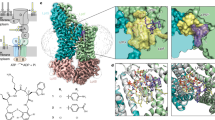
Abstract
Carbapenem-resistant Acinetobacter baumannii infections have limited treatment options. Synthesis, transport and placement of lipopolysaccharide or lipooligosaccharide (LOS) in the outer membrane of Gram-negative bacteria are important for bacterial virulence and survival. Here we describe the cerastecins, inhibitors of the A. baumannii transporter MsbA, an LOS flippase. These molecules are potent and bactericidal against A. baumannii, including clinical carbapenem-resistant Acinetobacter baumannii isolates. Using cryo-electron microscopy and biochemical analysis, we show that the cerastecins adopt a serpentine configuration in the central vault of the MsbA dimer, stalling the enzyme and uncoupling ATP hydrolysis from substrate flipping. A derivative with optimized potency and pharmacokinetic properties showed efficacy in murine models of bloodstream or pulmonary A. baumannii infection. While resistance development is inevitable, targeting a clinically unexploited mechanism avoids existing antibiotic resistance mechanisms. Although clinical validation of LOS transport remains undetermined, the cerastecins may open a path to narrow-spectrum treatment modalities for important nosocomial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study, including statistical analyses, are available within the article and its Supplementary Information or Source Data files. The cryo-EM structure and supporting data have been deposited to the PDB under the accession code 8GK7. Source data are provided with this paper.
Code availability
No proprietary code was used in this work.
References
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Ma, C. & McClean, S. Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines https://doi.org/10.3390/vaccines9060570 (2021).
Oldenkamp, R., Schultsz, C., Mancini, E. & Cappuccio, A. Filling the gaps in the global prevalence map of clinical antimicrobial resistance. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2013515118 (2021).
Nikaido, H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264, 382–388 (1994).
Zgurskaya, H. I. & Rybenkov, V. V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 1459, 5–18 (2020).
Walker, S. S. & Black, T. A. Are outer-membrane targets the solution for MDR Gram-negative bacteria? Drug Discov. Today 26, 2152–2158 (2021).
Doerrler, W. T., Gibbons, H. S. & Raetz, C. R. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J. Biol. Chem. 279, 45102–45109 (2004).
Guo, D. et al. Energetics of lipid transport by the ABC transporter MsbA is lipid dependent. Commun. Biol. 4, 1379 (2021).
Mi, W. et al. Structural basis of MsbA-mediated lipopolysaccharide transport. Nature 549, 233–237 (2017).
Zhou, Z., White, K. A., Polissi, A., Georgopoulos, C. & Raetz, C. R. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 273, 12466–12475 (1998).
Zhang, G. et al. Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. Proc. Natl Acad. Sci. USA 115, 6834–6839 (2018).
Ho, H. et al. Structural basis for dual-mode inhibition of the ABC transporter MsbA. Nature 557, 196–201 (2018).
Verma, V. A. et al. Discovery of inhibitors of the lipopolysaccharide transporter MsbA: from a screening hit to potent wild-type gram-negative activity. J. Med. Chem. 65, 4085–4120 (2022).
Zhang, G., Meredith, T. C. & Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 16, 779–785 (2013).
Boll, J. M. et al. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc. Natl Acad. Sci. USA. 113, E6228–E6237 (2016).
de Berardinis, V. et al. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol. Syst. Biol. 4, 174 (2008).
Moffatt, J. H. et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54, 4971–4977 (2010).
Peng, D., Hong, W., Choudhury, B. P., Carlson, R. W. & Gu, X. X. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect. Immun. 73, 7569–7577 (2005).
Richie, D. L. et al. Toxic accumulation of LPS pathway intermediates underlies the requirement of LpxH for growth of Acinetobacter baumannii ATCC 19606. PLoS ONE 11, e0160918 (2016).
Steeghs, L. et al. Meningitis bacterium is viable without endotoxin. Nature 392, 449–450 (1998).
Wei, J. R. et al. LpxK is essential for growth of Acinetobacter baumannii ATCC 19606: relationship to toxic accumulation of lipid A pathway intermediates. mSphere https://doi.org/10.1128/mSphere.00199-17 (2017).
Raetz, C. R. & Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002).
Beceiro, A. et al. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob. Agents Chemother. 58, 518–526 (2014).
Garcia-Quintanilla, M. et al. Lipopolysaccharide loss produces partial colistin dependence and collateral sensitivity to azithromycin, rifampicin and vancomycin in Acinetobacter baumannii. Int. J. Antimicrob. Agents 46, 696–702 (2015).
Barb, A. W. et al. Inhibition of lipid A biosynthesis as the primary mechanism of CHIR-090 antibiotic activity in Escherichia coli. Biochemistry 46, 3793–3802 (2007).
Bonifer, C. & Glaubitz, C. MsbA: an ABC transporter paradigm. Biochem. Soc. Trans. 49, 2917–2927 (2021).
Konovalova, A., Kahne, D. & Silhavy, T. J. Outer membrane biogenesis. Annu. Rev. Microbiol. 71, 539–556 (2017).
