cancers 13, 700 (2021).
Gu, Z. et al. Anlotinib inhibits tumor angiogenesis and promotes the anticancer effect of radiotherapy on esophageal cancer through inhibiting EphA2. J. Oncol. 2022, 5632744 (2022).
Janes, P. W. et al. EphA3 biology and cancer. Growth Factors 32, 176–189 (2014).
Gomez-Maldonado, L. et al. EFNA3 long noncoding RNAs induced by hypoxia promote metastatic dissemination. Oncogene 34, 2609–2620 (2015).
Husain, A. et al. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J. Hepatol. 77, 383–396 (2022). This study identifies EFNA3–EPHA2 as a hypoxia-driven signalling axis associated with more aggressive hepatocellular carcinoma through effects on cancer cell self-renewal, proliferation and migration that depend on de novo lipogenesis.
Fasanaro, P. et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 283, 15878–15883 (2008).
Fleuren, E. D., Zhang, L., Wu, J. & Daly, R. J. The kinome ‘at large’ in cancer. Nat. Rev. Cancer 16, 83–98 (2016).
Ashford, P., Pang, C. S. M., Moya-Garcia, A. A., Adeyelu, T. & Orengo, C. A. A CATH domain functional family based approach to identify putative cancer driver genes and driver mutations. Sci. Rep. 9, 263 (2019).
Martinez-Jimenez, F. et al. A compendium of mutational cancer driver genes. Nat. Rev. Cancer 20, 555–572 (2020).
Lisabeth, E. M., Fernandez, C. & Pasquale, E. B. Cancer somatic mutations disrupt functions of the EphA3 receptor tyrosine kinase through multiple mechanisms. Biochemistry 51, 1464–1475 (2012).
Zhuang, G. et al. Effects of cancer-associated EPHA3 mutations on lung cancer. J. Natl Cancer Inst. 104, 1182–1197 (2012).
Lahtela, J. et al. The putative tumor suppressor gene EphA3 fails to demonstrate a crucial role in murine lung tumorigenesis or morphogenesis. Dis. Model. Mech. 8, 393–401 (2015).
Mathot, L. et al. Somatic ephrin receptor mutations are associated with metastasis in primary colorectal cancer. Cancer Res. 77, 1730–1740 (2017).
Kim, Y., Ahmed, S. & Miller, W. T. Colorectal cancer-associated mutations impair EphB1 kinase function. J. Biol. Chem. 299, 105115 (2023).
Peifer, M. et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 44, 1110 (2012).
Zhang, Z. et al. EPHA7 mutation as a predictive biomarker for immune checkpoint inhibitors in multiple cancers. BMC Med. 19, 26 (2021).
Li, S. et al. Ligand-dependent EphA7 signaling inhibits prostate tumor growth and progression. Cell Death Dis. 8, e3122 (2017).
Lee, H., Park, E., Kim, Y. & Park, S. EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1. Mol. Cell 35, 450–455 (2013).
Jungas, T. et al. Eph-mediated tyrosine phosphorylation of citron kinase controls abscission. J. Cell Biol. 214, 555–569 (2016).
Chukkapalli, S. et al. Role of the EphB2 receptor in autophagy, apoptosis and invasion in human breast cancer cells. Exp. Cell Res. 320, 233–246 (2014).
Noren, N. K., Foos, G., Hauser, C. A. & Pasquale, E. B. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl–Crk pathway. Nat. Cell Biol. 8, 815–825 (2006).
Kuang, S. Q. et al. Aberrant DNA methylation and epigenetic inactivation of Eph receptor tyrosine kinases and ephrin ligands in acute lymphoblastic leukemia. Blood 115, 2412–2419 (2010).
Rutkowski, R., Mertens-Walker, I., Lisle, J. E., Herington, A. C. & Stephenson, S. A. Evidence for a dual function of EphB4 as tumor promoter and suppressor regulated by the absence or presence of the ephrin-B2 ligand. Int. J. Cancer 131, E614–624 (2012).
Aslam, M. I. et al. PDGFRβ reverses EphB4 signaling in alveolar rhabdomyosarcoma. Proc. Natl Acad. Sci. USA 111, 6383–6388 (2014).
Royet, A. et al. Ephrin-B3 supports glioblastoma growth by inhibiting apoptosis induced by the dependence receptor EphA4. Oncotarget 8, 23750–23759 (2017).
Raja, E. et al. Tyrosine kinase Eph receptor A6 sensitizes glioma-initiating cells towards bone morphogenetic protein-induced apoptosis. Cancer Sci. 110, 3486–3496 (2019).
El Zawily, A. M. et al. The intrinsically kinase-inactive EPHB6 receptor predisposes cancer cells to DR5-induced apoptosis by promoting mitochondrial fragmentation. Oncotarget 7, 77865–77877 (2016).
Amato, K. R. et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J. Clin. Invest. 124, 2037–2049 (2014).
Gong, S., Li, Y., Lv, L. & Men, W. Restored microRNA-519a enhances the radiosensitivity of non-small cell lung cancer via suppressing EphA2. Gene Ther. 29, 588–600 (2022).
Teramoto, K. & Katoh, H. The cystine/glutamate antiporter xCT is a key regulator of EphA2 S897 phosphorylation under glucose-limited conditions. Cell Signal. 62, 109329 (2019).
Harada, K., Hiramoto-Yamaki, N., Negishi, M. & Katoh, H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp. Cell Res. 317, 1701–1713 (2011).
Akada, M., Harada, K., Negishi, M. & Katoh, H. EphB6 promotes anoikis by modulating EphA2 signaling. Cell Signal. 26, 2879–2884 (2014).
Ji, X. D. et al. EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res. 71, 1156–1166 (2011).
Maddigan, A. et al. EphB receptors trigger Akt activation and suppress Fas receptor-induced apoptosis in malignant T lymphocytes. J. Immunol. 187, 5983–5994 (2011).
DiPrima, M. et al. Identification of Eph receptor signaling as a regulator of autophagy and a therapeutic target in colorectal carcinoma. Mol. Oncol. 13, 2441–2459 (2019).
McCall, J. L. et al. KSR1 and EPHB4 regulate Myc and PGC1β to promote survival of human colon tumors. Mol. Cell Biol. 36, 2246–2261 (2016).
Kumar, S. R. et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am. J. Pathol. 169, 279–293 (2006).
Xia, G. et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene 25, 769–780 (2006).
Spannuth, W. A. et al. Converging evidence for efficacy from parallel EphB4-targeted approaches in ovarian carcinoma. Mol. Cancer Ther. 9, 2377–2388 (2010).
Merchant, A. A. et al. EPHB4 is a therapeutic target in AML and promotes leukemia cell survival via AKT. Blood Adv. 1, 1635–1644 (2017).
Paul, J. M. et al. Targeting synthetic lethality between the SRC kinase and the EPHB6 receptor may benefit cancer treatment. Oncotarget 7, 50027–50042 (2016).
Schwill, M. et al. Systemic analysis of tyrosine kinase signaling reveals a common adaptive response program in a HER2-positive breast cancer. Sci. Signal. 12, eaau2875 (2019). This research is a system-wide analysis that identifies Eph receptor activation as part of an adaptive response program that prevents apoptosis in ERBB2-dependent breast cancer cells treated with the ERBB22 inhibitors trastuzumab and ARRY380 (which as single agents cause cell cycle arrest but not apoptosis). Thus, compensatory Eph receptor activation may facilitate escape from apoptosis and the development of acquired resistance to ERBB2-targeted therapies.
Wang, S. D. et al. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 31, 5132–5143 (2012).
Bhatia, S. et al. The effects of ephrinB2 signaling on proliferation and invasion in glioblastoma multiforme. Mol. Carcinog. 59, 1064–1075 (2020).
Song, W. et al. Phosphorylation of PLCγ1 by EphA2 receptor tyrosine kinase promotes tumor growth in lung cancer. Mol. Cancer Res. 18, 1735–1743 (2020).
Genander, M. et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 139, 679–692 (2009).
Xiao, Z. et al. EphB4 promotes or suppresses Ras/MEK/ERK pathway in a context-dependent manner: implications for EphB4 as a cancer target. Cancer Biol. Ther. 13, 630–637 (2012).
Hamaoka, Y., Negishi, M. & Katoh, H. EphA2 is a key effector of the MEK/ERK/RSK pathway regulating glioblastoma cell proliferation. Cell Signal. 28, 937–945 (2016).
Brannan, J. M. et al. EphA2 in the early pathogenesis and progression of non-small cell lung cancer. Cancer Prev. Res. 2, 1039–1049 (2009).
Song, W., Ma, Y., Wang, J., Brantley-Sieders, D. & Chen, J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 74, 2444–2454 (2014).
Huang, C. et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J. Cell Mol. Med. 25, 2967–2975 (2021).
Song, W. et al. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene 36, 5620–5630 (2017).
Kaibori, Y., Saito, Y. & Nakayama, Y. EphA2 phosphorylation at Ser897 by the Cdk1/MEK/ERK/RSK pathway regulates M-phase progression via maintenance of cortical rigidity. FASEB J. 33, 5334–5349 (2019).
Yu, L. et al. The notch pathway promotes osteosarcoma progression through activation of ephrin reverse signaling. Mol. Cancer Res. 17, 2383–2394 (2019).
Krusche, B. et al. EphrinB2 drives perivascular invasion and proliferation of glioblastoma stem-like cells. eLife 5, e14845 (2016). This study shows that EFNB2 upregulated in glioblastoma CSCs constitutively activates EPHB receptors through homotypic cell–cell interactions, rendering the CSCs insensitive to endothelial EFNB2 to enable perivascular invasion. EFNB2 expressed in CSCs also promotes tumorigenesis by increasing CSC proliferation via anchorage-independent cytokinesis.
Xu, C. et al. Adaptive activation of EFNB2/EPHB4 axis promotes post-metastatic growth of colorectal cancer liver metastases by LDLR-mediated cholesterol uptake. Oncogene 42, 99–112 (2023).
Sagar, V. et al. EPHB4 inhibition activates ER stress to promote immunogenic cell death of prostate cancer cells. Cell Death Dis. 10, 801 (2019).
Verschueren, E. et al. The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome. Cell 182, 329–344.e19 (2020). This study describes a systematic screen of single transmembrane proteins interacting across cell–cell contacts with 445 receptors of interest in oncology and identifies several Eph receptor and ephrin unconventional ligands, which were confirmed in orthogonal assays.
Ghoshdastider, U. et al. Pan-cancer analysis of ligand-receptor cross-talk in the tumor microenvironment. Cancer Res. 81, 1802–1812 (2021).
Adams, R. H. & Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2, a001875 (2010).
Kirschmann, D. A., Seftor, E. A., Hardy, K. M., Seftor, R. E. & Hendrix, M. J. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin. Cancer Res. 18, 2726–2732 (2012).
Wu, N. et al. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PLoS ONE 6, e16264 (2011).
Vail, M. E. et al. Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment. Cancer Res. 74, 4470–4481 (2014).
Lennon, F. E. et al. Transactivation of the receptor-tyrosine kinase ephrin receptor A2 is required for the low molecular weight hyaluronan-mediated angiogenesis that is implicated in tumor progression. J. Biol. Chem. 289, 24043–24058 (2014).
Chen, D. et al. Angiogenesis depends upon EPHB4-mediated export of collagen IV from vascular endothelial cells. JCI Insight 7, e156928 (2022). This study discovers a novel mechanism that regulates both normal angiogenesis and tumour angiogenesis and relies on EPHB4 receptor canonical signalling and its downstream target, RAS GTPase-activating protein 1 (RASA1), to promote the export of collagen IV from the endoplasmic reticulum so it can be released by vascular endothelial cells.
Sainz-Jaspeado, M. et al. EphA2-induced angiogenesis in Ewing sarcoma cells works through bFGF production and is dependent on caveolin-1. PLoS ONE 8, e71449 (2013).
Bhatia, S. et al. EphB4 and ephrinB2 act in opposition in the head and neck tumor microenvironment. Nat. Commun. 13, 3535 (2022).
Sawamiphak, S. et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465, 487–491 (2010).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Nakayama, M. et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 15, 249–260 (2013).
Nakayama, A. et al. Ephrin-B2 controls PDGFRβ internalization and signaling. Genes Dev. 27, 2576–2589 (2013).
Rupp, T. et al. Tenascin-C orchestrates glioblastoma angiogenesis by modulation of pro- and anti-angiogenic signaling. Cell Rep. 17, 2607–2619 (2016).
Han, B. et al. Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through ephrin A1-EPHA2 forward signaling. Theranostics 12, 4127–4146 (2022).
Sato, S. et al. EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI Insight 4, e132447 (2019). This study shows that EPHB2 released in extracellular vesicles from head and neck cancer cells promotes angiogenesis by activating ephrin-B–STAT3 reverse signaling in endothelial cells, even if distant from the cancer cells.
Shiuan, E. & Chen, J. Eph receptor tyrosine kinases in tumor immunity. Cancer Res. 76, 6452–6457 (2016).
Janes, P. W., Vail, M. E., Ernst, M. & Scott, A. M. Eph receptors in the immunosuppressive tumor microenvironment. Cancer Res. 81, 801–805 (2021).
Huang, W. et al. EPHA5 mutation predicts the durable clinical benefit of immune checkpoint inhibitors in patients with lung adenocarcinoma. Cancer Gene Ther. 28, 864–874 (2021).
Funk, S. D. et al. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 686–695 (2012).
Zhang, A. et al. EphA2 phosphorylates NLRP3 and inhibits inflammasomes in airway epithelial cells. EMBO Rep. 21, e49666 (2020).
Markosyan, N. et al. Tumor cell-intrinsic EPHA2 suppresses anti-tumor immunity by regulating PTGS2 (COX-2). J. Clin. Invest. 130, 3594–3609 (2019). This study reveals that EPHA2 is a driver of immunosuppression in pancreatic cancer through an EPHA2–TGFβ–COX2 pathway that inhibits T cell infiltration, rendering tumours resistant to immunotherapy.
Yang, W. H. et al. Juxtacrine signaling inhibits antitumor immunity by upregulating PD-L1 expression. Cancer Res. 78, 3761–3768 (2018).
Pfaff, D. et al. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J. Cell Sci. 121, 3842–3850 (2008).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117 (2014).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Hong, H. N. et al. Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner. J. Cancer Res. Clin. Oncol. 144, 1649–1663 (2018).
Wu, X. et al. Quantitative phosphoproteomic analysis reveals reciprocal activation of receptor tyrosine kinases between cancer epithelial cells and stromal fibroblasts. Clin. Proteom. 15, 21 (2018).
Curtis, M. et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 29, 141–155.e9 (2019).