Gross, S. et al. Improved broad-spectrum antibiotics against Gram-negative pathogens via darobactin biosynthetic pathway engineering. Chem. Sci. 12, 11882–11893 (2021).
Imai, Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019).
Kaur, H. et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 593, 125–129 (2021).
Wuisan, Z. G., Kresna, I. D. M., Bohringer, N., Lewis, K. & Schaberle, T. F. Optimization of heterologous darobactin A expression and identification of the minimal biosynthetic gene cluster. Metab. Eng. 66, 123–136 (2021).
Cohen, F. et al. Optimization of LpxC inhibitors for antibacterial activity and cardiovascular safety. ChemMedChem 14, 1560–1572 (2019).
Fujita, K. et al. Pharmacodynamic target assessment and prediction of clinically effective dosing regimen of TP0586532, a novel non-hydroxamate LpxC inhibitor, using a murine lung infection model. J. Infect. Chemother. 28, 635–642 (2022).
Piizzi, G. et al. Design, synthesis, and properties of a potent inhibitor of Pseudomonas aeruginosa deacetylase LpxC. J. Med. Chem. 60, 5002–5014 (2017).
Robinson, J. A. Folded synthetic peptides and other molecules targeting outer membrane protein complexes in gram-negative bacteria. Front. Chem. 7, 45 (2019).
Thélot, F. A. et al. Distinct allosteric mechanisms of first-generation MsbA inhibitors. Science 374, 580–585 (2021).
Carretero-Ledesma, M. et al. Phenotypic changes associated with colistin resistance due to lipopolysaccharide loss in Acinetobacter baumannii. Virulence 9, 930–942 (2018).
Coenye, T. & Vandamme, P. Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes. FEMS Microbiol. Lett. 228, 45–49 (2003).
Karakonstantis, S. A systematic review of implications, mechanisms, and stability of in vivo emergent resistance to colistin and tigecycline in Acinetobacter baumannii. J. Chemother. 33, 1–11 (2021).
Novovic, K. & Jovcic, B. Colistin resistance in Acinetobacter baumannii: molecular mechanisms and epidemiology. Antibiotics https://doi.org/10.3390/antibiotics12030516 (2023).
Shields, R. K., Paterson, D. L. & Tamma, P. D. Navigating available treatment options for carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex infections. Clin. Infect. Dis. 76, S179–S193 (2023).
Zampaloni, C. et al. A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 625, 566–571 (2024).
Lai, M. T. et al. Doravirine and islatravir have complementary resistance profiles and create a combination with a high barrier to resistance. Antimicrob. Agents Chemother. 66, e0222321 (2022).
Tripathi, P. K. et al. Screening and evaluation of approved drugs as inhibitors of main protease of SARS-CoV-2. Int. J. Biol. Macromol. 164, 2622–2631 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Stein, N. CHAINSAW: a program for mutating pdb files used as templates in molecular replacement. J. Appl. Crystallogr. 41, 641–643 (2008).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Drusano, G. L., Liu, W., Kulawy, R. & Louie, A. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob. Agents Chemother. 55, 5300–5305 (2011).
Zuluaga, A. F. et al. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6, 55 (2006).
Powers, M. J. & Trent, M. S. Expanding the paradigm for the outer membrane: Acinetobacter baumannii in the absence of endotoxin. Mol. Microbiol. 107, 47–56 (2018).
Acknowledgements
We thank D. Libardo and S. Dong for their careful review of the paper and helpful suggestions, as well as K. Smith, S. Zhou and I. Etim for their help with the plasma protein binding determinations. We thank the scientists at Evotec for their contributions to protein production and the scientists at HD Biosciences for testing the expanded strain panel including the CRAB isolates.
Author information
Authors and Affiliations
Contributions
S.S.W., C.J.B., P.S., H.W., J.S., I.R., R.T., T.A.B., A.I., Y.-T.C., D.J.K. and M.B. designed the experiments and wrote the paper. H.W. and R.E.P. designed and conducted microbial target identification and biological experiments. K.B., Z.W., J.S., A.W.S., C.W., L.T., M.L., H.J.M., D.S., J.M., T.M., W.L., J.M., A.C., A.B., L.-K.Z., M.X. and J.L. designed, constructed or analysed chemical matter. R.T., R.R.M., A.L. and T.D.C. designed and conducted formulation and pharmacokinetics studies, and analysed data. P.S., D.M. and H.L. designed, conducted and analysed in vivo efficacy studies. A.I., Y.-T.C., D.J.K. and G.S. designed and conducted cryo-EM structure experiments. C.B.-T., L.D. and M.B. designed and conducted biochemical experiments. Y.L., J.C.X., Q.S., P.A.M. and R.E.P. conducted antimicrobial and cell-based assays.
Corresponding author
Ethics declarations
Competing interests
All authors are or were, at the time this work was conducted, employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may be shareholders in Merck & Co., Inc., Rahway, NJ, USA. Cerastecin A–D are the subjects of two patent applications (PCT/US2023/022198 and PCT/US2023/022200) by Merck Sharp & Dohme LLC, Rahway, NJ, USA. I.R., C.J.B., J.S., M.L., H.J.M., L.T., H.W., C.W., A.B., L.-K.Z., J.L., K.B., Z.W., A.W.S. and A.C. are inventors on either or both applications.