Takasugi, M. et al. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 8, 15729 (2017).
Nakajima, K. et al. Neoadjuvant therapy alters the collagen architecture of pancreatic cancer tissue via ephrin-A5. Br. J. Cancer 126, 628–639 (2022).
Kakarla, M. et al. Ephrin B activate Src family kinases in fibroblasts inducing stromal remodeling in prostate cancer. Cancers 14, 2336 (2022).
Astin, J. W. et al. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 12, 1194–1204 (2010).
Lagares, D. et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat. Med. 23, 1405–1415 (2017).
Lennon, S. et al. Pancreatic tumor microenvironment modulation by EphB4-ephrinB2 inhibition and radiation combination. Clin. Cancer Res. 25, 3352–3365 (2019).
Mueller, A. C. et al. Induction of ADAM10 by radiation therapy drives fibrosis, resistance, and epithelial-to-mesenchymal transition in pancreatic cancer. Cancer Res. 81, 3255–3269 (2021).
Kwak, H. et al. Sinusoidal ephrin receptor EPHB4 controls hematopoietic progenitor cell mobilization from bone marrow. J. Clin. Invest. 126, 4554–4568 (2016).
Magnon, C. & Hondermarck, H. The neural addiction of cancer. Nat. Rev. Cancer 23, 317–334 (2023).
Madeo, M. et al. Cancer exosomes induce tumor innervation. Nat. Commun. 9, 4284 (2018).
Furuhashi, S. et al. Ephrin receptor A4 expression enhances migration, invasion and neurotropism in pancreatic ductal adenocarcinoma cells. Anticancer Res. 41, 1733–1744 (2021).
Hu, M., Carles-Kinch, K. L., Zelinski, D. P. & Kinch, M. S. EphA2 induction of fibronectin creates a permissive microenvironment for malignant cells. Mol. Cancer Res. 2, 533–540 (2004).
Finney, A. C. et al. EphA2 signaling within integrin adhesions regulates fibrillar adhesion elongation and fibronectin deposition. Matrix Biol. 103–104, 1–21 (2021).
Fattet, L. et al. Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev. Cell 54, 302–316.e7 (2020). This study demonstrates that ECM stiffness activates a mechanotransduction pathway in breast cancer that relies on EPHA2 non-canonical signalling to drive EMT, invasion and metastasis.
Duquet, A. et al. A novel genome-wide in vivo screen for metastatic suppressors in human colon cancer identifies the positive WNT-TCF pathway modulators TMED3 and SOX12. EMBO Mol. Med. 6, 882–901 (2014).
Birkbak, N. J. & McGranahan, N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell 37, 8–19 (2020).
Vecchi, M. et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene 27, 2148–2158 (2008).
Depner, C. et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat. Commun. 7, 12329 (2016).
Wolff, D. W. et al. Phosphorylation of guanosine monophosphate reductase triggers a GTP-dependent switch from pro- to anti-oncogenic function of EPHA4. Cell Chem. Biol. 29, 970–984.e6 (2022).
Chandrasekera, P. et al. Metalloprotease ADAM9 cleaves ephrin-B ligands and differentially regulates Wnt and mTOR signaling downstream of Akt kinase in colorectal cancer cells. J. Biol. Chem. 298, 102225 (2022).
Kawahara, Y. et al. Ligand-dependent EphB4 activation serves as an anchoring signal in glioma cells. Cancer Lett. 449, 56–65 (2019).
Broggini, T. et al. Ephrin-B2-EphB4 communication mediates tumor-endothelial cell interactions during hematogenous spread to spinal bone in a melanoma metastasis model. Oncogene 39, 7063–7075 (2020).
Yu, J. et al. The EPHB6 receptor tyrosine kinase is a metastasis suppressor that is frequently silenced by promoter DNA hypermethylation in non-small cell lung cancer. Clin. Cancer Res. 16, 2275–2283 (2010).
Bulk, E. et al. Mutations of the EPHB6 receptor tyrosine kinase induce a pro-metastatic phenotype in non-small cell lung cancer. PLoS ONE 7, e44591 (2012).
Chen, J. et al. Androgen-deprivation therapy with enzalutamide enhances prostate cancer metastasis via decreasing the EPHB6 suppressor expression. Cancer Lett. 408, 155–163 (2017).
Mateo-Lozano, S. et al. Loss of the EPH receptor B6 contributes to colorectal cancer metastasis. Sci. Rep. 7, 43702 (2017).
Truitt, L., Freywald, T., DeCoteau, J., Sharfe, N. & Freywald, A. The EphB6 receptor cooperates with c-Cbl to regulate the behavior of breast cancer cells. Cancer Res. 70, 1141–1153 (2010).
Locard-Paulet, M. et al. Phosphoproteomic analysis of interacting tumor and endothelial cells identifies regulatory mechanisms of transendothelial migration. Sci. Signal. 9, ra15 (2016). This phosphoproteomic analysis reveals that interaction of breast cancer cells with endothelial cells increases EPHA2 Y772 phosphorylation in the cancer cells, owing to interaction with endothelial EFNA1. This phosphorylation, which inhibits transendothelial migration in vitro and lung colonization in mice, is more transient in breast cancer cells that metastasize, owing to rapid and selective dephosphorylation.
Shiuan, E. et al. Host deficiency in ephrin-A1 inhibits breast cancer metastasis. F1000Res 9, 217 (2020).
Beauchamp, A. et al. EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol. Cell Biol. 32, 3253–3264 (2012).
Ieguchi, K. et al. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene 33, 2179–2190 (2014).
Liu, X. et al. Exosomal EphA2 promotes tumor metastasis of triple-negative breast cancer by damaging endothelial barrier. Clin. Exp. Metastasis 40, 105–116 (2022).
Aaron, P. A., Jamklang, M., Uhrig, J. P. & Gelli, A. The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor. Cell Microbiol. https://doi.org/10.1111/cmi.12811 (2018).
Darling, T. K. et al. EphA2 contributes to disruption of the blood-brain barrier in cerebral malaria. PLoS Pathog. 16, e1008261 (2020).
Heroult, M. et al. EphB4 promotes site-specific metastatic tumor cell dissemination by interacting with endothelial cell-expressed ephrinB2. Mol. Cancer Res. 8, 1297–1309 (2010).
Batson, J., Maccarthy-Morrogh, L., Archer, A., Tanton, H. & Nobes, C. D. EphA receptors regulate prostate cancer cell dissemination through Vav2-RhoA mediated cell-cell repulsion. Biol. Open 3, 453–462 (2014).
Lee, P. C. et al. C1GALT1 is associated with poor survival and promotes soluble ephrin A1-mediated cell migration through activation of EPHA2 in gastric cancer. Oncogene 39, 2724–2740 (2020).
Keskin, T. et al. A live single-cell reporter assay links intratumor heterogeneity to metastatic proclivity in Ewing sarcoma. Sci. Adv. 7, eabf9394 (2021).
Nakada, M. et al. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 64, 3179–3185 (2004).
Li, J. J. et al. EphB3 stimulates cell migration and metastasis in a kinase-dependent manner through Vav2-Rho GTPase axis in papillary thyroid cancer. J. Biol. Chem. 292, 1112–1121 (2017).
Miao, H. et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene 34, 558–567 (2015).
Koshikawa, N. et al. Proteolysis of EphA2 converts it from a tumor suppressor to an oncoprotein. Cancer Res. 75, 3327–3339 (2015).
Sachdeva, A. et al. Non-canonical EphA2 activation underpins PTEN-mediated metastatic migration and poor clinical outcome in prostate cancer. Br. J. Cancer 127, 1254–1262 (2022).
Garcia-Monclus, S. et al. EphA2 receptor is a key player in the metastatic onset of Ewing sarcoma. Int. J. Cancer 143, 1188–1201 (2018).
Asakura, N. et al. Expression of cancer stem cell markers EpCAM and CD90 is correlated with anti- and pro-oncogenic EphA2 signaling in hepatocellular carcinoma. Int. J. Mol. Sci. 22, 8652 (2021).
Gundry, C. et al. Phosphorylation of Rab-coupling protein by LMTK3 controls Rab14-dependent EphA2 trafficking to promote cell:cell repulsion. Nat. Commun. 8, 14646 (2017).
Marco, S. et al. Nuclear-capture of endosomes depletes nuclear G-actin to promote SRF/MRTF activation and cancer cell invasion. Nat. Commun. 12, 6829 (2021). This study shows that activation of the MET hepatocyte growth factor receptor promotes EPHA2 S897 phosphorylation and endocytosis, leading to EPHA2-mediated capture of endosomes on the outer surface of the nucleus. There, an EPHA2–RHOG–cofilin signalling axis promotes actin polymerization that inhibits nuclear import of G-actin, which drives transcription of genes encoding molecules that promote cancer cell scattering and invasion.
Feng, J. et al. ANXA1 binds and stabilizes EphA2 to promote nasopharyngeal carcinoma growth and metastasis. Cancer Res. 80, 4386–4398 (2020).
Mao, L. et al. EphA2-YES1-ANXA2 pathway promotes gastric cancer progression and metastasis. Oncogene 40, 3610–3623 (2021).
Huang, J. et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene 33, 2737–2747 (2014).
Hiramoto-Yamaki, N. et al. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 190, 461–477 (2010).
Zhang, C. et al. Noncanonical EphA2 signaling is a driver of tumor-endothelial cell interactions and metastatic dissemination in BRAF inhibitor-resistant melanoma. J. Invest. Dermatol. 141, 840–851.e4 (2021).
Sheng, Y. et al. Mutated EPHA2 is a target for combating lymphatic metastasis in intrahepatic cholangiocarcinoma. Int. J. Cancer 144, 2440–2452 (2019).
Wang, L. et al. Ligand-independent EphB1 signaling mediates TGF-β-activated CDH2 and promotes lung cancer cell invasion and migration. J. Cancer 11, 4123–4131 (2020).
Cho, H. J. et al. EphrinB1 promotes cancer cell migration and invasion through the interaction with RhoGDI1. Oncogene 37, 861–872 (2018).
Tanaka, M., Sasaki, K., Kamata, R. & Sakai, R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 120, 2179–2189 (2007).
Sasabe, E. et al. Ephrin-B2 reverse signaling regulates progression and lymph node metastasis of oral squamous cell carcinoma. PLoS ONE 12, e0188965 (2017).
Nakada, M., Drake, K. L., Nakada, S., Niska, J. A. & Berens, M. E. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 66, 8492–8500 (2006).
Nakada, M. et al. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int. J. Cancer 126, 1155–1165 (2010).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Wang, L. et al. Cell-cell contact-driven EphB1 cis- and trans-signalings regulate cancer stem cells enrichment after chemotherapy. Cell Death Dis. 13, 980 (2022).
Moyano-Galceran, L. et al. Adaptive RSK-EphA2-GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer. EMBO Mol. Med. 12, e11177 (2020).
Zhao, P. et al. Targeting the KLF5-EphA2 axis can restrain cancer stemness and overcome chemoresistance in basal-like breast cancer. Int. J. Biol. Sci. 19, 1861–1874 (2023).
Wen, Q. et al. EphA2 affects the sensitivity of oxaliplatin by inducing EMT in oxaliplatin-resistant gastric cancer cells. Oncotarget 8, 47998–48011 (2017).
Huang, C. et al. EphA2-to-YAP pathway drives gastric cancer growth and therapy resistance. Int. J. Cancer 146, 1937–1949 (2020).
Fan, J. et al. Chemoresistance transmission via exosome-mediated EphA2 transfer in pancreatic cancer. Theranostics 8, 5986–5994 (2018).
Yoon, S. et al. EPHB6 mutation induces cell adhesion-mediated paclitaxel resistance via EPHA2 and CDH11 expression. Exp. Mol. Med. 51, 1–12 (2019).
Owusu, M. et al. Mapping the human kinome in response to DNA damage. Cell Rep. 26, 555–563.e6 (2019).
Alam, S. K. et al. DNA damage-induced ephrin-B2 reverse signaling promotes chemoresistance and drives EMT in colorectal carcinoma harboring mutant p53. Cell Death Differ. 23, 707–722 (2016).
Peng, J. et al. EPHA3 regulates the multidrug resistance of small cell lung cancer via the PI3K/BMX/STAT3 signaling pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37, 11959–11971 (2016).
Kampen, K. R. et al. EphB1 suppression in acute myelogenous leukemia: regulating the DNA damage control system. Mol. Cancer Res. 13, 982–992 (2015).
Toosi, B. M. et al. EPHB6 augments both development and drug sensitivity of triple-negative breast cancer tumours. Oncogene 37, 4073–4093 (2018).
El Zawily, A. et al. The EphB6 receptor is overexpressed in pediatric T cell acute lymphoblastic leukemia and increases its sensitivity to doxorubicin treatment. Sci. Rep. 7, 14767 (2017).
Gabriel, N. et al. Loss of H3K27 trimethylation promotes radiotherapy resistance in medulloblastoma and induces an actionable vulnerability to BET inhibition. Cancer Res. 82, 2019–2030 (2022).
Bhatia, S. et al. Knockdown of EphB1 receptor decreases medulloblastoma cell growth and migration and increases cellular radiosensitization. Oncotarget 6, 8929–8946 (2015).
Stahl, S. et al. Inhibition of ephrin B3-mediated survival signaling contributes to increased cell death response of non-small cell lung carcinoma cells after combined treatment with ionizing radiation and PKC 412. Cell Death Dis. 4, e454 (2013).
Staquicini, F. I. et al. Receptor tyrosine kinase EphA5 is a functional molecular target in human lung cancer. J. Biol. Chem. 290, 7345–7359 (2015).
Kaminskyy, V. O. et al. EPHA2 interacts with DNA-PK(cs) in cell nucleus and controls ionizing radiation responses in non-small cell lung cancer cells. Cancers 13, 1010 (2021).
Martini, G. et al. EPHA2 is a predictive biomarker of resistance and a potential therapeutic target for improving antiepidermal growth factor receptor therapy in colorectal cancer. Mol. Cancer Ther. 18, 845–855 (2019).
Kuo, M. T. et al. Collaboration between RSK-EphA2 and Gas6-Axl RTK signaling in arginine starvation response that confers resistance to EGFR inhibitors. Transl. Oncol. 13, 355–364 (2020).
Zhuang, G. et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 70, 299–308 (2010).
Gai, Q. J. et al. EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma. Signal Transduct. Target. Ther. 7, 33 (2022).
Ruan, H., Li, S., Bao, L. & Zhang, X. Enhanced YB1/EphA2 axis signaling promotes acquired resistance to sunitinib and metastatic potential in renal cell carcinoma. Oncogene 39, 6113–6128 (2020).