Peer review
Peer review information
Nature Microbiology thanks Russell Bishop, Ian Seiple, Hendrik van Veen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bactericidal activity of cerastecin B.
The time-dependent effects of cerastecin B exposure at 8 X MIC on A. baumannii ATCC19606 and CLB 21655 cell viability. Results from independent single experiments using two A. baumannii strains, ATCC19606 and CLB21655 are shown.
Extended Data Fig. 2 Chemical genetic confirmation of cerastecin mechanism of action.
a, Sensitivity of wild-type (upper panel), cerastecin B-resistant (B5, middle panel), and lpxA deleted (lower panel) A. baumannii ATCC19606 to the indicated compounds. Dilutions of compounds from high (top) to low (bottom), indicated by black wedge, were placed directly on the agar surface. Col, colistin; Rif, rifampicin; Bac, bacitracin. Photographs are unprocessed and uncropped. b, Detection of LOS in wild-type and cerastecin B-resistant mutant B5 A. baumannii ATCC19606 by the HEK-Blue™ cell assay. Test done in duplicate, both data sets shown. c, RT-qPCR to measure relative expression of MsbA in wild-type, mutant B1 (V39F) and B3 (K6K) A. baumannii ATCC19606 (n = 4). Data plotted with mean +/- SEM. Statistical significance relative to the wild-type strain is denoted as * (p = 0.003) and *** (p = 0.00038) was analyzed in MS Excel (v. 2302) using a two-tailed Student t-test. d, Sensitivity of wild-type (upper panel), cerastecin B-resistant mutants B1 (V39F) and B3 (K6K) to the indicated compounds. Dilutions of compounds were placed directly on the agar surface from high to low concentration as in a. Cer. B, cerastecin B; other abbreviations as above. The LpxC inhibitor CHIR-9025 was used at 4 mg mL−1 in the agar. Photographs are unprocessed and cropped only to fit space constraints and allow easier comparisons between images.
Extended Data Fig. 3 MsbA-cerastecin B binding, kinetics, and activity in vitro.
a, Kinetic binding parameters and affinity of cerastecin B binding to wild-type MsbA in nanodiscs. b, Activation of the ATPase activity of MsbA in proteoliposome (lipo) or in amphipols (amphi). Tested in duplicate, each data point shown for comparison. c, Michaelis-Menten parameters for cerastecin B stimulation of wild-type MsbA in nanodiscs (mean ± std. dev.). Supporting data. d, Image of instrument output of single cycle binding kinetic data. Red line, experimental sensogram trace; black line, single cycle kinetic fit. Axis details enlarged for readability. e, Image of binding affinity fit data from panel d (red diamonds, experimental data; black line, fit of data). Axis details enlarged for readability.
Extended Data Fig. 4 Pharmacokinetics of cerastecin D in mice.
Three fasted C57BL/6 mice were dosed subcutaneously at 300 mg kg−1 and tail vein samples were taken at the indicated times for quantitation by LC-MS as described. Data for all three mice are shown with the mean values at each time point in grey. Dashed lavender line indicates the serum-shifted MIC for cerastecin D (Table 1).
Extended Data Fig. 5 Molecular interactions of inhibitors with MsbA.
a, Cryo-EM structure depicting important amino acid residues in both MsbA monomers involved in cerastecin binding. Predicted hydrogen bonds are shown as yellow dashed lines. b, Overlay of the cerastecin C binding pocket with the existing MsbA binding structures. LPS is shown in stick form, Genentech inhibitor (G907, Fig. 1b) is shown as cyan spheres (both from PDB ID 6BPL), cerastecin C and AMP-PNP are shown as purple and green spheres respectively. c, Overlay of cerastecin C binding with TBT1 (Fig. 1b) (PDB ID 7MET). Ordered (shown in red) and disordered (shown in yellow) protomers of the TBT1-bound structure are overlayed with cerastecin C bound MsbA. TBT1 and cerastecin C molecules are shown as sticks in green and magenta, respectively.
Supplementary information
Supplementary Information
Cryo-EM image capture information, RT-qPCR primer sequences and synthesis of cerastecin A–D.
Source data
Source Data Fig. 2b,c
MsbA ATPase activation and MsbA (V39F) characterization.
Source Data Fig. 4
In vivo efficacy data.
Source Data Extended Data Fig. 1
Bactericidal activity of cerastecin B.
Source Data Extended Data Fig. 2b,c
Chemical genetic confirmation of cerastecin mechanism of action.
Source Data Extended Data Fig. 3b
MsbA ATPase activation in lipid environments.
Source Data Extended Data Fig. 4
Pharmacokinetics of cerastecin D.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Ishchenko, A., Skudlarek, J. et al. Cerastecins inhibit membrane lipooligosaccharide transport in drug-resistant Acinetobacter baumannii. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01667-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41564-024-01667-0