Chen, C. T. et al. Quantitative phosphoproteomic analysis identifies the potential therapeutic target EphA2 for overcoming sorafenib resistance in hepatocellular carcinoma cells. Exp. Mol. Med. 52, 497–513 (2020).
Leung, H. W. et al. EPHB2 activates β-catenin to enhance cancer stem cell properties and drive sorafenib resistance in hepatocellular carcinoma. Cancer Res. 81, 3229–3240 (2021).
Li, C. et al. Inhibition of the erythropoietin-producing receptor EPHB4 antagonizes androgen receptor overexpression and reduces enzalutamide resistance. J. Biol. Chem. 295, 5470–5483 (2020).
Manso, L. et al. Analysis of paired primary-metastatic hormone-receptor positive breast tumors (HRPBC) uncovers potential novel drivers of hormonal resistance. PLoS ONE 11, e0155840 (2016).
Bilusic, M. et al. Molecular profiling of exceptional responders to cancer therapy. Oncologist 26, 186–195 (2021).
Noberini, R., Lamberto, I. & Pasquale, E. B. Targeting Eph receptors with peptides and small molecules: progress and challenges. Semin. Cell Dev. Biol. 23, 51–57 (2012).
Choi, Y. et al. Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 19, 4467–4470 (2009).
Martiny-Baron, G. et al. The small molecule specific EphB4 kinase inhibitor NVP-BHG712 inhibits VEGF driven angiogenesis. Angiogenesis 13, 259–267 (2010).
Davis, M. I. et al. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1046–1051 (2011).
Unzue, A. et al. Three stories on Eph kinase inhibitors: from in silico discovery to in vivo validation. Eur. J. Med. Chem. 112, 347–366 (2016).
Le Large, T. Y. et al. Microdissected pancreatic cancer proteomes reveal tumor heterogeneity and therapeutic targets. JCI Insight 5, e138290 (2020).
Wang, H. et al. Targeting EphA2 suppresses hepatocellular carcinoma initiation and progression by dual inhibition of JAK1/STAT3 and AKT signaling. Cell Rep. 34, 108765 (2021).
Yan, H. et al. Regorafenib inhibits EphA2 phosphorylation and leads to liver damage via the ERK/MDM2/p53 axis. Nat. Commun. 14, 2756 (2023). This study demonstrates that off-target inhibition of EPHA2 non-canonical signaling in liver cells is responsible for the hepatotoxicity of the multi-targeted kinase inhibitor regorafenib, which is an FDA-approved drug used to treat several cancer types. Thus, EPHA2 S897 phosphorylation can confer stress resilience and prevent cytotoxicity not only in cancer cells but also in non-cancer cells.
Troster, A. et al. NVP-BHG712: effects of regioisomers on the affinity and selectivity toward the EPHrin family. ChemMedChem 13, 1629–1633 (2018).
Zhou, Y. et al. Cellular stress induces non-canonical activation of the receptor tyrosine kinase EphA2 through the p38-MK2-RSK signaling pathway. J. Biol. Chem. 299, 104699 (2023). This study describes how different types of cellular stress upregulate EPHA2 S897 phosphorylation through p38 MAPK–MK2–RSK signalling. This pathway can mediate glioblastoma cell migration induced by temozolomide chemotherapy. Furthermore, it could operate not only in cancer cells but also in the TME.
Heinzlmeir, S. et al. Chemical proteomics and structural biology define EPHA2 inhibition by clinical kinase drugs. ACS Chem. Biol. 11, 3400–3411 (2016).
Zhang, C. et al. Structure-guided inhibitor design expands the scope of analog-sensitive kinase technology. ACS Chem. Biol. 8, 1931–1938 (2013).
Heinzlmeir, S. et al. Chemoproteomics-aided medicinal chemistry for the discovery of EPHA2 inhibitors. ChemMedChem 12, 999–1011 (2017).
Kung, A. et al. Development of specific, irreversible inhibitors for a receptor tyrosine kinase EphB3. J. Am. Chem. Soc. 138, 10554–10560 (2016).
Modukuri, R. K. et al. Discovery of highly potent and BMPR2-selective kinase inhibitors using DNA-encoded chemical library screening. J. Med. Chem. 66, 2143–2160 (2023).
Pan, Y. & Mader, M. M. Principles of kinase allosteric inhibition and pocket validation. J. Med. Chem. 65, 5288–5299 (2022).
Lodola, A., Giorgio, C., Incerti, M., Zanotti, I. & Tognolini, M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 142, 152–162 (2017).
Chen, Y., Zhang, H. & Zhang, Y. Targeting receptor tyrosine kinase EphB4 in cancer therapy. Semin. Cancer Biol. 56, 37–46 (2019).
Jackson, D. et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 68, 9367–9374 (2008).
Jacobson, O. et al. PET-guided evaluation and optimization of internalized antibody-drug conjugates targeting erythropoietin-producing hepatoma A2 receptor. J. Nucl. Med. 58, 1838–1844 (2017).
Kamoun, W. S. et al. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat. Biomed. Eng. 3, 264–280 (2019).
Hasegawa, J. et al. Novel anti-EPHA2 antibody, DS-8895a for cancer treatment. Cancer Biol. Ther. 17, 1158–1167 (2016).
Burvenich, I. J. et al. Molecular imaging and quantitation of EphA2 expression in xenograft models with 89Zr-DS-8895a. J. Nucl. Med. 57, 974–980 (2016).
Annunziata, C. M. et al. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest. N. Drugs 31, 77–84 (2013).
da Costa, R. et al. Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br. J. Pharmacol. 177, 3127–3146 (2020).
Dolgin, E. Bicyclic peptide makes targeting EphA2 possible. Cancer Discov. 11, 2951–2952 (2021).
Shitara, K. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: a first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 7, 219 (2019).
Day, B. W. et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell 23, 238–248 (2013).
Offenhauser, C. et al. EphA3 pay-loaded antibody therapeutics for the treatment of glioblastoma. Cancers 10, 519 (2018).
Nagano, K. et al. Ephrin receptor A10 is a promising drug target potentially useful for breast cancers including triple negative breast cancers. J. Control. Release 189, 72–79 (2014).
Cha, J. H. et al. Ephrin receptor A10 monoclonal antibodies and the derived chimeric antigen receptor T cells exert an antitumor response in mouse models of triple-negative breast cancer. J. Biol. Chem. 298, 101817 (2022).
Zang, X. et al. Anti-EphA10 antibody-conjugated pH-sensitive liposomes for specific intracellular delivery of siRNA. Int. J. Nanomed. 11, 3951–3967 (2016).
Krasnoperov, V. et al. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am. J. Pathol. 176, 2029–2038 (2010).
Liu, S. et al. PET imaging of colorectal and breast cancer by targeting EphB4 receptor with 64Cu-labeled hAb47 and hAb131 antibodies. J. Nucl. Med. 54, 1094–1100 (2013).
Stephenson, S. A. et al. Anti-tumour effects of antibodies targeting the extracellular cysteine-rich region of the receptor tyrosine kinase EphB4. Oncotarget 6, 7554–7569 (2015).
Qazi, M. A. et al. Cotargeting ephrin receptor tyrosine kinases A2 and A3 in cancer stem cells reduces growth of recurrent glioblastoma. Cancer Res. 78, 5023–5037 (2018).
El Zawily, A. et al. A multipronged unbiased strategy guides the development of an anti-EGFR/EPHA2-bispecific antibody for combination cancer therapy. Clin. Cancer Res. 29, 2686–2701 (2023).
Dimasi, N. et al. Development of a trispecific antibody designed to simultaneously and efficiently target three different antigens on tumor cells. Mol. Pharm. 12, 3490–3501 (2015).
Taki, S. et al. A novel bispecific antibody against human CD3 and ephrin receptor A10 for breast cancer therapy. PLoS ONE 10, e0144712 (2015).
Hammond, S. A. et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-bispecific single-chain antibody construct. Cancer Res. 67, 3927–3935 (2007).
Ferluga, S., Tome, C. M., Herpai, D. M., D’Agostino, R. & Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 7, 59860–59876 (2016).
Sharma, P. & Debinski, W. Receptor-targeted glial brain tumor therapies. Int. J. Mol. Sci. 19, 3326 (2018).
Riedl, S. J. & Pasquale, E. B. Targeting the Eph system with peptides and peptide conjugates. Curr. Drug Targets 16, 1031–1047 (2015).
Olson, E. J. et al. Modifications of a nanomolar cyclic peptide antagonist for the EphA4 receptor to achieve high plasma stability. ACS Med. Chem. Lett. 7, 841–846 (2016).
Gomez-Soler, M. et al. Engineering nanomolar peptide ligands that differentially modulate EphA2 receptor signaling. J. Biol. Chem. 294, 8791–8805 (2019).
Gambini, L. et al. Structure-based design of novel EphA2 agonistic agents with nanomolar affinity in vitro and in cell. ACS Chem. Biol. 13, 2633–2644 (2018).
Gomez-Soler, M. et al. Ligands with different dimeric configurations potently activate the EphA2 receptor and reveal its potential for biased signaling. iScience 25, 103870 (2022).
Baggio, C., Udompholkul, P., Gambini, L. & Pellecchia, M. Targefrin: a potent agent targeting the ligand binding domain of EphA2. J. Med. Chem. 24, 15443–15456 (2022).
Fan, T. et al. A synthetic bivalent peptide ligand of EphB4 with potent agonistic activity. Eur. J. Med. Chem. 244, 114804 (2022).
Noberini, R. et al. PEGylation potentiates the effectiveness of an antagonistic peptide that targets the EphB4 receptor with nanomolar affinity. PLoS ONE 6, e28611 (2011).
Wu, B. et al. Design and characterization of novel EphA2 agonists for targeted delivery of chemotherapy to cancer cells. Chem. Biol. 22, 876–887 (2015).
Huang, M. et al. Dual-modality micro-positron emission tomography/computed tomography and near-infrared fluorescence imaging of EphB4 in orthotopic glioblastoma xenograft models. Mol. Imaging Biol. 16, 74–84 (2014).
Guo, Z. et al. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int. J. Pharm. 493, 380–389 (2015).
Zhang, R. et al. Peptide-conjugated polymeric micellar nanoparticles for dual SPECT and optical imaging of EphB4 receptors in prostate cancer xenografts. Biomaterials 32, 5872–5879 (2011).
Bennett, G. et al. MMAE delivery using the bicycle toxin conjugate BT5528. Mol. Cancer Ther. 19, 1385–1394 (2020). This study describes the development and characterization of BT5528, a bicyclic peptide–toxin conjugate. The rapid uptake and prolonged persistence of BT5528 in tumours, despite its fast renal elimination in animal models, reduces systemic toxicity compared with an antibody–drug conjugate.
Alves, D. S. et al. A novel pH-dependent membrane peptide that binds to EphA2 and inhibits cell migration. eLife 7, e36645 (2018).
Petty, A. et al. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS ONE 7, e42120 (2012).
Guidetti, L. et al. Protein-protein interaction inhibitors targeting the Eph-ephrin system with a focus on amino acid conjugates of bile acids. Pharmaceuticals 15, 137 (2022).
Castelli, R. et al. Delta-cholenoyl-amino acids as selective and orally available antagonists of the Eph-ephrin system. Eur. J. Med. Chem. 103, 312–324 (2015).
Rusnati, M. et al. Cholenic acid derivative UniPR1331 impairs tumor angiogenesis via blockade of VEGF/VEGFR2 in addition to Eph/ephrin. Cancer Gene Ther. 29, 908–917 (2022).
Affinito, A. et al. Targeting ephrin receptor tyrosine kinase A2 with a selective aptamer for glioblastoma stem cells. Mol. Ther. Nucleic Acids 20, 176–185 (2020).
Li, W. et al. Identification of the target protein of the metastatic colorectal cancer-specific aptamer W3 as a biomarker by aptamer-based target cells sorting and functional characterization. Biosens. Bioelectron. 213, 114451 (2022).
Damelin, M. et al. Anti-EFNA4 calicheamicin conjugates effectively target triple-negative breast and ovarian tumor-initiating cells to result in sustained tumor regressions. Clin. Cancer Res. 21, 4165–4173 (2015).
Abengozar, M. A. et al. Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 119, 4565–4576 (2012).
Kertesz, N. et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-ephrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood 107, 2330–2338 (2006).
Liu, R. et al. EphB4 as a therapeutic target in mesothelioma. BMC Cancer 13, 269 (2013).
Ferguson, B. D. et al. The EphB4 receptor tyrosine kinase promotes lung cancer growth: a potential novel therapeutic target. PLoS ONE 8, e67668 (2013).
VanderWeele, D. J. et al. A phase II study of sEphB4-HSA in metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 20, 575–580 (2022).
Sadeghi, S. et al. EphrinB2 inhibition and pembrolizumab in metastatic urothelial carcinoma. J. Clin. Oncol. 41, 640–650 (2023).
Crnkovic, S. et al. Divergent roles of ephrin-B2/EphB4 guidance system in pulmonary hypertension. Hypertension 80, e17–e28 (2023).
Wang, Y. et al. EPHB4 protein expression in vascular smooth muscle cells regulates their contractility, and EPHB4 deletion leads to hypotension in mice. J. Biol. Chem. 290, 14235–14244 (2015).
Wang, Y. et al. Reduced blood pressure after smooth muscle EFNB2 deletion and the potential association of EFNB2 mutation with human hypertension risk. Eur. J. Hum. Genet. 24, 1817–1825 (2016).
Scalia, P., Pandini, G., Carnevale, V., Giordano, A. & Williams, S. J. Identification of a novel EphB4 phosphodegron regulated by the autocrine IGFII/IR(A) axis in malignant mesothelioma. Oncogene 38, 5987–6001 (2019).
Si, R. et al. Discovery of novel protein degraders based on bioorthogonal reaction-driven intracellular self-assembly strategy. Bioorg. Chem. 135, 106497 (2023).
Wagner, M. J. et al. Preclinical mammalian safety studies of EPHARNA (DOPC nanoliposomal EphA2-targeted siRNA). Mol. Cancer Ther. 16, 1114–1123 (2017).
Landen, C. N. Jr. et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 65, 6910–6918 (2005).
Tanaka, T. et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 70, 3687–3696 (2010).
Nishimura, M. et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 3, 1302–1315 (2013).
Shen, H. et al. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin. Cancer Res. 19, 1806–1815 (2013).
Zhang, T. et al. Inhibition of HDACs-EphA2 signaling axis with WW437 demonstrates promising preclinical antitumor activity in breast cancer. EBioMedicine 31, 276–286 (2018).
Hatano, M. et al. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J. Transl. Med. 2, 40 (2004).
Yamaguchi, S. et al. Immunotherapy of murine colon cancer using receptor tyrosine kinase EphA2-derived peptide-pulsed dendritic cell vaccines. Cancer 110, 1469–1477 (2007).
Yamaguchi, S. et al. Dendritic cell-based vaccines suppress metastatic liver tumor via activation of local innate and acquired immunity. Cancer Immunol. Immunother. 57, 1861–1869 (2008).
Chen, H. et al. A novel vaccine containing EphA2 epitope and LIGHT plasmid induces robust cellular immunity against glioma U251 cells. Cell Immunol. 272, 102–106 (2011).
Qin, V. M., D’Souza, C., Neeson, P. J. & Zhu, J. J. Chimeric antigen receptor beyond CAR-T cells. Cancers 13, 404 (2021).
Shum, T. et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 7, 1238–1247 (2017).
Yi, Z., Prinzing, B. L., Cao, F., Gottschalk, S. & Krenciute, G. Optimizing EphA2-CAR T cells for the adoptive immunotherapy of glioma. Mol. Ther. Methods Clin. Dev. 9, 70–80 (2018).
Donovan, L. K. et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 26, 720–731 (2020).
Hsu, K. et al. Chimeric antigen receptor-modified T cells targeting EphA2 for the immunotherapy of paediatric bone tumours. Cancer Gene Ther. 28, 321–334 (2021).
An, Z. et al. Antitumor activity of the third generation EphA2 CAR-T cells against glioblastoma is associated with interferon gamma induced PD-L1. Oncoimmunology 10, 1960728 (2021).
Kubo, H. et al. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Mol. Ther. Oncolytics 20, 646–658 (2021).
Upadhyaya, P. et al. Anticancer immunity induced by a synthetic tumor-targeted CD137 agonist. J. Immunother. Cancer 9, e001762 (2021).
Bhatia, S. et al. Inhibition of EphB4-ephrin-B2 signaling reprograms the tumor immune microenvironment in head and neck cancers. Cancer Res. 79, 2722–2735 (2019).
Torres-Adorno, A. M. et al. Eicosapentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux. Oncogene 38, 2135–2150 (2019).
Bhatia, S. et al. Enhancing radiosensitization in EphB4 receptor-expressing head and neck squamous cell carcinomas. Sci. Rep. 6, 38792 (2016).
Bhatia, S. et al. Inhibition of EphB4-ephrin-B2 signaling enhances response to cetuximab-radiation therapy in head and neck cancers. Clin. Cancer Res. 24, 4539–4550 (2018).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, ebaf1230 (2021).
Giladi, A. Digging for treasures in the tumour interactome. Nat. Rev. Cancer 22, 434–435 (2022).
Mason, E. O. et al. Structure of the EphB6 receptor ectodomain. PLoS ONE 16, e0247335 (2021).
Buraschi, S. et al. Progranulin/EphA2 axis: a novel oncogenic mechanism in bladder cancer. Matrix Biol. 93, 10–24 (2020).
Lee, H. H. et al. Human ribonuclease 1 serves as a secretory ligand of ephrin A4 receptor and induces breast tumor initiation. Nat. Commun. 12, 2788 (2021).
Pradeep, S. et al. Erythropoietin stimulates tumor growth via EphB4. Cancer Cell 28, 610–622 (2015).
Fukai, J. et al. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol. Cancer Ther. 7, 2768–2778 (2008).
Kennedy, S. P. et al. Targeting promiscuous heterodimerization overcomes innate resistance to ERBB2 dimerization inhibitors in breast cancer. Breast Cancer Res. 21, 43 (2019).
Jorgensen, C. et al. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science 326, 1502–1509 (2009).
Banerjee, S. L. et al. EPH receptor tyrosine kinases phosphorylate the PAR-3 scaffold protein to modulate downstream signaling networks. Cell Rep. 40, 111031 (2022).
Moore, L. et al. The mutational landscape of human somatic and germline cells. Nature 597, 381–386 (2021).
Hutchens, T. & Piston, D. W. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes 64, 3839–3851 (2015).
Volta, F. et al. Glucose homeostasis is regulated by pancreatic β-cell cilia via endosomal EphA-processing. Nat. Commun. 10, 5686 (2019).
Luxan, G. et al. Endothelial EphB4 maintains vascular integrity and transport function in adult heart. eLife 8, e45863 (2019).
Cayuso, J. et al. EphrinB1/EphB3b coordinate bidirectional epithelial-mesenchymal interactions controlling liver morphogenesis and laterality. Dev. Cell 39, 316–328 (2016).
Valenzuela, J. I. & Perez, F. Localized intercellular transfer of ephrin-as by trans-endocytosis enables long-term signaling. Dev. Cell 52, 104–117 e105 (2020).
Pasquale, E. B. Exosomes expand the sphere of influence of Eph receptors and ephrins. J. Cell Biol. 214, 5–7 (2016).
Goutas, D., Pergaris, A., Goutas, N. & Theocharis, S. Utilizing exosomal-EPHs/ephrins as biomarkers and as a potential platform for targeted delivery of therapeutic exosomes. Int. J. Mol. Sci. 23, 3551 (2022).
Boissier, P., Chen, J. & Huynh-Do, U. EphA2 signaling following endocytosis: role of Tiam1. Traffic 14, 1255–1271 (2013).
Mertens-Walker, I. et al. EphB4 localises to the nucleus of prostate cancer cells. Exp. Cell Res. 333, 105–115 (2015).
Javier-Torrent, M. et al. Presenilin/γ-secretase-dependent EphA3 processing mediates axon elongation through non-muscle myosin IIA. eLife 8, e43656 (2019).
Weiss, F., Lauffenburger, D. & Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 22, 157–173 (2022).
Sun, G. et al. A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J. Cell Biol. 216, 3355–3368 (2017).
Porazinski, S. et al. EphA2 drives the segregation of Ras-transformed epithelial cells from normal neighbors. Curr. Biol. 26, 3220–3229 (2016).
Volz, C. et al. Inhibition of tumor VEGFR2 induces serine 897 EphA2-dependent tumor cell invasion and metastasis in NSCLC. Cell Rep. 31, 107568 (2020).
Huang, Z., Liu, J., Zhang, C. & Yang, X. Lipofectamine 2000 at transfection dose promotes EphA2 transcription in an HDAC4-dependent manner to reduce its cytotoxicity. Heliyon 8, e12118 (2022).
Gao, Z. et al. Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-ephrin A1 reverse signaling. Cell Death Dis. 12, 414 (2021).
Binda, E. et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 22, 765–780 (2012).
Hu, A. X. et al. EPH profiling of BTIC populations in glioblastoma multiforme using CyTOF. Methods Mol. Biol. 1869, 155–168 (2019).
Loong, J. H. et al. Glucose deprivation-induced aberrant FUT1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma. J. Clin. Invest. 131, e143377 (2021). This study shows that aberrant fucosylation of EPHA2 and several other proteins, induced by low glucose availability in hepatocellular carcinoma, contributes to deregulation of AKT–mTOR–eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) signalling to drive cancer stemness and drug resistance.
Merlos-Suarez, A. et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011).
Chen, Y. L. et al. Ephrin A4-ephrin receptor A10 signaling promotes cell migration and spheroid formation by upregulating NANOG expression in oral squamous cell carcinoma cells. Sci. Rep. 11, 644 (2021).
Li, Y. et al. EPHA5 mediates trastuzumab resistance in HER2-positive breast cancers through regulating cancer stem cell-like properties. FASEB J. 33, 4851–4865 (2019).
Brantley-Sieders, D. M. et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 118, 64–78 (2008).
Larsen, A. B. et al. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol. Cancer Res. 5, 283–293 (2007).
Gusenbauer, S., Vlaicu, P. & Ullrich, A. HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene 32, 3846–3856 (2013).
Paul, M. D., Grubb, H. N. & Hristova, K. Quantifying the strength of heterointeractions among receptor tyrosine kinases from different subfamilies: implications for cell signaling. J. Biol. Chem. 295, 9917–9933 (2020).
Kim, J., Chang, I. Y. & You, H. J. Interactions between EGFR and EphA2 promote tumorigenesis through the action of ephexin1. Cell Death Dis. 13, 528 (2022).
Bruggemann, Y., Karajannis, L. S., Stanoev, A., Stallaert, W. & Bastiaens, P. I. H. Growth factor-dependent ErbB vesicular dynamics couple receptor signaling to spatially and functionally distinct Erk pools. Sci. Signal. 14, eabd9943 (2021). This study demonstrates that EPHA2 S897 phosphorylation, and hence non-canonical signalling, can be induced by activated EGFR family members located at the plasma membrane, but not those in endosomes, owing to activation of spatially distinct ERK pools that leads to distinct phenotypes.
Tamura, Y., Nakamizo, Y., Watanabe, Y., Kimura, I. & Katoh, H. Filamin A forms a complex with EphA2 and regulates EphA2 serine 897 phosphorylation and glioblastoma cell proliferation. Biochem. Biophys. Res. Commun. 597, 64–70 (2022).
Oh, D. et al. Competition for shared downstream signaling molecules establishes indirect negative feedback between EGFR and EphA2. Biophys. J. 121, 1897–1908 (2022).
Miao, H. et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3, 527–530 (2001).
Stallaert, W. et al. Contact inhibitory Eph signaling suppresses EGF-promoted cell migration by decoupling EGFR activity from vesicular recycling. Sci. Signal. 11, eaat0114 (2018).
Leveque, R. et al. ProNGF increases breast tumor aggressiveness through functional association of TrkA with EphA2. Cancer Lett. 449, 196–206 (2019).
Srivastava, S. et al. Activation of EPHA2-ROBO1 heterodimer by SLIT2 attenuates non-canonical signaling and proliferation in squamous cell carcinomas. iScience 23, 101692 (2020).
Acknowledgements
Work in the author’s laboratory is supported by grants from the NIH.
Author information
Authors and Affiliations
Contributions
The author researched data for the article, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The author holds several patents on Eph receptor-targeting peptides.
Peer review
Peer review information
Nature Reviews Cancer thanks Jin Chen, Peter Janes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AI-Driver: aidriver.maolab.org
cBioPortal: cbioportal.org
Integrative Onco Genomics (IntOGen): www.intogen.org
Supplementary information
Glossary
- Allosteric inhibitors
-
Inhibitors that bind to a site different from the active site of an enzyme (the ATP-binding pocket in the case of kinases). Allosteric kinase inhibitors can bind near (type III kinase inhibitor) or far (type IV kinase inhibitor) from the ATP-binding pocket.
- Anoikis
-
Cell death induced by lack of cell–substrate and cell–cell adhesion.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). Immune response causing the death of cells, such as cancer cells, coated with an antibody bound to a cell surface antigen. This involves the binding of Fcγ receptors on natural killer (NK) cells and other immune cells to the Fc portion of the antibody, especially when the antibody lacks fucosylation.
- Aptamers
-
Single-stranded oligonucleotides that bind to target molecules with high affinity and specificity through secondary structural motifs.
- Autophagy
-
Involves the incorporation of cellular components into double-membrane vesicles called autophagosomes, which eventually fuse with lysosomes enabling degradation and recycling of the cellular components.
- Cancer stem-like cells
-
(CSCs). Subpopulations of cancer cells with characteristics similar to stem and progenitor cells, including slow growth and the ability to self-renew and differentiate. Thus, CSCs can survive cancer therapies and drive tumour growth and heterogeneity.
- Chimeric antigen receptor
-
(CAR). Engineered receptor or antibody that can be expressed in T cells, dendritic cells and other immune cells for cancer immunotherapy. CARs recognize a molecule on the surface of another cell, such as a tumour cell, triggering signalling in the immune cell.
- Cytokinesis
-
Ultimate step of cell division, leading to the physical separation of daughter cells at the end of mitosis.
- Dependence receptor
-
Receptor that can mediate signals triggering programmed cell death when not bound to certain ligands.
- Epithelial-to-mesenchymal transition
-
(EMT). Process by which polarized epithelial cells lose cell–cell junctions and attachment to a basement membrane, acquiring a migratory and invasive phenotype characteristic of mesenchymal cells.
- Exosomes
-
Small (30–150 nm) extracellular vesicles that contain proteins, nucleic acids, lipids and metabolites. Produced in the endosomal compartment, exosomes are released from cells and can make contact with and be taken up by other cells.
- Inflammasome
-
Large cytosolic multiprotein complex that forms in response to infection, tissue damage or metabolic imbalance and activates inflammatory caspases.
- Long non-coding RNAs
-
(lncRNAs). Transcripts longer than 200 bp that do not code for proteins. lncRNAs can regulate gene expression by acting as endogenous ‘sponges’ that bind miRNAs, thus preventing miRNA binding to target mRNAs.
- MicroRNAs
-
(miRNAs). Single-stranded small non-coding RNAs, approximately 20 nucleotides long, that function by interacting with target mRNAs to repress translation or promote mRNA cleavage. miRNAs can function as oncogenes or tumour suppressors, and their expression is accordingly upregulated or downregulated in cancer cells.
- Neoadjuvant therapy
-
Chemotherapy, radiotherapy or other therapies given before a main treatment, for example, to shrink a tumour before surgery.
- Nonsense-mediated mRNA decay
-
Mechanism that eliminates mRNAs with a stop codon that prematurely terminates translation.
- Polyploidy
-
State in which a cell has more than two sets of chromosomes.
- Proteolysis-targeting chimeras
-
(PROTACs). Molecules that consist of a ligand for a target protein to be degraded, a linker and a ligand that recruits a E3 ubiquitin ligase triggering proteasomal degradation of the target protein.
- Single-chain variable fragment (scFv) antibody
-
Antibody that contains only the variable (antigen-binding) domain of the light and heavy chains of a monoclonal antibody, typically linked together into a single molecule.
- Synthetic lethality
-
Cell death induced by the concurrent disruption of two (or more) genes but not by the disruption of each individual gene.
- Transendocytosis
-
Process through which a plasma membrane fragment and other material from one cell is taken up by another cell, for example, through internalization of full-length Eph receptor–ephrin complexes assembled at sites of cell–cell contact. Internalization can occur in either the Eph receptor-expressing cell or the ephrin-expressing cells.
- Type I kinase inhibitors
-
Bind to the active conformation of a kinase domain, in which the aspartic acid of the DFG (aspartic acid–phenylalanine–glycine) motif in the activation loop points towards the ATP-binding pocket (DFG-in).
- Type II kinase inhibitors
-
Bind to the inactive kinase conformation, in which the aspartic acid is oriented away from the ATP-binding pocket (DFG-out).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat Rev Cancer 24, 5–27 (2024). https://doi.org/10.1038/s41568-023-00634-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00634-x